Abstract
Mechanisms underlying the female preponderance in affective disorders are poorly understood. Here we show that hippocampal nitric oxide (NO) plays a role in the sex difference of depression-like behaviors in rodents. Female mice had substantially lower NO production in the hippocampus and were significantly more likely to display negative affective behaviors than their male littermates. Eliminating the difference in the basal hippocampal NO level between male and female mice mended the sex gap of affective behaviors. Estradiol exerted a positive control on hippocampal NO production via estrogen receptor-β–mediated neuronal NO synthase expression. Thus, low estrogen in the female hippocampus accounts for lower local NO than in the male hippocampus. Although estrogen has important significance in modulating affective behaviors, it is not estrogen but NO in the hippocampus that mediates the sex difference of affective behaviors directly, because hippocampal NO was necessary for the behavioral effects of estradiol, and NO was an independent factor in modulating behaviors. Stress promoted hippocampal NO production in males because of glucocorticoid release, thus leading to local NO excess. In contrast, stress suppressed NO production in females because of decreased estrogen, thereby resulting in hippocampal NO shortage. Whereas activating cAMP response element binding protein (CREB) rescued the depression-like effects of the intrahippocampal NO donor diethylenetriamine/nitric oxide adduct (DETA/NONOate), inactivating CREB abolished the antidepressant-like effects of the intrahippocampal NO donor DETA/NONOate. Our findings suggest a molecular mechanism underlying the sex difference of affective behaviors.
Keywords: gender difference, psychiatric disorders, chronic mild stress, depressive behaviors, corticosterone
Affective disorders such as depression and anxiety, life-threatening and highly prevalent stress-related disorders, rank among the top causes of worldwide disease burden and disability (1, 2). It is commonly suggested that a female preponderance in depression and anxiety is universal and substantial. The prevalence of depression and anxiety for women is approximately twice that for men (2–6). Although there are advocates for the implications of hypothalamic–pituitary–adrenal axis hyperactivity in the sex differentiation in some expressions of both depression and anxiety (7), it is not clear what underlies the sex gap in these affective disorders.
Neuronal nitric oxide synthase (nNOS) has been implicated in the psychiatric disorders characterized by depression (8, 9), schizophrenia (10), and aggressiveness (11). We recently demonstrated that hippocampal nNOS mediates the stress-related depressive behaviors of glucocorticoids (9) and the role of 5-hydroxytryptamine receptor 1A (5-HT1A) receptor in modulating anxiety-related behaviors (12). Evidence from clinical and biological studies indicates that the hippocampus plays an important role in major depression disorder (13). We found that chronic mild stress (CMS) increased hippocampal NO production by nNOS in male mice (6–9). In contrast, CMS diminished hippocampal NO production by nNOS in female mice (14). Here we show that hippocampal NO plays a role in the sex difference of depression-like behaviors in rodents. The basal level of hippocampal NO in female mice was substantially lower than that in male mice. Abolishing the difference in NO levels in the hippocampus between male and female mice mended the sex gap of depression-like behaviors. Because 17β-estradiol (E2) exerted a positive control on nNOS expression, the lower E2 level in the female hippocampus (15) accounts for the sex difference in local NO levels. However, the sex difference of depression-like behaviors was not caused by estrogen but NO in the hippocampus, because hippocampal NO was essential for the behavioral effects of estrogen. Whereas stress promoted hippocampal NO production in males because of glucocorticoid release, thus leading to local NO excess, it suppressed NO production in female because of decreased estrogen, thereby resulting in hippocampal NO shortage. Both NO excess and shortage resulted in depression-like behaviors through affecting cAMP response element binding protein (CREB) activation. Our findings suggest a mechanism underlying the vulnerability of females to affective disorders.
Results
Hippocampal NO Is Essential for the Sex Difference of Affective Behaviors.
