Abstract
Triclosan (TCS), a high-production-volume chemical used as a bactericide in personal care products, is a priority pollutant of growing concern to human and environmental health. TCS is capable of altering the activity of type 1 ryanodine receptor (RyR1), but its potential to influence physiological excitation–contraction coupling (ECC) and muscle function has not been investigated. Here, we report that TCS impairs ECC of both cardiac and skeletal muscle in vitro and in vivo. TCS acutely depresses hemodynamics and grip strength in mice at doses ≥12.5 mg/kg i.p., and a concentration ≥0.52 μM in water compromises swimming performance in larval fathead minnow. In isolated ventricular cardiomyocytes, skeletal myotubes, and adult flexor digitorum brevis fibers TCS depresses electrically evoked ECC within ∼10–20 min. In myotubes, nanomolar to low micromolar TCS initially potentiates electrically evoked Ca2+ transients followed by complete failure of ECC, independent of Ca2+ store depletion or block of RyR1 channels. TCS also completely blocks excitation-coupled Ca2+ entry. Voltage clamp experiments showed that TCS partially inhibits L-type Ca2+ currents of cardiac and skeletal muscle, and [3H]PN200 binding to skeletal membranes is noncompetitively inhibited by TCS in the same concentration range that enhances [3H]ryanodine binding. TCS potently impairs orthograde and retrograde signaling between L-type Ca2+ and RyR channels in skeletal muscle, and L-type Ca2+ entry in cardiac muscle, revealing a mechanism by which TCS weakens cardiac and skeletal muscle contractility in a manner that may negatively impact muscle health, especially in susceptible populations.
Keywords: cachexia, calcium regulation, heart failure, muscle contraction, L-type current
Triclosan [5-chloro-2-(2,4-dichlorophenoxy)phenol; TCS] is a bactericide found in many personal care products and has been incorporated into cleaning supplies, bedding, clothes, fabrics, shoes, carpets, plastics, and medical devices (1–3). Introduced over 40 y ago, TCS production has dramatically increased over the past two decades, exceeding 1 million pounds produced annually when assessed in 1998 by the US Environmental Protection Agency (USEPA) (1). Based on the available data, TCS is well tolerated following oral or dermal exposure, and several aspects of safety have been assessed in animal models (2). However, TCS has been detected in raw and treated waste water, natural streams, sewage sludge, fish, and human samples of urine, plasma, and breast milk (4–9), raising concerns of its potential long-term adverse impacts on environmentally sensitive species and human health. There is emerging evidence that TCS can disrupt endocrine signaling and immune function, inhibit carboxylesterase activity, bioaccumulate in organisms, and cause bacterial resistance (10–15).
Structurally TCS, a polychlorinated diphenyl ether, shares chemical properties with nondioxin-like persistent organic pollutants, such as the hydroxylated metabolites of ortho-substituted polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). Receptor-based screens (12, 16) revealed that similar to PCBs (17) and PBDEs (18), TCS has potential for direct interaction with type 1 and type 2 ryanodine receptors (RyR1 and RyR2), supporting a possible convergent mechanism of action among structurally related noncoplanar halogenated persistent organic pollutants. Despite extensive analysis of TCS efficacy and toxicity, the potential of TCS to alter cardiac and skeletal muscle physiology have not been previously reported (2). Considering that RyRs are intracellular channels that mediate the release of Ca2+ from the sarcoplasmic reticulum (SR), necessary for excitation–contraction coupling (ECC) in skeletal and cardiac muscle, alteration in ECC by TCS may promote acute or long-term impacts on muscle health in human populations and environmentally sensitive species. Moreover, mutations in the RYR1 gene are linked to dysregulated Ca2+ homeostasis in heritable diseases of skeletal muscle, including malignant hyperthermia and central core disease (19, 20), and aging related muscle weakness (21). Mutations in the RYR2 gene are responsible for arrhythmogenic disorders, including catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular dysplasia type 2 (20, 22), and are etiological contributors to ischemic heart failure (23). Considering the critical role of RyR channels in muscle physiology and pathophysiology, we deemed it necessary to investigate whether TCS has the potential of impairing ECC of cardiac and skeletal muscle in vivo and in vitro to better assess the potential risks associated with its pervasive use.
Results
TCS Depresses Cardiac Hemodynamics and Skeletal Muscle Contractility in Vivo.
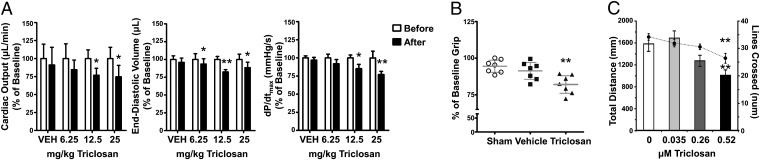
Whether acute exposures to TCS were capable of altering functional parameters of striated muscle were investigated using three in vivo tests. First, anesthetized mice instrumented with a pressure-sensitive catheter were exposed to a single intraperitoneal dose of TCS (6.25, 12.5, or 25 mg/kg) or an equal volume of vehicle (20% vol/vol DMSO in saline, 200 μL). In contrast to vehicle control, mice receiving TCS showed significantly impaired hemodynamic functions within 10 min of exposure in a dose-dependent manner. Cardiovascular impairments included significantly reduced cardiac output, lower left ventricular end-diastolic volume, and reduction in the maximum time-derivative of the left ventricular pressure development (Fig. 1A). Of note, the highest-dose group (25 mg/kg) exhibited a 25.3 ± 15.7% decrease in cardiac output, implying that TCS was capable of eliciting severe cardiovascular impairments. The administration of TCS to mice led to dose-dependent plasma levels of unconjugated TCS, with up to 0.31 ± 0.09 μM detected in the samples from the 25-mg/kg dose group (Fig. S1).
