Abstract
The sequence of the first 149 nucleotides of the major leftward RNA of bacteriophage lambda has been determined. Preliminary sequence information was also obtained for a portion of the untranscribed area immediately upstream of the point on the template when RNA synthesis normally starts. Several restriction endonuclease sites, deletion endpoints, and single base changes have been localized within the sequence. The first potential translation initiation codon which is not followed by an in-phase termination codon is a GUG located 90 nucleotides from the transcription startpoint.
Full text
PDF





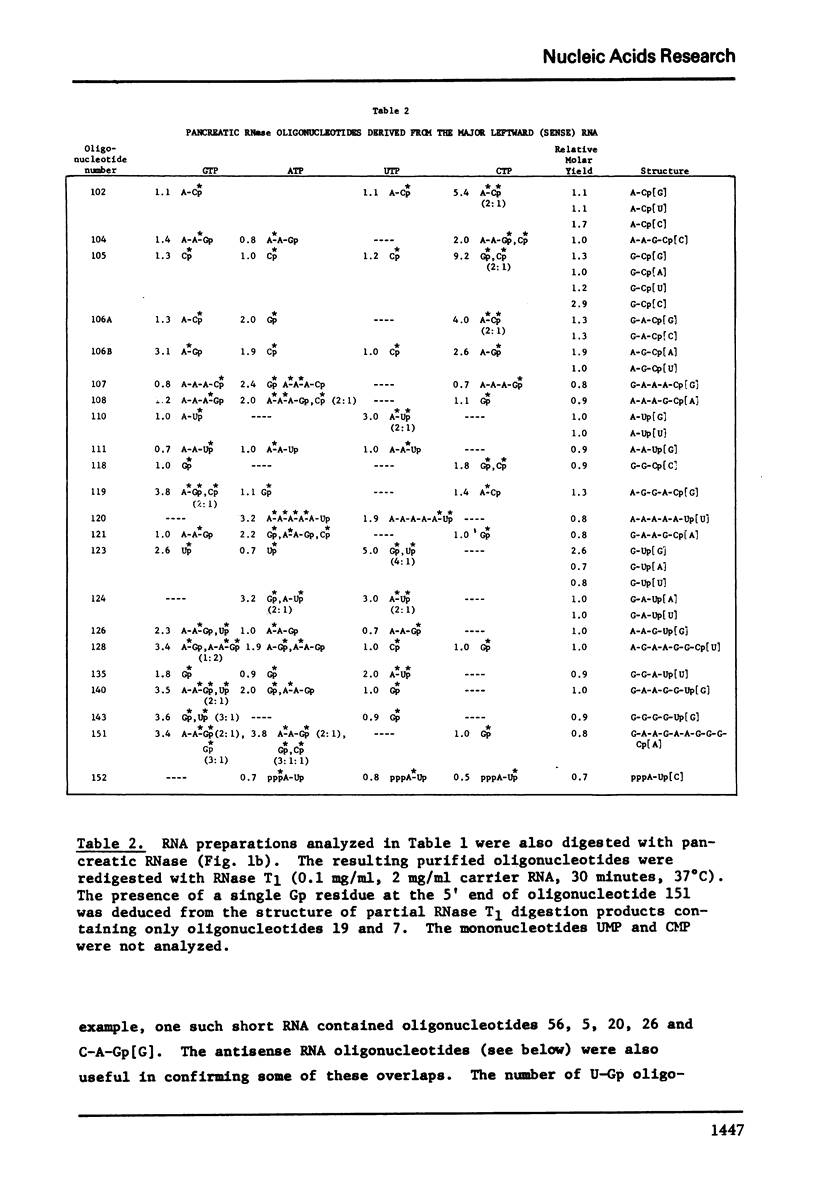











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B. Fragments produced by cleavage of lambda deoxyribonucleic acid with the Hemophilus parainfluenzae restriction enzyme Hpa II. Biochemistry. 1973 Sep 25;12(20):3972–3977. doi: 10.1021/bi00744a029. [DOI] [PubMed] [Google Scholar]
- Allet B., Solem R. Separation and analysis of promoter sites in bacteriophage lambda DNA by specific endonucleases. J Mol Biol. 1974 Jan 5;85(4):475–484. doi: 10.1016/0022-2836(74)90310-6. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Dahlberg J. E., Goodman H. M., Hindley J., Weissmann C. Nucleotide sequence analysis of an enzymatically synthesized RNA corresponding to the 5'-terminal region of Q beta RNA. Cold Spring Harb Symp Quant Biol. 1969;34:635–646. doi: 10.1101/sqb.1969.034.01.073. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E., Boettiger J. K., Fiandt M., Szybalski W. Distance from a promoter mutation to an RNA synthesis startpoint on bacteriophage lambda DNA. Nat New Biol. 1972 Jun 21;237(77):232–236. doi: 10.1038/newbio237232a0. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E. RNA synthesis startpoints in bacteriophage lambda: are the promoter and operator transcribed? Nat New Biol. 1972 Jun 21;237(77):227–232. doi: 10.1038/newbio237227a0. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Fiandt M., Hass K. K., Twose P. A., Szybalski W. Deletions and insertions in the immunity region of coliphage lambda: revised measurement of the promoter-startpoint distance. Virology. 1974 Dec;62(2):458–471. doi: 10.1016/0042-6822(74)90407-3. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Contreras R., Fiers W. A new method for partial digestion useful for sequence analysis of polynucleotides. FEBS Lett. 1971 Sep 1;16(4):281–283. doi: 10.1016/0014-5793(71)80370-8. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M., Radding C. M. Nucleotide sequence of a ribonucleic acid transcribed in vitro from lambda phage deoxyribonucleic acid. J Biol Chem. 1971 Aug 25;246(16):5120–5139. [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Maurer R. Control elements in the DNA of bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1974;38:857–868. doi: 10.1101/sqb.1974.038.01.088. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M. Structure of the lambda operators. Nature. 1973 Nov 16;246(5429):133–136. doi: 10.1038/246133a0. [DOI] [PubMed] [Google Scholar]
- Marcaud L., Portier M. M., Kourilsky P., Barrell B. G., Gros F. A low molecular weight species of RNA synthesized early after induction of the prophage lambda. J Mol Biol. 1971 Apr 28;57(2):247–261. doi: 10.1016/0022-2836(71)90344-5. [DOI] [PubMed] [Google Scholar]
- Maurer R., Maniatis T., Ptashne M. Promoters are in the operators in phage lambda. Nature. 1974 May 17;249(454):221–223. doi: 10.1038/249221a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Preparation of [alpha-32P]nucleoside and deoxynucleoside 5'-triphosphates from 32Pi and protected and unprotected nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):548–550. doi: 10.1016/0005-2787(69)90105-1. [DOI] [PubMed] [Google Scholar]
- Szybalski W., Bovre K., Fiandt M., Guha A., Hradecna Z., Kumar S., Lozeron H. A., Sr, Maher V. M., Nijkamp H. J., Summers W. C. Transcriptional controls in developing bacteriophages. J Cell Physiol. 1969 Oct;74(2 Suppl):33–70. doi: 10.1002/jcp.1040740405. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]