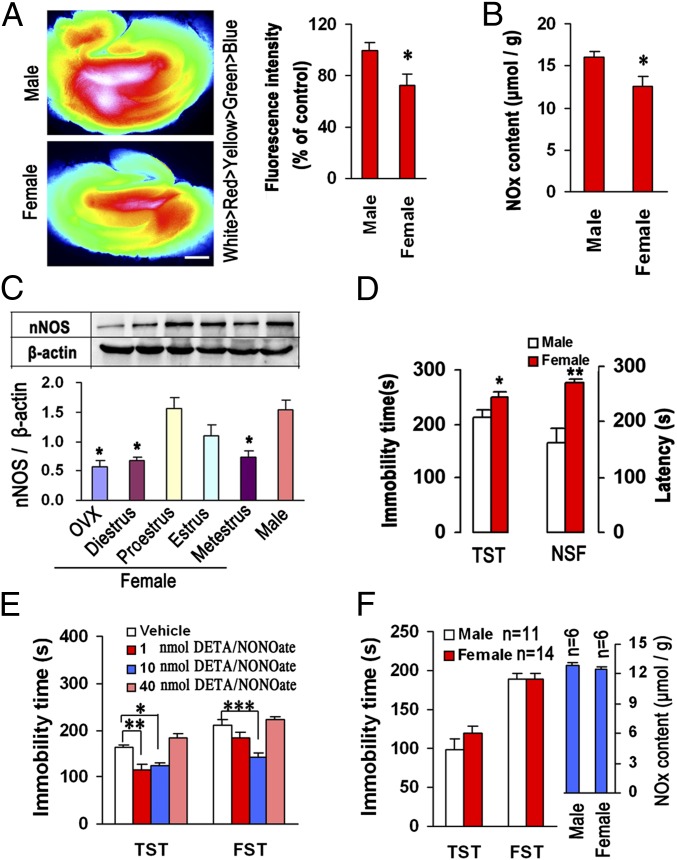
To determine whether hippocampal NO contributes to the sex difference in affective behaviors, we measured nNOS and NO levels in the hippocampus in male and female mice and compared their immobility in the tail suspension test (TST) and latency to feed in the novelty suppressed feeding (NSF) test, previously used to assess depressive behaviors in rodents (16). Compared with their male littermates, female mice had significantly scanty hippocampal NO production (Fig. 1 A and B), decreased nNOS expression during the diestrus and metestrus (Fig. 1C), and increased immobility in the TST and latency in the NSF (Fig. 1D), suggesting sex differences. To test whether the different basal levels of hippocampal NO between males and females account for the sex difference of affective behaviors, we microinjected the NO donor DETA/NONOate into the bilateral hippocampi of the female mice subjected to ovariectomy (OVX-female mice) to elevate local NO level, and measured hippocampal NO and assessed immobility in the TST and forced swimming test (FST) at 48 h after treatment. Fig. 1E shows that intrahippocampal NO donor at doses of 1.0 or 10 nmol diminished immobility in the TST and FST significantly. Most importantly, when approximating the NO level in female hippocampus to that in male by microinjecting 10 nmol DETA/NONOate into the hippocampi of OVX-female mice, the sex difference in immobility in the TST and FST disappeared (Fig. 1F). Thus, hippocampal NO contributes to the sex difference of affective behaviors.
Fig. 1.
Hippocampal NO is crucial for sex differences in affective behaviors. (A) Fluorescence images of a cultured rat hippocampal slice loaded with an NO-sensitive fluorescent dye, 3-Amino, 4-aminomethyl-2′,7′-difluorofluorescein Diacetate (DAF-FM DA). Fluorescence intensity shown in pseudocolor (Left), and a statistical graph showing relative fluorescence intensity (Right) (n = 4). For females, one mouse each stage in estrous cycle. (Scale bar, 400 μm.) (B) NOx contents in male and female hippocampus (n = 9; for female, diestrus, proestrus, or metestrus stage, n = 2, estrus stage, n = 3). (C) Immunoblots showing hippocampal nNOS levels in male or the female mice being within an estrus cycle or subjected to OVX (n = 3). (D) Immobility in the TST and latency in the NSF test in male and female mice (n = 10; for female, diestrus or proestrus stage, n = 2, metestrus or estrus stage, n = 3). (E) Immobility in the TST and FST for the DETA/NONOate-treated OVX-female mice (intrahippocampal infusion, n = 12). (F) Immobility in the TST and FST and hippocampal NOx contents in male and 10 nmol DETA/NONOate-treated OVX-female mice (intrahippocampal infusion). Means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 in A–D, compared with male.
Estrogen Is Implicated in the Hippocampal NO-Mediated Sex Difference in Affective Behaviors.