Fig. 1.
TCS depresses hemodynamics and in vivo skeletal muscle function. (A) Mice (vehicle: n = 3; TCS: n = 4–5) were anesthetized with ketamine/xylazine and instrumented with recording PV catheter advanced into the left ventricle via the carotid artery. After dosing with TCS (20% DMSO vol/vol in 200 μL) intraperitoneally, both 12.5 and 25 mg/kg groups experienced significant reductions in cardiac output, ventricular filling, and left ventricular developed pressure. Error bars represent SEM. *P < 0.05; **P < 0.01, one-tailed paired t test. (B) Grip strength was assessed in mice (n = 7 per group) before and after sham, vehicle (30 μL DMSO), or 40 mg/kg TCS intraperitoneal injection. Postdose grip was significantly lower in the TCS group compared with both sham and vehicle. Gray bars represent mean and 95% CI. **P < 0.01, ANOVA. (C) Larval fathead minnow (n = 36 per concentration) were exposed up to 7 d to vehicle (0.01% MeOH), or TCS (0.035, 0.26, or 0.52 μM). Swimming behavior was assessed by nonprovoked (distance traveled, bar graph) and forced (lines crossed in 60 s, line graph) activity. A decreasing trend was seen for both swimming parameters, with significance found in the 0.52-μM group. Error bars represent SEM. **P < 0.01, Kruskal-Wallis.
Second, skeletal muscle function was assessed using a grip-strength meter by measuring the total force produced by the limbs of mice at test times up to 60 min after a single 40 mg/kg i.p. TCS dose. Normalized to predose baseline, TCS-treated animals had a mean decrease of 18% [95% confidence interval (CI): 12.0–24.0%] in grip strength, which was significantly lower than both sham- and vehicle-injected groups (Fig. 1B). No statistical difference was seen between sham- and vehicle-injected groups. These impairments were transitory and exposed mice recovered grip strength comparable to baseline 24 h after dosing.
Third, the fathead minnow (Pimephales promelas) has been extensively used as model organism to study the potential impacts of aquatic pollutants on teleost (24). Considering the pervasive nature of TCS in the aquatic environment, and TCS-mediated impairments detected in mammalian striated muscle, we investigated whether TCS adversely affected fish swimming performance. Larval fathead minnow were exposed to TCS (0.035, 0.26, or 0.52 μM) for up to 7 d and swimming performance assessed at test termination. At the highest TCS concentration tested, swimming activity, as well as predator avoidance and endurance, were significantly reduced when measured as total distance swam during nonprovoked swimming (Fig. 1C, bar graph) and the number of lines crossed during a forced swim test (Fig. 1C, line graph), respectively. Minnows exposed to 0.26 μM TCS exhibited reduced nonprovoked and forced swimming behavior, although the magnitude of these impairments did not reach statistical significance (Fig. 1C).
TCS Impairs ECC in Vitro.
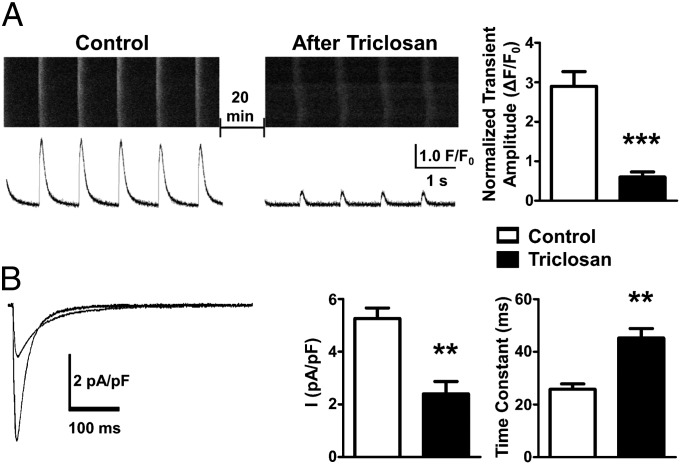
The molecular mechanisms responsible for the impairments in muscle performance observed in vivo were investigated using primary cells isolated from murine cardiac and skeletal muscle. Cardiomyocytes were isolated from excised naive mouse hearts, loaded with the fluorescent Ca2+ indicator Fluo-4, and electrically paced at 1 Hz to evoke Ca2+ transients. After 20 min of exposure to 10 μM TCS, the evoked Ca2+ transients were greatly depressed with normalized peak amplitudes reduced by 79.3 ± 5.2% compared with cells exposed to equivalent vehicle as control (Fig. 2A). L-type Ca2+ entry through Cav1.2 (also termed the dihydropyridine receptor; DHPR) is essential for initiating cardiac ECC through a process termed Ca2+-induced Ca2+-release mediated as a consequence of RyR2 activation (25). Using the same TCS exposure protocol, voltage-clamped cardiomyocytes showed that 20 min after exposure to 10 μM TCS the peak L-type Ca2+ current was attenuated by 54.4 ± 9.6% compared with cardiomyocytes exposed to vehicle (Fig. 2B, Center), and the inactivation time constant was prolonged from 25.8 ± 2.0 ms to 45.2 ± 3.6 ms (Fig. 2B, Right).
