Abstract
Phosphatidylinositol phosphate kinase type 1γ (PIPKIγ) is a key enzyme in the generation of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and is expressed at high levels in the nervous system. Homozygous knockout mice lacking this enzyme die postnatally within 24 h, whereas PIPKIγ+/− siblings breed normally and have no reported phenotype. Here we show that adult PIPKIγ+/− mice have dramatically elevated hearing thresholds for high-frequency sounds. During the first postnatal week we observed a reduction of ATP-dependent Ca2+ signaling activity in cochlear nonsensory cells. Because Ca2+ signaling under these conditions depends on inositol-1,4,5-trisphosphate generation from phospholipase C (PLC)-dependent hydrolysis of PI(4,5)P2, we conclude that (i) PIPKIγ is primarily responsible for the synthesis of the receptor-regulated PLC-sensitive PI(4,5)P2 pool in the cell syncytia that supports auditory hair cells; (ii) spatially graded impairment of this signaling pathway in cochlear nonsensory cells causes a selective alteration in the acquisition of hearing in PIPKIγ+/− mice. This mouse model also suggests that PIPKIγ may determine the level of gap junction contribution to cochlear development.
Keywords: connexins, deafness, phosphoinositides, Ca2+ oscillations, NF-κB
Cell stimulation with a variety of agents triggers signaling cascades that involve PI(4,5)P2, a minor glycerophospholipid of the inner leaflet of the plasma membrane. PI(4,5)P2 contributes to these processes by being converted into second messengers, by controlling the activity of PI(4,5)P2-binding proteins, or by acting as the substrate of PI(3,4,5)P3 kinase (1). Phospholipase C (PLC)-dependent hydrolysis of PI(4,5)P2 generates the second messenger molecules diacylglycerol and IP3; the latter binds to IP3 receptors (IP3R) to activate Ca2+ efflux from the endoplasmic reticulum, raising the cytosolic free Ca2+ concentration ([Ca2+]i) (2).
Most plasma membrane PI(4,5)P2 is generated by phosphorylation of PI(4)P at the D-5 position of the inositol ring, a reaction catalyzed by type I PI(4)P 5-kinases (PIPKI) (1). Distinct genes encode the three PIPKI isoforms, named α, β, and γ (note that nomenclature for the murine and human isoforms of PIPKIα and PIPKIβ were previously inconsistent; in this article, the murine nomenclature is used); the three isoforms localize within different compartments and play specific roles in individual cell types, because one isoform cannot compensate for the loss of another (3, 4). Of the three isoforms, PIPKIγ is the one expressed at highest concentration in the nervous system (5) in platelets and megakaryocytes (6).
Knockout mice have been generated for all three isoforms of PIPKI. PIPKIα−/− mice develop normally but show increased degranulation and cytokine production by mast cells activated via the Fcε receptor (7). PIPKIβ−/− mice have a normal phenotype except for decreased fertility; however, platelets from these mice show diminished PI(4,5)P2 production, PLC activation, and IP3 production after thrombin treatment (6). PIPKIγ−/− mice, in which exons 2–6 of the PIPKIγ gene (Pip5k1c) that encode most of the catalytic region were deleted by homologous recombination, present decreased levels of PI(4,5)P2 in the nervous system (5). Whereas PIPKIγ+/− mice breed normally and have no obvious phenotype, all PIPKIγ−/− mice are recognizable soon after birth by their impaired motility, and they die postnatally within 24 h. The inability of these mice to feed after birth strongly suggested the occurrence of major neurological defects. Indeed, cortical neurons in primary cultures of from PIPKIγ−/− mice revealed defects in synaptic transmission that may result, at least in part, from an impairment of synaptic vesicle traffic (5). Note that another PIPKIγ knockout mouse generated by random insertional mutagenesis exhibits embryonic lethality at midgestation (8); the reason for this discrepancy is not known.