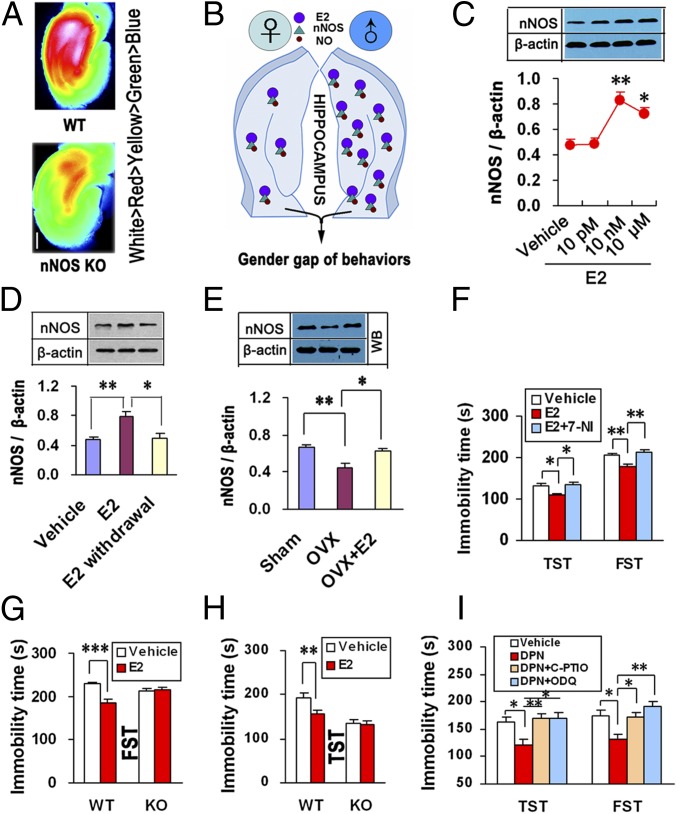
Why do females have relatively low hippocampal NO? Because E2 in the female hippocampus was low relative to males (approximately 8 nM for males and 0.5–2 nM for females) (15), and because the hippocampal nNOS level fluctuated as E2 did (Fig. 1C) and NO was predominantly derived from nNOS in the hippocampus (Fig. 2A), we hypothesized that estrogen-mediated nNOS expression in the hippocampus is critical (Fig. 2B). To address this notion, we investigated the effect of E2 on nNOS expression. We incubated hippocampal neurons with E2 for 24 h and found that E2 up-regulated nNOS dose-dependently (Fig. 2C) and that nNOS returned to the basal level after E2 withdrawal (Fig. 2D). To further confirm these findings in vivo, we treated the OVX-female mice with systemic E2 for 7 d starting at 2 h after surgery. E2 reversed OVX-induced hippocampal nNOS decrease (Fig. 2E). The actions of estrogen are ascribed to two nuclear estrogen receptors (ERs), ERα and ERβ (17). Beneficial effects of estrogens on mood are mainly due to ERβ signaling (18). By incubating the cultured hippocampal neurons with ICI182,780 (a nonselective ER antagonist), 4,4′,4′′-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) (an ERα-selective agonist), or diarylpropionitrile (DPN) (an ERβ-selective agonist), we demonstrated that E2 promoted nNOS expression via activating ERβ (SI Appendix, Fig. S1). Delivering DPN into the female hippocampus up-regulated nNOS and diminished immobility in the TST and FST, whereas PPT had no effect (SI Appendix, Fig. S2 A–D). Moreover, intrahippocampal E2 increased nNOS and decreased immobility in the TST dose-dependently, and the effects of E2 on nNOS and immobility were abolished by LV-ERβ-shRNA, a lentiviral vector containing the shRNA of ERβ (SI Appendix, Fig. S2 E–J), further supporting the significance of ERβ.
Fig. 2.
Estrogen is implicated in the hippocampal NO-mediated sex difference in affective behaviors. (A) Fluorescence images of cultured rat hippocampal slices from nNOS KO or WT male mice loaded with DAF-FM DA. (Scale bar, 400 μm.) (B) A hypothesized mechanism underlying the sex difference in depression-like behaviors. (C) Immunoblots showing nNOS levels in E2-treated hippocampal neurons (n = 4). (D) Immunoblots showing nNOS levels in the hippocampal neurons incubated with 10 nM E2 for 24 h or withdrawal (n = 5). (E) Immunoblots showing hippocampal nNOS levels in OVX-female mice treated with E2 or vehicle (n = 4). (F) Immobility time in the TST and FST for OVX-female mice treated with intrahippocampal E2 alone or in combination with 7-NI (n = 13–19). (G and H) Immobility time in the FST (G) and TST (H) of nNOS KO (n = 12–14) and WT (n = 17–18) mice treated with intrahippocampal E2. (I) Immobility time in the TST and FST of OVX-female mice treated with intrahippocampal DPN alone or in combination with C-PTIO or ODQ (n = 14–16). Means ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001. In C, vs. vehicle.