Fig. 2.
TCS impairs cardiac ECC. (A) Isolated mouse cardiomyocytes (n = 5) were loaded with the fluorescent Ca2+ indicator Fluo-4. RyR2-mediated Ca2+ transients were evoked by field stimulation and measured by line-scan confocal imaging. Twenty minutes after applying 10 μM TCS, Ca2+ transient amplitudes were significantly reduced by 79.3 ± 5.2%. (B) Cardiomyocytes (n = 5) were patch-clamped in whole-cell configuration, and depolarized by voltage protocol. Exposure to 10 μM TCS for 20 min significantly reduced peak inward currents through Cav1.2 by 54.4 ± 9.6% and prolonged the inactivation time constant from 25.8 ± 2.0 ms to 45.2 ± 3.6 ms. All error bars represent SEM. **P < 0.01; ***P < 0.001, t test.
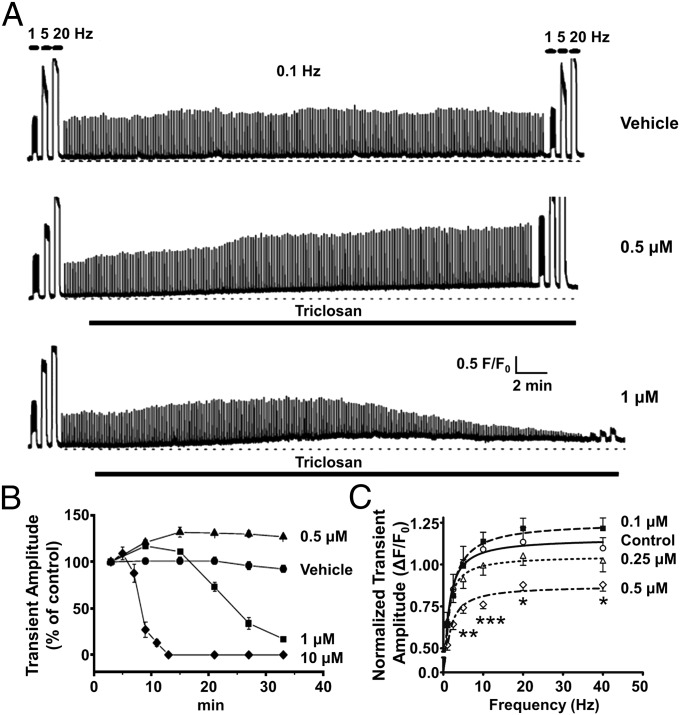
The acute influences of TCS on ECC were assessed in more detail with mouse embryonic skeletal muscle cells (myotubes). Myotubes were stimulated with a standardized protocol that included electrical pulse trains ranging from 0.1 to 20 Hz over an ∼30-min test period. As shown in Fig. 3A (Top), perfusion of vehicle had no measurable influence on the amplitude or kinetics of Ca2+ transients applied during the protocol. However, inclusion of TCS (0.5 μM) in the perfusate caused a gradual rise in baseline Ca2+ and significantly potentiated the maximum amplitude (corrected for baseline) of electrically evoked Ca2+ transients for the duration of the experiment (Fig. 3A, Middle). In contrast, 1 μM TCS initially potentiated Ca2+ transient amplitudes but invariably produced a subsequent diminution and eventual loss of electrically evoked Ca2+ transients (Fig. 3A, Bottom). The rapidity with which ECC was lost was highly dependent on the TCS concentration, with 10 μM TCS causing a complete failure of ECC within ∼10 min (Fig. 3B; see also Fig. 6).
Fig. 3.
TCS impairs skeletal ECC. (A) Mouse skeletal myotubes were loaded with the Ca2+ indicator Fluo-4 and electrically stimulated. Compared with vehicle (0.1% DMSO; Top) perfusion, 0.5 μM TCS significantly potentiated Ca2+ transient amplitudes (Middle). A similar potentiation was initially seen with 1 μM TCS, but this invariably led to the diminution and complete abrogation of electrically evoked Ca2+ transients (Bottom). (B) The effect of TCS on ECC is highly dose-dependent, with 10 μM causing rapid ECC failure within 12.5 min. All TCS exposures significantly altered the Ca2+ transient amplitude responses to single electrical test pulses (P < 0.01). Error bars represent SEM. (C) Exposure to submicromolar TCS for 24 h was sufficient to alter transient amplitudes in myotubes, with 0.5 μM TCS producing significant reductions at 5–40 Hz stimulus frequencies. Error bars represent SEM. *P < 0.05; **P < 0.01; ***P < 0.001, two-way ANOVA.