Here we report that PIPKIγ deficiency in mice results in a sizeable and spatially graded down-regulation of IP3R Ca2+ signaling along the sensory epithelium of the cochlea (inner ear) during a crucial postnatal period that precedes acquisition of hearing, which in mice occurs around postnatal day 12 (P12, where P0 indicates day of birth). This reduction of Ca2+ signaling correlates with the appearance of a specific deafness phenotype in adult PIPKIγ+/− mice, characterized by normal sensitivity to low-frequency sounds but a dramatic reduction of the sensitivity to frequencies in excess of 20 kHz. These data are discussed in terms of present models of inner ear development and sound perception.
Results
Hearing relies on a sensitive mechanoelectrical transduction process in the cochlea of the inner ear, in which the organ of Corti encompasses highly specialized sensory hair cells that are embedded in a matrix of supporting and epithelial cells, henceforth designated as cochlear nonsensory cells. The functional maturation of the cochlea relies not only on the differentiation of hair cells and the formation of coordinately polarized hair bundles, but also on the formation of nonsensory cell networks, which form vast functional syncytia coupling transfer of ions, signaling molecules and nutrients through gap junction channels (9). Our recent work has linked alterations of IP3R Ca2+ signaling in nonsensory cells of the developing cochlea to impaired hearing acquisition in mouse models of human hereditary deafness (10). On the basis of the primary role played by PIPKIγ in IP3 signaling, although no phenotype has been described in PIPKIγ+/− mice, we would predict that a reduction of PI(4,5)P2 levels may affect the development of hearing capability. Accordingly, it seemed of potentially great interest to investigate the hearing performances of transgenic mice with PIPKIγ deficiency.
Hearing Loss in PIPKIγ+/− Mice.
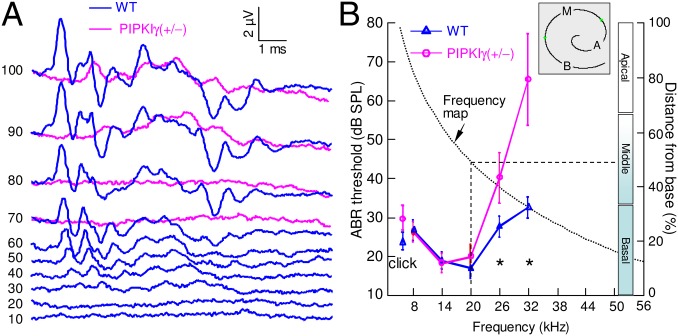
Initially, we examined hearing in adult PIPKIγ+/− mice by recording auditory brainstem responses (ABRs), which are electrical signals evoked from the brainstem by the presentation of sound stimuli (Fig. 1). We assessed ABR thresholds in (n = 6) PIPKIγ+/− mice and (n = 4) WT siblings and found similar values for click and tone burst stimuli at 8, 14, and 20 kHz. However, thresholds where significantly elevated in PIPKIγ+/− mice compared with WT animals, by more than 12 and 33 dB sound pressure level (SPL), respectively, at 26 kHz (P = 0.003) and 32 kHz (P = 0.0002). These results demonstrate that PIPKIγ deficiency ensues in a unique phenotype of hearing loss at high sound frequencies, which are mapped onto the basal 50% of the length of the mouse cochlea (11) (Fig. 1B). Note that the structure of ABR waveforms, when identifiable, was well preserved at all frequencies (Fig. 1A). This behavior suggests normal general functioning of the nervous system and in particular of the neural elements contributing to the generation of synchronized discharges along the auditory nerve and brainstem pathway; and this is also consistent with the lack of an overt neurologic phenotype in these heterozygous mice. Confocal microscopy in adult PIPKIγ+/− mice revealed neither hair cell loss nor morphological abnormalities in spiral ganglion neurons and organ of Corti, which appeared normally innervated (SI Appendix, Fig. S1). In particular, the density of nerve fibers in tangential sections of the osseous spiral lamina (expressed as number of fibers per mm2) was (1.6 ± 0.2) × 105 in WT and (1.6 ± 0.4) × 105 in PIPKIγ+/− mice.
Fig. 1.