Is it NO or estrogen in the hippocampus that is critical for the sex difference of affective behaviors? To answer this question, we infused E2 with or without the nNOS inhibitor 7-Nitroindazole (7-NI) (given at 30 min before E2) into the bilateral hippocampi of OVX-female mice and assessed immobility in the TST and FST 48 h after microinjection. Pretreatment with 7-NI abolished the behavioral effects of E2 (Fig. 2F). Next we infused E2 into the bilateral hippocampi of nNOS KO and WT OVX-female mice and assessed immobility in the TST and FST 48 h after microinjection. Intrahippocampal E2 significantly decreased immobility in WT mice but was ineffective in KO mice (Fig. 2 G and H). Similarly, 7-NI pretreatment or deleting nNOS abolished the behavioral effects of DPN (SI Appendix, Fig. S3). To further link nNOS-derived NO and ERβ-mediated behavioral effects, we infused DPN with or without 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (C-PTIO) (given at 30 min before DPN), a cell-impermeable NO scavenger, into the bilateral hippocampi of OVX-female mice and tested the TST and FST 48 h after microinjections. The NO-mediated actions are mostly due to cGMP formation (9). Accordingly, we also pretreated the hippocampus with 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one(ODQ) (given at 30 min before DPN), a soluble guanylate cyclase inhibitor, to inhibit cGMP production. Interestingly, both C-PTIO and ODQ abolished the DNP-induced immobility decreases in the TST and FST (Fig. 2I), suggesting a requirement of hippocampal NO for estrogen-mediated behavioral modifications.
Hippocampal NO Shortage Contributes to Stress-Induced Depressive Behaviors in Females.
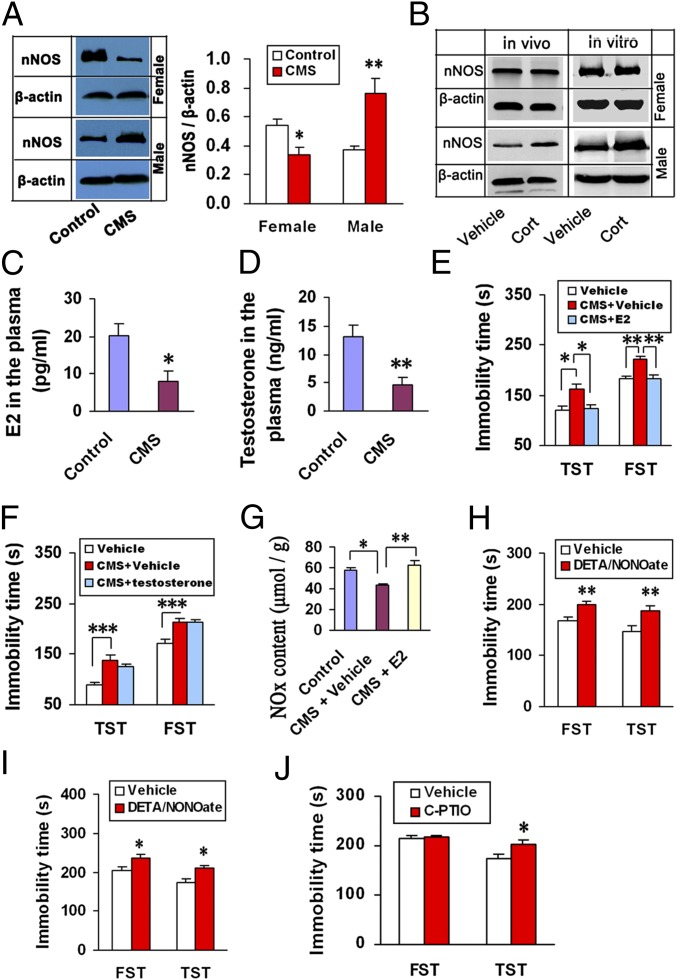
Stressful life events have a substantial causal association with affective disorders (19). Previously, we found that CMS caused hippocampal nNOS and NO up-regulation in male mice (8, 9). We thus investigated whether there is a sex difference of nNOS expression in the hippocampus after exposure to CMS. Interestingly, CMS up-regulated nNOS in male mice, whereas it significantly down-regulated nNOS in female mice (Fig. 3A). To answer why stress has the opposite effects on nNOS, we investigated effects of glucocorticoid owing to its significant role in mediating stress-induced behavioral modifications (20) and nNOS expression in males (9). We measured nNOS expression after incubating organotypic hippocampal slice cultures from male or female mice with 10 μM corticosterone for 24 h, or microinjecting corticosterone into the hippocampus of male or OVX-female mice for 24 h. Corticosterone up-regulated hippocampal nNOS in male mice and had no effect in female mice both in vivo and in vitro (Fig. 3B and SI Appendix, Fig. S4), suggesting the contribution of glucocorticoid to nNOS regulation for males only. Given that corticosterone induced hippocampal nNOS expression via activating mineralocorticoid receptor (MR) (9), the failure of corticosterone to regulate nNOS in females may be explained by low MR in the female hippocampus (21) (SI Appendix, Fig. S5).
Fig. 3.