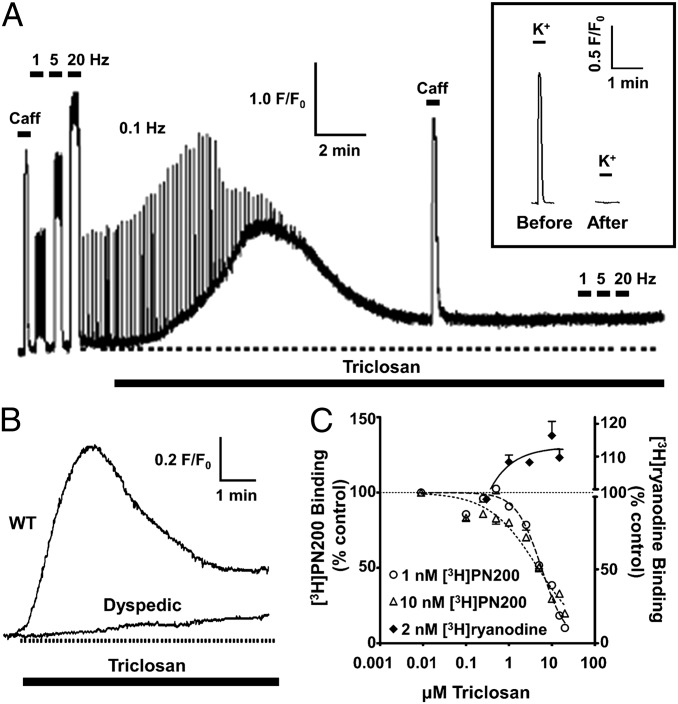
Fig. 6.
TCS concomitantly affects both RyR and DHPR activity without depleting SR Ca2+ stores. (A) Mouse skeletal myotubes were loaded with the Ca2+ indicator Fluo-4 and electrically stimulated. Perfusion with 10 μM TCS resulted in a profound change in resting Ca2+, as well as a rapid diminution and abrogation of Ca2+ transients. Challenge with 20 mM caffeine (Caff) indicates SR Ca2+ stores were not depleted. (Inset) In separate experiments, after TCS caused failure of myotubes to respond to electrical stimuli, challenge with 60 mM KCl (K+) also failed to elicit ECC. (B) Dyspedic myotubes lacking RyR1 exhibited little change in cytosolic Ca2+ when exposed to TCS, implicating RyR1 as a molecular target of TCS. (C) Mouse skeletal muscle preparations were incubated with [3H]ryanodine or [3H]PN200 in the presence of TCS. TCS increased specific [3H]ryanodine binding with respect to control, whereas specific [3H]PN200 binding was inhibited noncompetitively across similar TCS concentrations.
Myotubes exposed to TCS for 24 h before testing ECC revealed the drug’s higher potency with longer (subacute) exposures. Exposure to as low as 0.1 μM TCS enhanced myotube Ca2+ transient amplitudes tested at ≥10 Hz stimuli, although this effect did not reach statistical significance. Nevertheless, TCS (0.5 μM) caused a significant depression of Ca2+ transient amplitudes at ≥5 Hz stimulus frequencies tested (Fig. 3C).
Acute exposure to TCS (10 μM) also caused a rapid failure of ECC in flexor digitorum brevis (FDB) muscle fibers isolated from adult mice (Fig. S2A), without causing either an initial increase in baseline Ca2+ or a potentiation of the Ca2+ transient amplitude, as was observed with myotubes. Subacute (24 h) exposure to lower TCS (0.5 μM) significantly reduced the number of responsive FDB fibers by 44.7 ± 8.6% (Fig. S2B).
TCS is known to tightly bind serum protein (2), and we therefore tested if the presence of BSA could prevent its acute actions on ECC in myotubes. BSA perfused in the imaging buffer at either 0.1% or 0.5% (final concentration) did not alter EC coupling responses (Fig. S3A). Even in the presence of 0.1% BSA, TCS (10 μM) impaired ECC in 67% of the cells (Fig. S3B), whereas TCS in the presence of 0.5% BSA impaired ECC in 22% of myotubes treated with 10 μM TCS (Fig. S3C). In all cases, however, serum albumin was unable to repress increases in resting Ca2+ levels, indicating that serum proteins alone cannot fully abate the adverse effects of TCS in myotubes.
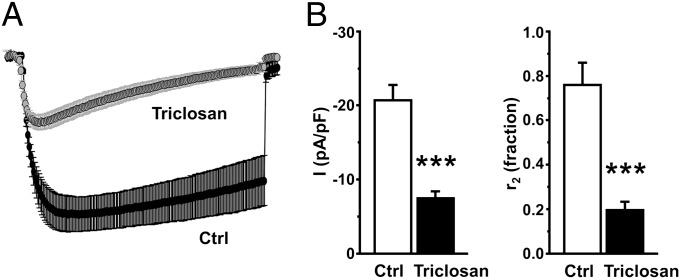
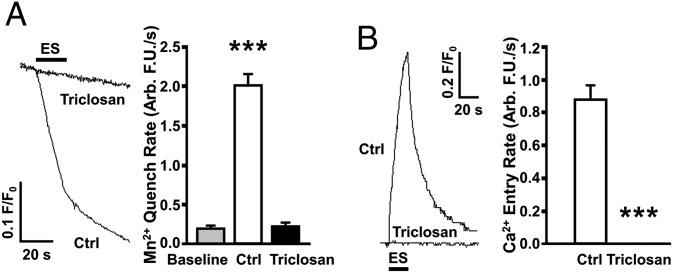
In contrast to cardiac ECC, L-type Ca2+ entry through Cav1.1 is not required for initiating skeletal muscle ECC (26, 27). Instead, a form of conformational coupling engages bidirectional signaling between the voltage sensor of Cav1.1 and RyR1 activation (28, 29). Exposure to 10 μM TCS for 10 min before measuring Ca2+ current attenuated peak L-type Ca2+ current by nearly threefold compared with myotubes exposed to vehicle control (Fig. 4B, Left), and also caused a ∼fourfold acceleration of inactivation (Fig. 4B, Right). Although Ca2+ entry through Cav1.1 is not necessary to intially trigger ECC in skeletal muscle cells, the entry of Ca2+ through a mechanism requiring Cav1.1 appears to be essential for maintaining Ca2+ stores during prolonged physiological stimuli in a process termed excitation-coupled Ca2+ entry (ECCE) (30, 31). We therefore tested if TCS interfered with myotube ECCE using two approaches. First, ECCE was measured as the rate of quench of cytoplasmic fura-2 fluorescence as extracellular Mn2+ enters the myotube in response to a train of electrical pulses (40 Hz). As shown in Fig. 5A, 10 μM TCS caused a complete block of ECCE. Second, myotubes were pretreated with 500 μM ryanodine (30 min) to block RyR1, which was verified by the loss of responses to challenge with caffeine. In contrast to control cells that responded to a 40-Hz electrical pulse with a large Ca2+ entry, myotubes exposed to TCS (10 μM) failed to respond to an electrical pulse train (Fig. 5B). Importantly, inhibition of ECCE appears to be a selective action of TCS because it failed to inhibit store-operated Ca2+ entry (SOCE) subsequent to depletion of SR Ca2+ stores by thapsigargin (Fig. S4).