Hearing loss in adult PIPKIγ+/− mice. (A) Representative ABR waveforms evoked by a 32-kHz tone burst in a WT mouse (blue) and a PIPKIγ+/− mouse (magenta); numbers indicate stimulus intensity in dB sound pressure level (SPL). (B) Average hearing thresholds (left ordinates) of wave IV ABR for clicks and tone bursts of 8, 14, 20, 26, and 32 kHz in WT mice (blue) and PIPKIγ+/− mice (magenta) between P27 and P50; black asterisks indicate significant differences (ANOVA); error bars, SD. The black dotted curve (right ordinates) is a portion of the mouse position vs. frequency map d = 156.5 − 82.5 · log10(f), where d is the normalized distance along the coiling axis of the cochlea from its base, in percent, and f is the frequency in kHz (11); dashed lines delimit the portion of the map where hearing loss in PIPKIγ+/− mice is noticeable; Apical (A), Middle (M), and Basal (B) refer to the three segments of the cochlear duct length shown schematically (Inset) (SI Appendix, Fig. S2).
PIPKIγ Deficiency Impairs Intercellular Ca2+ Waves and Intracellular [Ca2+]i Oscillations Evoked by ATP in the Postnatal Cochlea.
For these experiments, we used organotypic cultures of P0–P5 cochlear tissue (SI Appendix, Fig. S2) prepared according to standard protocols, because this preparation preserves the architecture and functional relationships among the cells observed in vivo (12). Cultures comprised the lesser epithelial ridge (LER), which is thought to give rise to the outer hair cells and lateral nonsensory cells, and the adjacent greater epithelial ridge (GER), which gives rise to the inner hair cells and medial nonsensory cells (13). After overnight incubation in vitro, we loaded cochlear cultures with the Ca2+ indicator fura-2 and performed ratiometric imaging of the cytosolic free Ca2+ concentration ([Ca2+]i) at room temperature (22−24 °C).
We and others have previously demonstrated that the binding of extracellular ATP to G protein-coupled P2Y2 and P2Y4 receptors, expressed on the endolymphatic surface of the developing sensory epithelium, activates PLC-dependent generation of IP3, which in turn triggers a regenerative Ca2+ wave that propagates for hundreds of micrometers from the site of stimulation (14–18). Using previously developed image processing algorithms (16, 17), we examined Ca2+ wave propagation elicited by a puff of ATP (4 μM, 50 ms) in cultures from (n = 3) WT, (n = 5) PIPKIγ+/−, and (n = 3) PIPKIγ−/− mice (Fig. 2 A and B). Data analysis revealed a significant reduction of the propagation index in LER cells of PIPKIγ+/− (P = 0.003) and PIPKIγ−/− (P = 0.001) cultures compared with WT cultures; the reduction was more pronounced in PIPKIγ−/− cultures, and differences between these and PIPKIγ+/− cultures were statistically significant (P = 0.011) (Fig. 2C). Thus, only quantitative and not qualitative differences were observed, related to this parameter, between the heterozygous and homozygous animals. In addition, because of the postnatal lethality of PIPKIγ−/− mice (5), not only could we analyze only P0 tissues, but later alterations in cochlea physiology were prevented. Clearly complete loss of PIPKIγ may be particularly toxic for the whole organisms, whereas the reduction in the gene dosage in the heterozygote may be more informative of some key feature and specific of the enzymes for cochlear functions. Therefore, we concentrated exclusively on PIPKIγ+/− mice.
Fig. 2.