Hippocampal NO shortage explains stress-induced depressive behaviors for females. (A) Immunoblots showing hippocampal nNOS levels in male and female mice exposed to CMS for 21 d (n = 4). For females, one mouse each stage in estrous cycle. (B) Representative immunoblots showing nNOS levels in the corticosterone-treated hippocampus from male and female mice in vitro and in vivo. (Histograms showing statistical analysis of nNOS levels are shown in SI Appendix, Fig. S4). (C and D) Blood E2 Levels in female mice (n = 6–7) (C) and testosterone levels in male mice (n = 7–8) (D). These mice were exposed to CMS for 21 d. (E) Immobility in the TST and FST of female mice exposed to CMS for 21 d and treated with E2 or vehicle (n = 11–13). (F) Immobility in the TST and FST of male mice exposed to CMS for 21 d and treated with testosterone or vehicle (n = 11–13). (G) NOx contents in the hippocampus of female mice exposed to CMS for 21 d and treated with E2 or vehicle (n = 5). (H) Immobility in the TST and FST of male mice treated with DETA/NONOate (n = 13–14). (I) Immobility in the TST and FST of female mice subjected to OVX and treated with 100 nmol DETA/NONOate (n = 12–13). (J) Immobility in the TST and FST of female mice treated with C-PTIO (n = 16, 4 mice each stage in estrous cycle). In H and J, drugs were microinjected into the hippocampus. Cort, corticosterone. Means ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001. In A–D, vs. control; in H–J, vs. vehicle.
Next, we measured gonadal hormone changes in the blood of mice after exposure to CMS for 21 d. The CMS caused dramatic decreases in E2 for females (Fig. 3C) and testosterone for males (Fig. 3D) and significant increases in immobility in the TST and FST in both male and female mice. Replacement therapy with E2 for females reversed the CMS-induced behavioral modifications and hippocampal NO reduction (Fig. 3 E and G), suggesting the importance of estrogen decrease for females. However, replacement therapy with testosterone did not change the CMS-induced behavioral modifications for males (Fig. 3F), and castration in male mice did not change nNOS expression and behaviors (SI Appendix, Fig. S6 A–C), excluding significance of androgen. Although E2 up-regulated nNOS in the male hippocampus (SI Appendix, Fig. S6D), it significantly increased immobility in the TST for males (SI Appendix, Fig. S6 E and F), suggesting different effects of E2 between males and females. Additionally, endothelial NOS (eNOS) is not involved in CMS-induced depressive behaviors and the effects of gonadal hormone (SI Appendix, Fig. S7).
To know the physiological meaning of the CMS-induced nNOS up-regulation for males and down-regulation for females, we delivered 10 nmol DETA/NONOate into the male hippocampus or 100 nmol DETA/NONOate into the hippocampus of OVX-female mice. As expected, the intrahippocampal NO donor significantly increased immobility in the TST and FST both in males (Fig. 3H) and females (Fig. 3I). Moreover, microinjecting 10 nmol C-PTIO into the bilateral hippocampi of intact female mice increased immobility in the TST too (Fig. 3J). Thus, both NO excess and shortage in the hippocampus bring about depression-like behaviors.
CREB Is Necessary for the Hippocampal NO-Mediated Sex Difference in Affective Behaviors.
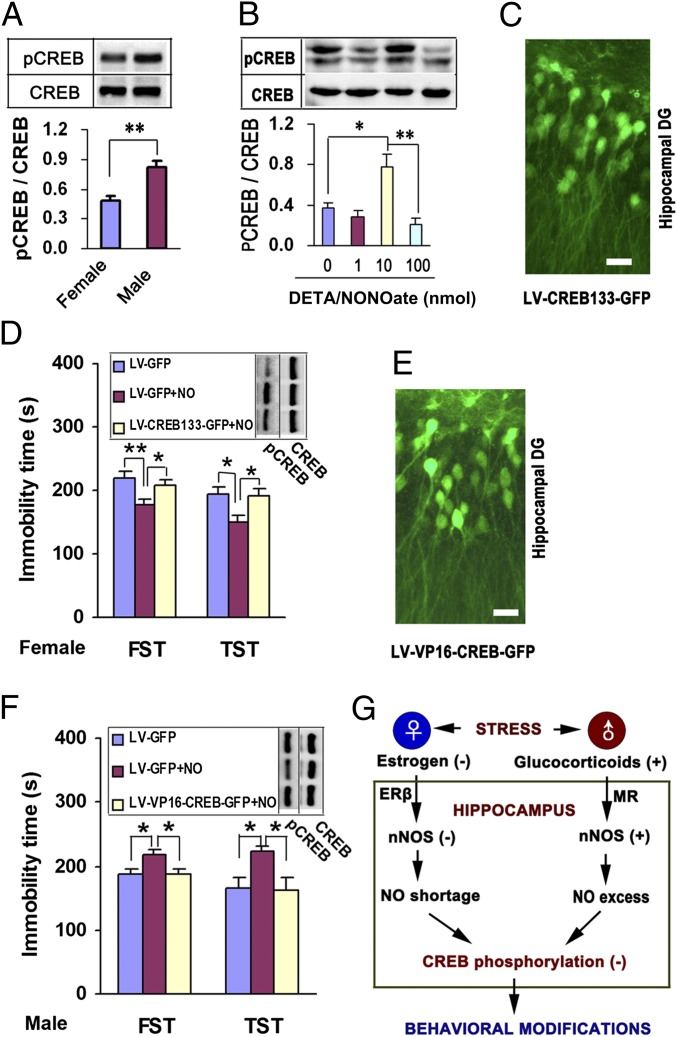