Fig. 4.
TCS attenuates skeletal muscle L-type Ca2+ current. (A) Averaged recordings of L-type Ca2+ currents elicited by 2-s depolarizations from −50 mV to +30 mV are shown for untreated normal myotubes (black circle, n = 4) or normal myotubes exposed to 10 μM TCS for 15–20 min at ∼25 °C (gray circle, n = 9). (B) Summary of peak current densities (Left) and r2 values (Right) for myotubes in the presence and absence of 10 μM TCS. r2 is ratio of the current remaining at 2 s to the peak current. Error bars represent SEM. ***P < 0.001, t test.
Fig. 5.
TCS inhibits excitation-coupled Ca2+ entry. (A) The rate of ECCE was measured using Mn2+ quench of Fura-2 fluorescence at the isosbestic wavelength. Electrical stimuli (ES) were applied to myotubes perfused with 500 μM Mn2+. Myotubes exposed to 10 μM TCS had negligible fluorescence quench compared with control, suggesting an inhibition of ECCE. (B) Ca2+ entry was monitored in ryanodine-treated myotubes loaded with the Ca2+ indicator Fluo-4. Ca2+ entry was induced by ES. Ryanodine-treated myotubes did not respond to caffeine, hence the rising signal represented pure ECCE, a process completely suppressed by 10 μM TCS. All error bars represent SEM. ***P < 0.001, t test.
Molecular Targets of TCS-Mediated ECC Impairment.
ECC in skeletal muscle invariably relies on bidirectional signaling between Cav1.1 and RyR1, which form a conformationally coupled unit. To further investigate whether TCS directly targets Cav1.1, RyR1, or both, we investigated its mechanism of action in wild-type and dyspedic (RyR1-null) myotubes. In addition to sequentially potentiating then blocking ECC, TCS (10 μM) caused a pronounced although transient rise in resting Ca2+ (Fig. 6A). After recovery to a slightly elevated baseline, brief challenge with caffeine (20 mM) produced a Ca2+ transient, the magnitude of which was not different compared with the caffeine response before TCS (area under curve = 72.5 ± 6.3 × 106 units before TCS treatment vs. 60.3 ± 8.3 × 106 units after TCS treatment; n = 9, P = 0.26), indicating that TCS causes ECC failure without depleting SR Ca2+ stores nor by block of RyR1 activity. Moreover, the loss of ECC in skeletal myotubes exposed to TCS was not the result of Na+ channel block because depolarization of the myotube surface membrane with externally applied K+ (40 mM) failed to elicit a Ca2+ transient (Fig. 6A, Inset). Dyspedic myotubes did not respond to 10 μM TCS, as did RyR1-expressing myotubes (compare wild-type and dyspedic in Fig. 6B), indicating that TCS directly targeted RyR1 to produce a transient rise in baseline Ca2+. In support of this interpretation, direct radioligand-receptor binding analysis showed that TCS (1–20 μM) significantly enhanced specific [3H]ryanodine binding to mouse skeletal muscle membrane preparations in a concentration-dependent manner (Fig. 6C). Specific binding of [3H]PN200, a dihydropyridine that binds specifically to high-affinity sites on DHPR in skeletal muscle membrane preparations, was inhibited by TCS in a concentration-dependent but noncompetitive (allosteric) manner (Fig. 6C).
Discussion
We report that TCS is capable of disrupting ECC in both cardiac and skeletal muscle, resulting in impaired grip strength and hemodynamics, and mobility in a model fish species. These results were unexpected given the extensive toxicological testing of TCS recently reviewed (2). In fact, there is a dearth of information regarding the myotoxicity of TCS and its potential impacts on human and environmental health. An earlier study reported TCS to have an inhibitory effect on the excitability of rat phrenic nerve-diaphragm preparations; however, the mechanisms behind these findings were not explored and the study was largely ignored (32). Our present findings identify a potential for TCS to impair physiological muscle functions in vivo and identify proteins essential for ECC as targets, namely Cav1.1, Cav1.2, and RyR channels.