ATP-evoked Ca2+ signaling in the LER of cochlear organotypic cultures from postnatal WT and PIPKIγ+/− mice. (A) Samples of a representative false-color image sequence showing fura-2 fluorescence ratio changes (ΔR), encoded as shown by the color scale bar, recorded in a WT culture after delivery of a brief puff (50 ms) of ATP (4 μM) through a glass micropipette (white asterisk); time in seconds from puff offset is shown in the lower right corner of the corresponding frame. (Scale bar, 100 μm.) (B) Waveforms of Ca2+ responses obtained by averaging signals within the nine color-matched regions of interest shown over individual cells at increasing distance from micropipette opening in A. (C) propagation index, defined as the maximal area invaded by the intercellular Ca2+ wave divided by the area directly stimulated by the puff (pooled data); an index of 1 indicates no propagation; black asterisks indicate significant differences (ANOVA); error bars, SD. (D) Sample traces of Ca2+ responses obtained by averaging fura-2 ratio signals within 15 randomly selected cells in representative apical turn cultures from P5 WT and PIPKIγ+/− mice during sustained application of extracellular ATP (200 nm, gray bars). (E) Frequency histograms of ATP-evoked [Ca2+]i oscillations (events) in cultures from P5 WT and PIPKIγ+/− mice (pooled data); in each graph, the solid black line (right ordinates) is the time integral of the corresponding frequency histogram and, as such, it tracks the mean number of events in the cell population from the onset of the ATP application.
Intercellular Ca2+ waves are often accompanied by a trail of [Ca2+]i oscillations, which can also be reliably evoked by the application of extracellular ATP at nanomolar levels (16–18). When stimulated by prolonged application of ATP (200 nM), nonsensory LER cells in cultures from P5 WT mice developed [Ca2+]i oscillations that persisted for periods in excess of 10 min (Fig. 2 D and E, Left). By contrast, [Ca2+]i oscillations in LER cells from age-matched PIPKIγ+/− mice ceased almost abruptly after variable time intervals that rarely exceeded 5 min (Fig. 2 D and E, Right). Specifically, the duration of Ca2+ oscillation bursts evoked by application of 200 nM ATP in the LER of PIPKIγ+/− cultures were 169 ± 37 s in the apical turn, 244 ± 40 s in the middle turn, and 171 ± 38 s in the basal turn (m = 100 cells in each of n = 3 cultures for each turn and each genotype).
Next, we constructed ATP dose–response functions by plotting the maximal fura-2 ratio change (ΔRmax) vs. [ATP] in LER cells from P5 mice (Fig. 3; each data point is the average of m = 15 cells in n = 3 cultures). In the apical turn, the dose–response of WT and PIPKIγ+/− cultures superimposed almost perfectly, with similar half-maximal effective concentration: EC50 = 93 ± 4 nM in WT and EC50 = 101 ± 9 nM in PIPKIγ+/− cultures. In the middle turn, the EC50 shifted significantly from 96 ± 5 nM of WT to 353 ± 13 nM of PIPKIγ+/− cultures (P = 2 × 10−4). In the basal turn, the shift was even more dramatic: from 72 ± 4 nM of WT to 448 ± 18 nM of PIPKIγ+/− cultures (P = 5 × 10−4). Quantitative real-time PCR (qPCR) analysis of cochlear ducts explanted from (n = 4) PIPKIγ+/− mice at P5 showed the expected reduction of PIPKIγ mRNA level compared with (n = 4) age-matched WT controls (Fig. 3B). The reduction in the basal turn was significantly more pronounced than in the middle and apical turn (P < 0.05), in accord with the ATP dose–response analysis (Fig. 3A).
Fig. 3.
ATP dose–response curves in the LER of organotypic cultures (A) and qPCR analysis of PIPKIγ mRNA level in the sensory epithelium (B) from P5 WT and PIPKIγ+/− cochleae (pooled data). Lines through data in A are least-square fits with the sum of two scaled logistic functions. Black asterisks in B indicate significant differences (ANOVA); error bars, SD.
The question thus arises as to whether PIPKIγ reduction also affects the amplitude of the [Ca2+]i oscillations evoked by ATP in the LER (Fig. 4). Averaged waveforms had similar amplitudes in apical turn of WT and PIPKIγ+/− cultures; by contrast, amplitudes in middle and basal turn PIPKIγ+/− cultures were reduced by 24% and 22% compared with WT controls (Fig. 4, Left; each waveform was obtained in LER cells by averaging more than 1,500 peak-aligned [Ca2+]i oscillations within 3 min from the onset of a 200-nM ATP application). Accordingly, distributions of [Ca2+]i oscillation amplitudes in WT and PIPKIγ+/− LER cultures (Fig. 4, Right) overlapped in the apical turn but were shifted toward significantly lower values in middle and basal turns (P = 2.2 × 10−16).