Because hippocampal CREB plays an important role in the pathophysiology and treatment of depression (22) and is essential for the behavioral effects of nNOS (12), it was appropriate to determine whether CREB is critical for the hippocampal NO-mediated sex difference in affective behaviors. In female hippocampus, the phosphorylated cAMP response element binding protein (pCREB) level was significantly lower than that in males (Fig. 4A). DETA/NONOate changed pCREB levels bidirectionally in the hippocampus of OVX-female mice (Fig. 4B) and in the cultured hippocampal neurons (SI Appendix, Fig. S8), implicating CREB in the effect of NO. To prevent CREB activation specifically, we generated a recombinant lentivirus, LV-CREB133-GFP, expressing a mutant variant of CREB protein that could not be phosphorylated at Ser133. It effectively infected the hippocampal dentate gyrus (Fig. 4C). We infused LV-CREB133-GFP or its control LV-GFP into the bilateral hippocampi of OVX-female mice. Seven days later, we delivered 10 nmol DETA/NONOate into the bilateral hippocampi by microinjection and assessed immobility in the TST and FST and pCREB levels 48 h after treatments. DETA/NONOate caused significant increases in pCREB levels and decreases in immobility in the TST and FST. Pretreatment with LV-CREB133-GFP abolished these effects of DETA/NONOate (Fig. 4D). To activate CREB specifically, we generated a recombinant lentivirus, LV-VP16-CREB-GFP, expressing constitutively active CREB, VP16-CREB. We infused it or its control LV-GFP into the bilateral hippocampi of male mice. Seven days later, we delivered 10 nmol DETA/NONOate into the bilateral hippocampi by microinjection and assessed immobility in the TST and FST and pCREB levels 48 h after delivering DETA/NONOate. LV-VP16-CREB-GFP effectively infected the hippocampal dentate gyrus (Fig. 4E). Intrahippocampal DETA/NONOate caused significant decreases in pCREB levels and increases in immobility in the TST and FST. Using LV-VP16-CREB-GFP rescued the behavioral effects of DETA/NONOate (Fig. 4F). Levels of pCREB in the hippocampus of female mice fluctuated within an oestrous cycle. Moreover, in the OVX-female mice, E2 and DPN up-regulated pCREB, and pretreatment with N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89), a protein kinase A (PKA) inhibitor, neutralized the behavioral effects of DPN (SI Appendix, Fig. S9 A–C). The effect of DPN on pCREB disappeared in female mice lacking nNOS (SI Appendix, Fig. S9D), suggesting a requirement of nNOS for the ERβ-mediated CREB activation. However, inactivating CREB by microinjecting 10 nmol H89 into the hippocampus of female mice or pretreating the cultured hippocampal neurons with LV-CREB133-GFP did not influence E2- or DPN-induced nNOS expression (SI Appendix, Fig. S9 E–G), putting CREB downstream of E2–nNOS signaling. In addition, in the male hippocampus, corticosterone did not affect ERβ expression, and LV-ERβ-shRNA did not change corticosterone-induced nNOS expression (SI Appendix, Fig. S9 H and I). Our data suggest a model of signal pathways whereby stress causes depressive behaviors in males and females (Fig. 4G).
Fig. 4.
CREB function is essential for the behavioral effects of hippocampal NO. (A) Immunoblots showing pCREB levels in the hippocampus from male and OVX-female mice (n = 4). (B) Immunoblots showing pCREB levels in the hippocampus of mice treated by intrahippocampal DETA/NONOate at 48 h after infusion (n = 4). (C) Representative hippocampal slice infected with LV-CREB133-GFP. (Scale bar, 20 μm.) (D) Hippocampal pCREB levels (n = 3) and immobility in the TST and FST (n = 14–15) of OVX-female mice that received intrahippocampal DETA/NONOate with LV-CREB133-GFP or LV-GFP infection. (E) Representative hippocampal slice infected with LV-VP16-CREB-GFP. (Scale bar, 20 μm.) (F) Hippocampal pCREB levels (n = 3) and immobility in the TST and FST (n = 13) of male mice that received intrahippocampal DETA/NONOate with LV-VP16-CREB-GFP or LV-GFP infection. (G) A model of different signal pathways whereby stress causes depressive behaviors in males and females. NO, DTEA/NONOate in D and F; (−), negative regulation; (+), positive regulation. Means ± SEM. *P < 0.05; **P < 0.01.
