When fighting an infection, a host must balance microbe clearance with self-damage, and this balance presumably changes dynamically over the course of the infection. Ideally, the host will kill the pathogens early during infection and near the end, focus on repairing any resulting damage to return to its original state (1). The rules governing this balance will change with environmental conditions; for example, the balance might be altered by changes in host nutrition or stress level. The work by Ko et al. (2) identifies a link between metabolism and immunity that has the potential to balance damage vs. clearance while listening to the nutritive status of the host.
Salmonella typhimurium invades host cells and lives within the resulting membrane-enclosed vesicles (3). Subsequent programmed cell death of the host cell has a complex affect on infection dynamics; host cell death eliminates the niche, but it also spreads the pathogen and causes damage to the host. The work by Ko et al. (2) searches for human variants with an altered programmed cell death response to S. typhimurium infection. Genetic analysis gives its practitioners the unique anxiety of being thrown into unexpected fields and having to make sense of the connection. The work by Ko et al. (2) identifies an SNP implicating the enzyme methylthioribulose-1-phosphate dehydratase (APIP) as a modulator of pyroptosis. Although lacking an immediately obvious connection, the work by Ko et al. (2) establishes a link between this enzyme and immunity.
APIP is required for methionine salvage, a pathway responsible for recycling polyamines like spermine, spermidine, and putrescene back into methionine (4). The work by Ko et al. (2) finds that the concentration of the substrate of this enzyme, 5′-methylthioadenosine (MTA), regulates programmed cell death. High levels of MTA and low levels of the enzyme APIP each correlate with increased pyroptosis.
MTA was previously implicated as protective against inflammation; MTA injection into LPS-treated mice helps protect the mice against shock, and inflammatory indicators like TNF are reduced (5). It is hard to compare the Salmonella and LPS studies. The LPS study induced a response in whole animals using a pathogen associated molecular pattern (PAMP) and not a pathology-causing microbe that can grow and needs to be cleared. In contrast, the Salmonella study focuses on a single step of infection in cultured cells, which cannot easily predict the consequences on microbe clearance and pathology in a whole animal (2). Both studies, however, suggest that the host will modulate its immune response in response to MTA concentrations (2, 5).
Why would a cell be wired this way? Why might pyroptosis be favored when MTA levels are high? The work by Ko et al. (2) makes an argument about the host balancing clearance of microbes with damage caused by the immune response. The finding of an association between patients surviving systemic inflammatory response syndrome (SIRS) and carrying the APIP allele that should increase MTA concentrations bolsters this idea (2); the hypothesis is that patients survive, because they might be better at clearing their pathogen. The idea is plausible, and analysis of both resistance and infection tolerance is measurable in their system. The work by Ko et al. (2), however, stops shy of measuring these outputs. To measure infection tolerance, the dose–response curve of health vs. microbe load, one has to measure microbe load and damage (6, 7). The work by Ko et al. (2) does not report the microbe loads under varying MTA levels, and although their quantification of pyroptosis is a reasonable output for cellular health, extrapolating this outcome to the whole organism health is complicated. Pyroptosis causes immediate cell damage, destroying a potential niche and potentially clearing microbes, but it also spreads the bacteria. Predicting the effects of altered pyroptosis on the dynamics of the infection in a whole animal is difficult.
Even if MTA concentrations regulate the infection tolerance of the system causing predictable alterations of the immune response, this finding only addresses part of the equation. We still need to know why methionine salvage is a useful rheostat for programmed cell death. The work by Ko et al. (2) argues that this pathway might help regulate immune responses in a starving host. If resources are limiting, perhaps the host cannot afford to repair damage, and thus, it adopts a more tolerance-based immune response with lower pyroptosis (Fig. 1). Alternatively, the work by Ko et al. (2) suggests that the MTA concentration might change dynamically during the course of the infection, leading to a retuning of pyroptosis during distinct stages of infection. Perhaps MTA provides a cue to prompt the switch from clearance to repair as an infection resolves? A third alternative, which is not mentioned in the work by Ko et al. (2), is that methionine salvage could directly affect the pathogen. For example, polyamines potentiate the virulence of Salmonella and a variety of other pathogens (8). Assessing MTA levels might help the host monitor microbe manipulation of polyamine metabolism. This hypothesis could be a vertebrate example of the Guard Hypothesis that is used to describe plant immunity; the host might trigger a more desperate immune response if manipulation of infected cells is detected (9). To distinguish between these hypotheses, we need to know how methionine salvage and the metabolites involved change during the course of infection or during starvation. We know that this pathway contributes to immunity, but to manipulate these pathways in a clinical way, we need to understand the dynamics of the system. Should we concentrate on raising or lowering MTA levels? Will these rules change as the infection progresses from clearance to recover? Will these rules apply for all infections?