Fig. 4.
Amplitudes of [Ca2+]i oscillations evoked by 200 nM ATP in the LER of organotypic cochlear cultures from P5 WT and PIPKIγ+/− mice (pooled data). Left: Average waveforms; Right: amplitude distributions (for box plot symbolism; see, e.g., http://en.wikipedia.org/wiki/Box_plot); black asterisks indicate significant differences between data distributions (Mann−Whitney U test; SI Appendix, SI Materials and Methods).
PIPKIγ Deficiency Impairs Spontaneous [Ca2+]i Transients in the Postnatal Cochlea.
Spontaneous [Ca2+]i oscillations in LER cells are rarely observed while working with cochlear organotypic cultures at room temperature, but their frequency is drastically increased upon blockade of ectoATPases (17). Conversely, spontaneous [Ca2+]i transients were always observed in the GER (Fig. 5A). These spontaneous events have been attributed by us and other investigators to spontaneous release of ATP (19, 20) through connexin hemichannels (21). Not only have we confirmed these data in these GER preparations (sensitivity to ATP receptor antagonists and to connexin hemichannel inhibitors), but we could also show that focal UV photolysis of a caged intracellular IP3 precursor in the GER evokes [Ca2+]i transients similar to the spontaneous ones (Movie S1). In addition, and similarly to [Ca2+]i oscillations induced by exogenous ATP in LER cells, (i) the spontaneous [Ca2+]i transients showed no sign of fatigue in recordings lasting 15 min or more in the GER of (n = 3) WT cultures (SI Appendix, Fig. S3A, Left); (ii) the spontaneous [Ca2+]i transients in the GER ceased within 5 min in (n = 3) WT cultures after addition of 200 nM thapsigargin (SI Appendix, Fig. S3A, Right) a noncompetitive inhibitor of the Ca2+-ATPase (SERCA) that causes the complete and irreversible depletion of Ca2+ from the endoplasmic reticulum; (iii) the PLC inhibitor U73122 (2.5 μM) significantly (P = 0.001) and reversibly reduced the frequency of spontaneous [Ca2+]i transients in the GER (n = 3; SI Appendix, Fig. S3B, Left); and (iv) the spontaneous [Ca2+]i transients in the GER were also drastically reduced by the potent (although not completely specific) IP3R antagonist 2-aminoethoxydiphenyl borate (2-APB, 100 μM; n = 3; SI Appendix, Fig. S3B, Right). Altogether, the results of these and previously published experiments (17, 21) indicate that ATP-evoked [Ca2+]i oscillations in the LER and spontaneous [Ca2+]i transients in the GER are generated by similar mechanisms [i.e., ATP activation of P2Y receptors (by exogenous addition of ATP in the LER or local ATP release through connexin hemichannels in the GER), followed by IP3 generation and Ca2+ release from the endoplasmic reticulum].
Fig. 5.
Spontaneous [Ca2+]i transients in the GER of WT and PIPKIγ+/− postnatal cochlear cultures. (A) Left: representative false-color image of fura-2 fluorescence ratio changes (ΔR), encoded as shown by the color scale bar, obtained as maximal projection rendering of all frames recorded in a basal turn culture from a WT P5 mouse imaged for 4 min at eight frames per second; 20 regions of interests are shown superimposed on different Ca2+ hot spots. (Scale bar, 100 μm.) Right: Fura-2 traces generated as pixel averages from the 20 color-matched regions of interests shown at Left. (B) Frequency histograms of spontaneous [Ca2+]i transients (events) in cultures from P5 WT and PIPKIγ+/− mice (pooled data); in each graph, the solid black line (right ordinates) is the time integral of the corresponding frequency histogram and, as such, it tracks the mean number of events in the cell population from the onset of the recording.