The molecular mechanisms by which TCS impairs ECC in skeletal muscle appear to stem from a unique functional dissociation (uncoupling) of bidirectional signaling between Cav1.1 and RyR1 Ca2+ release units (CRUs). Several observations support that TCS functionally disrupts ECC by selective interactions with the CRU: (i) TCS-induced failure of ECC is not associated with depletion of SR Ca2+ stores; (ii) TCS, even at high concentration (10 μM), fails to block RyR1 channels (myotubes remain responsive to caffeine and [3H]ryanodine binding is enhanced); (iii) The loss of ECC can be demonstrated even with external additions of K+ to depolarize the myotubes, which bypasses the Na+ action potential; (iv) TCS influences [3H]PN200 binding to DHPR in a noncompetitive manner and enhances [3H]ryanodine binding to RyR1 in the same concentration range; (v) The TCS-modified skeletal L-type Ca2+ current is reduced in density; (vi) The transient rise in basal Ca2+ caused by TCS is dependent on RyR1 expression and tightly coincides with loss of ECC and ECCE, but not SOCE, suggesting that TCS influences both proteins at sites critical for CRU interactions. Regardless of the exact molecular mechanisms leading to loss of CRU function, impairment of bidirectional signaling in even a fraction of skeletal muscle CRUs would be expected to result in weakened contractility, promoting fatigue, sarcopenia, or cachexia, a conclusion verified in grip-strength measurements made following acute exposure to TCS. Prolonged increases in cytosolic Ca2+ have been shown to promote contractile fatigue in adult skinned muscle fibers and ECC failure (33, 34). Our current results in adult FDB fibers and myotubes indicate that at lower concentrations (≤1 μM) of TCS impairs CRU functions without overtly promoting chronically elevated cytoplasmic Ca2+, suggesting TCS-mediated CRU dysfunction is mediated by a unique mechanism that chemically interferes with CRU function.
TCS also interferes with cardiac ECC, even though cardiac CRUs have fundamental differences in physical organization and functional coupling between Cav1.2 and RyR2. The activation of RyR2 depends solely on Ca2+ entry through Cav1.2, and block of Ca2+ entry prevents RyR2-mediated Ca2+ release. Because TCS reduces Cav1.2 current by ∼50%, direct block of L-type Ca2+ current may represent the primary mechanism by which TCS impairs cardiac ECC. TCS also retards inactivation of Cav1.2, an action that is likely secondary to Ca2+ transient attenuation, because RyR2-mediated Ca2+ release from the SR plays major feedback role in the inactivation of L-type Ca2+ current (35). Indeed, the in vitro effects of TCS on cardiac ECC are further supported by the in vivo analyses using hemodynamic monitoring. TCS results in a suppression of the maximal left ventricular pressure development in a dose-dependent manner. This effect is associated with a significant reduction in cardiac output.
Dysregulation of cardiac ECC has been implicated in heart failure, cardiac arrhythmias, and cardiomyopathies (36). A prominent comorbidity of heart failure with poor prognosis is cachexia (37). TCS-mediated ECC failure could promote adverse cardiac outcomes, especially in such susceptible populations with mutations in CRU proteins known to confer stress-triggered arrhythmias.
Commensurate with the increased production of TCS over the past four decades (1–3) are increased exposures, as evidenced in human samples of urine, plasma, and breast milk (7–9). Typical routes of exposure to TCS (oral, dermal) are sufficient in bringing the compound into systemic circulation (38, 39). Importantly, one study reported plasma Cmax of nearly 1 μM within 1–3 h after administering a 4-mg oral dose in human subjects (38). Our acute in vivo experiments were aimed at understanding mechanisms and potential risks, and therefore used an intraperitoneal route of exposure. However, the exposures tested here produced TCS blood plasma concentrations consistent with levels found in some humans. Therefore, disruption of ECC in skeletal and cardiac muscle may also inform about previously unappreciated risk factors associated with pervasive exposures to TCS in both humans and environmentally sensitive species (e.g., fish). TCS has been detected in sewage sludge and sediment, as well as in organisms ranging from algae to fish to dolphins (3, 6). Using fathead minnows as a marker for aquatic toxicology, Schultz et al. recently reported that TCS decreased aggression and predator-avoidance performance in the minnow (40). Consistent with this report, our study revealed a negative impact on swimming performance in larval fathead minnows exposed to TCS, an effect attributable to perhaps a combination of both cardiac and skeletal muscle impairments. In this regard, recent studies have found TCS in both raw and treated drinking water, as well as in >60% of streams across 30 states (1, 4, 5), suggesting that ecosystems are also impacted by exposure to this high-volume chemical.
In mammals, TCS is primarily metabolized to glucuronide and sulfate conjugates, yet details regarding the extent of conjugation have been equivocal. Reports about human exposure range from very little or no detectable levels of parent compound to 38% unconjugated after oral ingestion of TCS (2, 38, 41). The basis for this discrepancy is not known, but a possible explanation may involve disparate glucuronidation or sulfation capacities in the subjects tested. Indeed, UDP-glucuronosyltransferases are highly polymorphic (42) and genetic disorders, like Gilbert-Meulengracht syndrome, which affects 5–10% of the population, can lead to decreased UDP-glucuronosyltransferase activity (43). Overall, these genetic variations could dramatically influence the capacity to clear TCS from systemic circulation by conjugation. Thus, a segment of the human population with impaired phase II metabolism might be at a higher risk of adverse TCS effects described in this article. Plasma protein binding has been suggested to mitigate the possible effects of TCS, because more than 95% of the compound is protein-bound in human blood (2). However, our results demonstrated that TCS disrupts skeletal ECC even in the presence of excess serum protein.