Discussion
It is well established that women have a higher prevalence of depression and anxiety disorders (2–6), but the biological mechanisms underlying the sex gap in these stress-related disorders have been less well researched. We have shown that hippocampal NO production is implicated in anxiety-like and depression-like behaviors in male rodents (8, 9, 11). Here, we demonstrated that hippocampal NO contributes to the sex difference of depression-like behavior in mice. Causality is supported by four arguments: (i) the basal NO level in the female hippocampus was substantially lower than that in the male hippocampus; (ii) female animals were significantly more likely to display negative affective behaviors than male; (iii) whereas CMS, a classic chronic stress model of depression, promoted nNOS expression and led to NO excess in male hippocampus, it inhibited nNOS expression and caused NO shortage in the female hippocampus; and, most importantly, (iv) eliminating the difference in hippocampal NO levels between males and females by delivering an NO donor into the female hippocampus mended the sex gap of affective behaviors.
Not surprisingly, many researchers have paid close attention to the functional roles of estrogens in the vulnerability of women to affective disorders. Dozens of reports indicate that females are vulnerable to affective disorders when dramatic estrogen fluctuation occurs (18, 23–25). Consistent with our current observation that immobility in the TST and FST in female mice changed as estrogen fluctuated (SI Appendix, Fig. S10), a large body of evidence (18) demonstrates that depression-like behavior in the TST and FST varies as a function of the estrous cycle. Furthermore, there are several studies that report that estrogen replacement therapy is beneficial to women who suffered postpartum and perimenopausal affective disorders (18, 26, 27). We show here that E2 reverses CMS-induced behavioral modifications in female mice. Thus, the fluctuation of estrogen may be a primary contributor to the onset of mood disorders in females. In essence, however, it is not estrogen but NO in the hippocampus that is a direct mediator for the sex gap in affective behaviors, because hippocampal NO is essential for the behavioral effects of estrogen.
Interestingly, similar to the E2 level (15), the basal level of NO in the female hippocampus is substantially low. We demonstrate that estrogen exerts positive control on hippocampal NO production. In the female hippocampus, a low NO level is due to low E2. The biological effects of estrogen are mediated by binding to two intracellular ERs, ERα and ERβ (17). ERα is critical for reproductive function. In contrast, ERβ is important for modulating affective behaviors (18). The effects of E2 on hippocampal NO production, as we have shown, is mediated by ERβ. ERβ may mediate hippocampal NO production for males too, because ERβ expressed in the male hippocampus at an extent comparable to that in the female hippocampus (28) (SI Appendix, Fig. S6G).
Both gonadal hormones and glucocorticoid participate in stress responses (18). We have shown that glucocorticoid is responsible for stress-induced nNOS expression and NO production in the male hippocampus (9). Here, we demonstrate that for females, estrogen decrease accounts for stress-induced behavioral modifications and hippicampal nNOS reduction. For males suffering from stress, however, androgen decrease has no effect on behaviors and hippocampal nNOS. Furthermore, we demonstrate that both NO excess and shortage in the hippocampus led to depression-like behaviors, likely reflecting an exquisite sensitivity of the brain to both high and low levels of hippocampal NO for correctly modulating effective behaviors. Considering the interaction of hormonal status with stress in the female (18), the vulnerability of females to affective disorders may be due to intense fluctuation of hippocampal NO during periods of marked hormonal fluctuations. Furthermore, given the high basal level of NO in the male hippocampus (15), NO excess owing to the E2-mediated nNOS up-regulation may explain why E2-treated male mice displayed depression-like behavior.
CREB is a well-known nuclear transcription factor contributing to the pathophysiology and treatment of depression (23). Estrogen has been shown to elicit phosphorylation of CREB in the hippocampal neurons (29). A previous report (30) and our results indicate that males have more pCREB than females in the hippocampus, which may be due to lower estrogen levels in the female hippocampus than in male. Here, we demonstrate that estrogen mediates CREB activation by activating ERβ, and most importantly, nNOS is necessary for the ERβ-mediated CREB activation in the female hippocampus. Stress causes NO shortage in the female hippocampus, whereas it leads to NO overproduction in the male hippocampus. However, either NO shortage or overproduction impairs CREB activation, thereby causing depression-like behaviors (Fig. 4G). Additionally, OVX may be another variable in addition to sex. Because of the difficulty in avoiding the interference of the estrous cycle for gonadally intact female mice, it is acceptable to investigate the effects of intrahippocampal drugs using OVX-mice to make their estrogen levels similar.