The prediction can thus be made that PIPKIγ deficiency should also affect the pattern of spontaneous events in GER cells. We thus examined the frequency of spontaneous [Ca2+]i transients in each cochlear turn by counting all events within the portion of the GER in the field view while imaging cultures obtained from P5 WT mice (n = 4) and age-matched PIPKIγ+/− siblings (n = 4) (Fig. 5B). Even considering the different areas of the GER regions along the coiling axis of the cochlea (SI Appendix, SI Materials and Methods), the 1.85-fold lower mean frequency in the basal turn with respect to the apical turn reflects a genuine reduction of spontaneous Ca2+ signaling activity in the different GER regions of WT cultures (Fig. 5B, Left). We then performed the same analysis in GER cells from PIPKIγ+/− cultures (Fig. 5B, Right). In the apical turn, we found similar mean frequencies of occurrence (39 ± 4 events per minute in WT vs. 38 ± 11 events per minute in PIPKIγ+/− cultures). Differences become discernible, although not statistically significant, in the middle turn (32 ± 7 events per minute in WT vs. 25 ± 8 events per minute in PIPKIγ+/− cultures; P = 0.32). They were evident (fivefold reduction) and highly significant in the basal turn (21 ± 2 events per minute in WT vs. 4 ± 1 events per minute in PIPKIγ+/− cultures; P = 2 × 10−4), in accord with the more pronounced reduction of PIPKIγ mRNA level in this turn (Fig. 3B).
As far as amplitudes of spontaneous [Ca2+]i transients are concerned (Fig. 6), akin to what we noticed in LER cells, averaged waveforms had similar amplitudes in apical turn GER of WT and PIPKIγ+/− cultures; by contrast, amplitudes in middle and basal turn GER of PIPKIγ+/− cultures were reduced by 21% and 33% compared with WT controls (Fig. 6, Left; waveforms were obtained by averaging 448, 385, and 389 peak-aligned [Ca2+]i transients in WT cultures, 557, 392, and 62 transients in PIPKIγ+/− cultures from the apical, middle, and basal turn, respectively). Accordingly, amplitude distributions of spontaneous [Ca2+]i transients in the GER of PIPKIγ+/− cultures overlapped with WT distributions in the apical turn but were shifted toward significantly lower values (P < 5.0 × 10−5) both in middle and basal turns (Fig. 6, Right). [Ca2+]i oscillations induced by exogenous ATP were not investigated in GER cells, because they superimpose to spontaneous events.
Fig. 6.
Amplitudes of spontaneous [Ca2+]i transients in the GER of cochlear organotypic cultures from P5 WT and PIPKIγ+/− mice (pooled data). Left: Average waveforms; Right: amplitude distributions; black asterisks indicate significant differences between data distributions (Mann−Whitney U test).
To summarize, both the amplitude and frequency of spontaneous [Ca2+]i transients were drastically reduced in the basal turn and hardly affected in the apical turn of the GER. This correlates with the reduced sensitivity to ATP and the more marked reduction in the level of PIPKIγ in the basal region, suggesting that this enzyme is critical for producing the PI(4,5)P2 pool responsible for IP3 generation in cochlear nonsensory cells.
PIPKIγ Deficiency Causes Down-Regulation of Connexin Transcripts in the Postnatal Cochlea.
In nonexcitable cells, such as cochlear nonsensory cells, [Ca2+]i controls the dynamics of gene expression through the activation of multiple transcription factors (22, 23). A widely held hypothesis is that information is encoded mainly by the frequency of [Ca2+]i oscillations (22); however, a possible role of amplitudes and duration in signal transduction has been discussed (24, 25). It has also been argued that amplitude modulation and frequency modulation differentially regulate distinct targets (26).