Fig. 1.
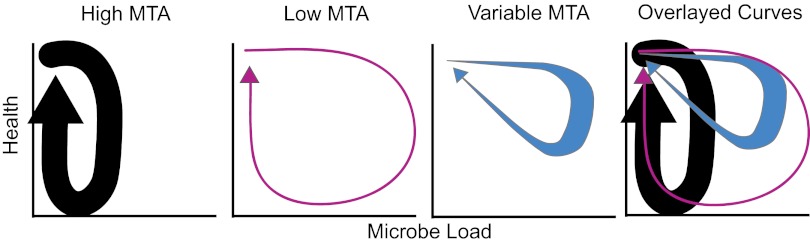
Potential impact of changes in MTA concentrations on an infection. This figure imagines the path that a Salmonella-infected host might take through health-by-microbe phase space (1). The host starts in the upper left corner, the microbes grow, the individual sickens, and then, the microbes are cleared; the host recovers, producing a looping resilient curve. Using the conditions suggested in the work by Ko et al. (2), a constitutively high MTA level might result in an increased ability to clear the microbes at a cost of increased damage. These hosts would have low infection tolerance. Under starvation conditions, the host might clear the pathogens less well but would be more tolerant, because it would take more microbes to cause damage. If MTA levels change dynamically, they could tune the phase curve to minimize both microbe load and damage.
The work by Ko et al. (2) concludes with an intriguing evolutionary argument. The work by Ko et al. (2) finds a difference in APIP allele prevalence between African and European populations, and this work proposes that selection of the high cell death allele might have been driven by the development of agricultural techniques, which allowed humans to live in denser populations. The resulting emergence of
The work by Ko et al. provides a compelling mechanistic link between MTA concentrations and programmed cell death.
crowd epidemic infections may have increased the fitness of carriers of this allele in European populations if the allele helped fight these infections. This proposal seems plausible, but because the host seems to be monitoring a metabolite, there may be a more direct explanation. Perhaps the agricultural improvements themselves induced a change in diet, and this change altered baseline MTA levels, requiring the system to be retuned. This proposal makes us wonder—do our current diets provide optimal MTA levels to deal with modern pathogens? This question is already being extensively studied in the aging field, because methionine restriction explains many of the physiological changes seen in diet-restricted animals (10). Diet restriction affects survival of animals to infection, and combining these studies with the findings in the work by Ko et al. (2) suggests that we should look at the effects of diet restriction on MTA levels and infection tolerance.
The work by Ko et al. (2) provides a compelling mechanistic link between MTA concentrations and programmed cell death. This study should prompt the generation of hypotheses to explain the functional significance of this mechanism (2). Metabolites may be modulated by simple changes in diet, which would allow us to treat infections more inexpensively. Because cost is often a determining factor in treating infection throughout the world, developing treatments that rely on diet instead of blockbuster drugs has the potential to save lives.
Footnotes
References
- 1.Schneider DS. Tracing personalized health curves during infections. PLoS Biol. 2011;9:e1001158. doi: 10.1371/journal.pbio.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko DC, et al. Functional genetic screen of human diversity reveals that a methionine salvage enzyme regulates inflammatory cell death. Proc Natl Acad Sci USA. 2012;109:E2343–E2352. doi: 10.1073/pnas.1206701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 4.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hevia H, et al. 5′-methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology. 2004;39:1088–1098. doi: 10.1002/hep.20154. [DOI] [PubMed] [Google Scholar]
- 6.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jelsbak L, Thomsen LE, Wallrodt I, Jensen PR, Olsen JE. Polyamines are required for virulence in Salmonella enterica serovar Typhimurium. PLoS One. 2012;7:e36149. doi: 10.1371/journal.pone.0036149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 10.Miller RA, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