Experimental Procedures
Animals.
Young adult (6- to 7-wk-old) homozygous nNOS-deficient mice (B6;129S4-Nos1tm1Plh, KO, stock number: 002633) and their wild-type controls of similar genetic background (B6129SF2, WT) (both from Jackson Laboratories; maintained at the Model Animal Research Center of Nanjing University, Nanjing, China), and young adult (6- to 7-wk-old), newborn (postnatal day 0), and juvenile (15-d-old) ICR (swiss Hauschka) mice (from the Model Animal Research Center of Nanjing University, Nanjing, China) were used in this study. Animals were housed in an air-conditioned room (20 ± 2 °C, 12 h light/dark cycle), with food and water ad libitum, except when specified otherwise. The female mice received ovariectomy (OVX) and the male mice received castration were allowed an adaptation period of one week before the beginning of the experiments. All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. Every effort was made to minimize the number of animals used and their suffering.
Drugs.
Systematic administration.
E2 (160 μg/kg per day) and testosterone (160 μg/kg per day) were s.c. injected. All drugs were consecutively administrated for 7 d.
Hippocampal infusion.
Stereotaxic surgery was used to deliver 20 nmol E2, 20 nmol DPN, 20 nmol PPT, 0.1 nmol corticosterone, 1.0, 10, or 100 nmol DETA/NONOate, 10 nmol 7-NI, 10 nmol C-PTIO, 10 nmol ODQ, or 10 nmol H89 into the hippocampus (coordinates: 2.3 mm posterior to bregma, 1.3 mm lateral to the midline, and 2.0 mm below dura, 0.2 μL/min). If one drug was microinjected alone, the volume was 2 μL. If one drug was used in combination with another drug, the volume of each drug was 1 μL. For stereotaxic surgery, adult mice were anesthetized with 0.2% chloral hydrate (0.2 mL/10 g body weight).
Hippocampal slice treatments.
Corticosterone was dissolved in DMSO and diluted with serum-free culture medium to the final concentration.
Hippocampal neurons treatments.
E2, DPN, PPT, or ICI 182,780 were dissolved in DMSO and diluted with serum-free culture medium to the final concentration. DETA/NONOate was dissolved in physiological saline and diluted with serum-free culture medium to the final concentration.
Other Experiments.
Other experiments are described in SI Appendix, SI Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 30870900 and 81030023, National Basic Research Program of China (973 Program) Grant 2011CB504404, and Natural Science Foundation of Jiangsu Province–Special Program Grant BK2011029.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207461109/-/DCSupplemental.
References
- 1.Duric V, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragam N, Wang KS, Pan Y. Genome-wide association analysis of gender differences in major depressive disorder in the Netherlands NESDA and NTR population-based samples. J Affect Disord. 2011;133:516–521. doi: 10.1016/j.jad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 4.Essau CA, Lewinsohn PM, Seeley JR, Sasagawa S. Gender differences in the developmental course of depression. J Affect Disord. 2010;127:185–190. doi: 10.1016/j.jad.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder EB, et al. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34:99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
- 8.Zhou QG, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression in mice. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou QG, et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci. 2011;31:7579–7590. doi: 10.1523/JNEUROSCI.0004-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donovan MC, et al. Molecular Genetics of Schizophrenia Collaboration Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 11.Nelson RJ, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J Neurosci. 2010;30:2433–2441. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacQueen G, Frodl T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo ML, et al. Loss of hippocampal neuronal nitric oxide synthase contributes to the stress-related deficit in learning and memory. J Neurochem. 2007;102:261–274. doi: 10.1111/j.1471-4159.2007.04528.x. [DOI] [PubMed] [Google Scholar]
- 15.Mukai H, et al. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim Biophys Acta. 2010;1800:1030–1044. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 17.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 20.Revest JM, et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 21.Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Voleti B, Tanis KQ, Newton SS, Duman RS. Analysis of target genes regulated by chronic electroconvulsive therapy reveals role for Fzd6 in depression. Biol Psychiatry. 2012;71:51–58. doi: 10.1016/j.biopsych.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shansky RM, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 25.Mahon PB, et al. NIMH Genetics Initiative Bipolar Disorder Consortium BiGS Consortium Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166:1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen LS, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519–1522. doi: 10.1176/appi.ajp.160.8.1519. [DOI] [PubMed] [Google Scholar]
- 27.Parry BL. Perimenopausal depression. Am J Psychiatry. 2008;165:23–27. doi: 10.1176/appi.ajp.2007.07071152. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 29.Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.