Our prior work with cochlear organotypic cultures had noted that [Ca2+]i oscillations in nonsensory cells participate in the coordinated regulation of connexin26 (Cx26) and connexin30 (Cx30) expression through the nuclear factor-κ light chain enhancer of activated B cells (NF-κB) (27), a thoroughly investigated Ca2+-dependent transcription factor (22, 23, 28). These are the only connexins expressed in the organ of Corti nonsensory cells (29), and they are essential for the cell-to-cell propagation of Ca2+ signals (14, 17, 18). Here we analyzed samples from (n = 8) P5 PIPKIγ+/− mice and compared mRNA levels of both connexins with the corresponding cochlear turn in (n = 8) age-matched WT controls. The residual level of Cx26 mRNA was 75% ± 7% in the apical turn, 65% ± 7% in the middle, and 65% ± 6% in the basal turn. The corresponding figures for Cx30 mRNA were 70% ± 5%, 79% ± 5%, and 67% ± 4% in the apical, middle, and basal turn, respectively. Thus, impaired IP3R Ca2+ signaling in cochlear nonsensory cells due to PIPKIγ deficiency causes significant down-regulation of both connexin transcripts (P < 0.005), whereas our prior work determined that deletion of either Cx26 or Cx30 impairs IP3R Ca2+ signaling in cochlear nonsensory cells (17, 27). A seemingly logical conclusion is that IP3R Ca2+ signaling establishes a tight feedback loop between channel function and transcript levels of Cx26 and Cx30 in the developing cochlea.
To corroborate this conclusion, we analyzed also Cx30+/− and Cx30−/− mice. Note that normal auditory thresholds were reported for adult Cx30+/− mice (30), whereas both Cx30−/− mice (21, 30) and mice with targeted ablation of Cx26 in the inner ear (31, 32) are profoundly deaf at all tested frequencies. We also showed that whole-cochlea samples from postnatal Cx30−/− mice have only ∼8% residual Cx26 mRNA (27). Here we found significantly reduced levels (P < 0.001) of Cx26 (58% ± 8%) and Cx30 (54% ± 9%) in the cochlea of (n = 4) P5 Cx30+/− mice compared with (n = 4) age-matched WT controls. The frequencies of occurrence of spontaneous [Ca2+]i transients in GER cells of cochlear organotypic cultures from (n = 4) P5 Cx30+/− mice were indistinguishable from those of (n = 4) WT controls; by contrast, frequencies in (n = 4) Cx30−/− cultures were significantly reduced (P < 0.001) to levels resembling those of (n = 4) basal turn PIPKIγ+/− cultures (SI Appendix, Fig. S4). Finally, amplitudes of these spontaneous [Ca2+]i transients were reduced in the middle and basal turn of Cx30+/− cultures (P < 0.005) and in all turns of Cx30−/− cultures (P < 0.001) (SI Appendix, Fig. S5).
To consolidate these findings we exploited the previously demonstrated capability of flufenamic acid, a connexin hemichannel blocker, to reversibly suppress both intercellular Ca2+ waves (17) and spontaneous [Ca2+]i transients (21). In this study, we used a canalostomy approach for drug delivery to the inner ear via the posterior semicircular canal of WT mouse pups aged P4 or P5 (Movie S2). Four weeks after this single-shot injection, we tested hearing by recording ABRs and determined that auditory thresholds in (n = 25) mice injected with artificial endolymph (vehicle) were indistinguishable from those of (n = 12) noninjected controls; in contrast, thresholds in (n = 4) mice that had received flufenamic acid (250 μM) were significantly elevated (P < 0.05) by more than 15 dB SPL at all tested frequencies (SI Appendix, Fig. S6).
Discussion
This study reveals a unique hearing loss phenotype in adult PIPKIγ+/− mice at sound frequencies in excess of 20 kHz (Fig. 1) and demonstrates a key role of PIPKIγ in the inner ear and its essential function for the acquisition of hearing. It has been hypothesized that different enzymes contribute to the local control of PI(4,5)P2 synthesis and confinement, thus generating distinct pools of PI(4,5)P2 in the plasma membrane, each controlling specific functions (1). On the basis of studies in platelets it has been suggested that PIPKIα and PIP5KIβ contribute to the rapid synthesis of the PI(4,5)P2 pool required for IP3 and diacylglycerol generation upon agonist stimulation, whereas the PI(4,5)P2 pool synthesized by PIP5KIγ does not play a major role in this process (6). At odds with this conclusion, inhibition of PIPKIγ expression in HeLa cells by RNA interference, although marginally reducing the total cellular PI(4,5)P2 level, dramatically reduces IP3 formation after histamine stimulation (33). The present data concur with the results in HeLa cells, demonstrating that Ca2+ signaling is selectively affected in nonsensory cells of PIPKIγ+/− and PIPKIγ−/− cochlear tissue.