In conclusion, we show that TCS potently impairs striated muscle ECC by interfering with signaling between DHPR and RyR, resulting in weakened contractility and depressed hemodynamics. Considering that exposures to TCS are pervasive, these recent finding may present a concern to both human and environmental health.
Materials and Methods
More detailed descriptions can be found in SI Materials and Methods.
Animal Use.
All mouse and fathead minnow experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of California at Davis.
Hemodynamic Measurement.
Hemodynamic measurements were performed as described previously (44). After signal stabilization for 10–15 min, the baseline pressure-volume (PV) loops at a steady state were recorded followed by intraperitoneal administration of 6.25, 12.5, or 25 mg/kg of TCS or vehicle. PV loops were recorded after 15 min of drug administration. A stock of TCS was prepared in DMSO and diluted [20% (vol/vol)] into saline to give a total volume of 200 μL before injection.
Determination of TCS Concentration in Mouse Plasma.
Fifteen minutes after intraperitoneal administration of TCS to mice, blood was collected by cardiac puncture and directly processed to plasma by centrifugation. TCS levels in plasma were determined by liquid chromatography electrospray-ionization tandem mass spectrometry with online solid-phase extraction, as previously described (45).
Mouse in Vivo Grip Strength.
Grip strength was measured with 3-mo-old wild-type male C57BL/6 mice using a grip-strength meter with metal wire mesh (Columbus Instruments). Upon acquiring baseline readings, the mice were administered sham, vehicle, or 40 mg/kg TCS by intraperitoneal injection, and grip strength was evaluated up to 1 h. Sham, vehicle, and TCS groups were compared via one-way ANOVA with Newman-Keuls posttest using Prism (GraphPad Software).
Swimming Behavior in Larval Fathead Minnow.
Larval fathead minnow (Aquatox, Inc.), 7 d posthatch, were exposed for up to 7 d to dilution water, 0.01% methanol, or 0.035, 0.26, and 0.52 μM TCS in 0.01% methanol. Control and dilution water consisted of deionized water, modified to USEPA moderately hard standards (46), and tests followed standard USEPA (46, 47) protocols. Swimming behavior was assessed in two stages: (i) nonprovoked swimming activity captured using video monitoring and analyzed using Ethovision Behavior Software (Noldus Information Technology); (ii) provoked swimming behavior techniques designed by Heath et al. (48) as described by Beggel and colleagues (49). All time points were combined (n = 36) by concentration for each swimming parameter (n = 36) and effects were determined using a Kruskal-Wallis followed by a Dunn’s posttest.
Isolation of Adult Mouse Ventricular Myocytes.
Single-mouse free-wall left ventricular myocytes were isolated from 10- to 12-wk-old C57BL/6J mice, as previously described (50).
Confocal Ca2+ Imaging of Mouse Ventricular Myocytes.
Ca2+ responses from isolated left ventricular myocytes were evoked and measured, as previously described (51).
Measurement of Cardiac L-type Ca2+ Current.
L-type currents were recorded from isolated left ventricular myocytes, as previously described (52).
Preparation and Culture of Primary Myotubes and Adult FDB Fibers.
Primary skeletal myoblasts were isolated from wild-type and dyspedic (RyR1-null) mouse neonates and cultured, as previously described (31). FDB muscles were dissected from 3- to 6-mo-old WT male mice, and single myofibers were enzymatically isolated and cultured, as previously described (53).
Imaging of Primary Myotubes and Adult FDB Fibers.
Ca2+ responses from myotubes and FDB fibers were evoked and measured, as previously described (54).
Measurement of Skeletal L-type Ca2+ Current.
L-type currents were recorded from skeletal myotubes as described previously (55).
Preparations of Membrane Fractions from Mouse Skeletal Muscle.
SR microsomes were isolated from mouse skeletal muscle tissue, as previously described (54).
Radioligand Binding Assay.
Radioligand binding assays with [3H]ryanodine and [3H]PN200 were performed as previously described (54, 56).
Statistical Analysis.
All data are presented as mean ± SEM. All comparisons were made by two-tailed unpaired t test unless otherwise indicated; P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants 1P01 AR52354 (to I.N.P. and K.G.B.), 2P01 ES011269 (to I.N.P.), P42 ES004699 (to B.D.H., I.N.P., and N.C.), R03 AG038778 (to R.A.B), R01 AR055104 (to K.G.B.), R01 ES002710 (to B.D.H.), and R01 HL85727, R01 HL85844, and VA Merit Grant 5 I01BX000576 (to N.C.); Muscular Dystrophy Association Grants 176448 (to K.G.B.) and T32 HL86350 (to R.Z.); and the J. B. Johnson Foundation (I.N.P.). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211314109/-/DCSupplemental.
References
- 1.Fang JL, et al. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2010;28:147–171. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- 2.Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 3.Dann AB, Hontela A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 4.Loraine GA, Pettigrove ME. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in southern California. Environ Sci Technol. 2006;40:687–695. doi: 10.1021/es051380x. [DOI] [PubMed] [Google Scholar]
- 5.Kolpin DW, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 6.Chu S, Metcalfe CD. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2007;1164:212–218. doi: 10.1016/j.chroma.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovander L, et al. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch Environ Contam Toxicol. 2002;42:105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- 9.Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–1489. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 10.Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat . Toxicol Sci. 2010;117(1):45–53. doi: 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- 11.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Perspect. 2011;119:390–396. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisseau C, et al. Toxicology in the fast lane: Application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect. 2009;117:1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannu MW, O’Connor GA, Toor GS. Toxicity and bioaccumulation of biosolids-borne triclosan in terrestrial organisms. Environ Toxicol Chem. 2012;31:646–653. doi: 10.1002/etc.1721. [DOI] [PubMed] [Google Scholar]
- 14.Russell AD. Whither triclosan? J Antimicrob Chemother. 2004;53:693–695. doi: 10.1093/jac/dkh171. [DOI] [PubMed] [Google Scholar]
- 15.Zorrilla LM, et al. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107(1):56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 16.Ahn KC, et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ Health Perspect. 2008;116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pessah IN, et al. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- 18.Kim KH, et al. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect. 2011;119:519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pessah IN, Lynch C, 3rd, Gronert GA. Complex pharmacology of malignant hyperthermia. Anesthesiology. 1996;84:1275–1279. doi: 10.1097/00000542-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Allen PD, Pessah IN, Naguib M. Miller’s Anesthesia. 7th Ed. Philadelphia, PA: Churchill Livingstone; 2010. [Google Scholar]
- 21.Andersson DC, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan J, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ankley GT, Villeneuve DL. The fathead minnow in aquatic toxicology: past, present and future. Aquat Toxicol. 2006;78:91–102. doi: 10.1016/j.aquatox.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Serratos H, Valle-Aguilera R, Lathrop DA, Garcia MC. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982;298:292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 28.Nakai J, et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 30.Bannister RA, Pessah IN, Beam KG. The skeletal L-type Ca(2+) current is a major contributor to excitation-coupled Ca(2+) entry. J Gen Physiol. 2009;133:79–91. doi: 10.1085/jgp.200810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherednichenko G, et al. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kjaerheim V, Röed A, Brodin P, Rölla G. Effects of triclosan on the rat phrenic nerve-diaphragm preparation. J Clin Periodontol. 1995;22:488–493. doi: 10.1111/j.1600-051x.1995.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 33.Verburg E, Dutka TL, Lamb GD. Long-lasting muscle fatigue: Partial disruption of excitation-contraction coupling by elevated cytosolic Ca2+ concentration during contractions. Am J Physiol Cell Physiol. 2006;290:C1199–C1208. doi: 10.1152/ajpcell.00469.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirksen RT. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front Biosci. 2002;7:d659–d670. doi: 10.2741/A802. [DOI] [PubMed] [Google Scholar]
- 36.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 37.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 38.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 39.Queckenberg C, et al. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother. 2010;54:570–572. doi: 10.1128/AAC.00615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz MM, Bartell SE, Schoenfuss HL. Effects of triclosan and triclocarban, two ubiquitous environmental contaminants, on anatomy, physiology, and behavior of the fathead minnow (Pimephales promelas) Arch Environ Contam Toxicol. 2012;63:114–124. doi: 10.1007/s00244-011-9748-x. [DOI] [PubMed] [Google Scholar]
- 41.Lin YJ. Buccal absorption of triclosan following topical mouthrinse application. Am J Dent. 2000;13:215–217. [PubMed] [Google Scholar]
- 42.Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181-182:453–456. doi: 10.1016/s0300-483x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 43.Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome) Best Pract Res Clin Gastroenterol. 2010;24:555–571. doi: 10.1016/j.bpg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schebb NH, et al. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol. 2011;45:3109–3115. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.USEPA . Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms. Washington, DC: US Environmental Protection Agency; 2002. [Google Scholar]
- 47.USEPA . Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater Organisms. Washington, DC: US Environmental Protection Agency; 2002. [Google Scholar]
- 48.Heath AG, Cech JJ, Jr, Zinkl JG, Steele MD. Sublethal effects of three pesticides on Japanese medaka. Arch Environ Contam Toxicol. 1993;25:485–491. doi: 10.1007/BF00214337. [DOI] [PubMed] [Google Scholar]
- 49.Beggel S, Werner I, Connon RE, Geist JP. Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas) Sci Total Environ. 2010;408:3169–3175. doi: 10.1016/j.scitotenv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Li N, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, et al. The effects of intracellular Ca2+ on cardiac K+ channel expression and activity: Novel insights from genetically altered mice. J Physiol. 2005;562:745–758. doi: 10.1113/jphysiol.2004.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, et al. Functional Roles of Ca(v)1.3 (α(1D)) calcium channel in sinoatrial nodes: Insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 53.Brown LD, Rodney GG, Hernández-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am J Physiol Cell Physiol. 2007;292:C1156–C1166. doi: 10.1152/ajpcell.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng W, et al. Functional and biochemical properties of ryanodine receptor type 1 channels from heterozygous R163C malignant hyperthermia-susceptible mice. Mol Pharmacol. 2011;79:420–431. doi: 10.1124/mol.110.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eltit JM, et al. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci USA. 2012;109:7923–7928. doi: 10.1073/pnas.1119207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buck ED, Nguyen HT, Pessah IN, Allen PD. Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function. J Biol Chem. 1997;272:7360–7367. doi: 10.1074/jbc.272.11.7360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.