Taken together, the simplest interpretation of the data presented in Figs. 2 and 3 is that reduction in gene dosage of PIPKIγ in the heterozygote causes a corresponding reduction in PI(4,5)P2 levels in nonsensory cells of the developing cochlea. Consequently, PLC-mediated PI(4,5)P2 hydrolysis in PIPKIγ+/− cultures more rapidly depletes a specific pool of PI(4,5)P2 that is necessary for ATP-dependent intercellular Ca2+ wave propagation and maintenance of [Ca2+]i oscillations in nonsensory LER cells. The apparent decrease in affinity for ATP in the medial and basal turn (Fig. 3A) most likely depends on the more marked reduction in PIPKIγ in these locations (Fig. 3B). Thus, below a critical enzyme level, PI(4,5)P2 becomes the limiting factor, and optimal levels of IP3 can only be produced when a larger number of P2 receptors are engaged by the ligand. This conclusion is also consistent with the reduced amplitudes of [Ca2+]i oscillations evoked by 200 nM ATP (Fig. 4), which is a nearly saturating concentration in all cochlear turns of WT cultures, but submaximal for medial and basal turn PIPKIγ+/− cultures (Fig. 3A). Most relevant from a functional point of view, the hearing loss detected by our ABR measurements in adult PIPKIγ+/− mice maps precisely to this basal portion of the cochlea (Fig. 1), in a region comprising part of the middle and the whole basal turn where we detected the most pronounced Ca2+ signaling alterations (Figs. 3–6).
To summarize, the results presented here demonstrate that cochlear nonsensory cells of the LER and the GER share the same PLC- and IP3R-dependent signal transduction cascade activated by ATP (SI Appendix, Fig. S3) and suggest that reduction in PIPKIγ gene dosage in the heterozygote causes a corresponding reduction in the critical pool of PI(4,5)P2 necessary for normal Ca2+ signaling. We can speculate that the lack of spontaneous oscillations in LER cells in these experimental conditions (unlike those of GER) is due to (i) more efficient ATP hydrolysis by ectoATPases; (ii) reduced number and/or decreased opening probability of Cx hemichannel; or (iii) a combination of these mechanisms.
PIPKIγ reduction affects not only Ca2+ signaling directly (by reducing the precursor of IP3) but also the expression of another key component of Ca2+ signaling, the channel-forming proteins Cx26 and Cx30. Indeed, we have shown previously that genetic manipulation of these connexins strongly affects Ca2+ signal generation and propagation (17, 21, 27). The present results single out Cx26 and Cx30 as both targets and effectors of Ca2+ signaling in the developing cochlea. As for the pathogenesis of deafness, our comparative analysis of PIPKIγ+/− and Cx30+/− postnatal cultures (SI Appendix, Figs. S4 and S5) indicates that down-regulation of Cx26 and Cx30 mRNA level, by as much as ∼50%, is insufficient per se to cause hearing loss. Instead, hearing acquisition is disrupted when Ca2+ signaling in cochlear nonsensory cells falls below a critical threshold. In Cx30−/− postnatal cultures the criticality is imparted by the uniform deletion of Cx30 and the drastic reduction of the residual Cx26 in the sensory epithelium. Indeed, in all turns of the Cx30−/− postnatal cochlea, IP3R Ca2+ signaling in nonsensory cells is reduced to levels comparable to or lower than those of basal turn PIPKIγ+/− cochlea. As predicted, adult Cx30−/− mice are doomed to deafness at all frequencies (21, 30). In PIPKIγ+/− mice, the criticality is imparted by the apparent decrease in affinity for ATP only in the medial and basal turn, and the hearing defect is limited to the sound frequencies mapping in this region of the coiled sensory epithelium (Fig. 1). The importance of connexin hemichannel functionality in the critical period of hearing acquisition is further supported by the results with the connexin hemichannel inhibitor flufenamic acid (SI Appendix, Fig. S6), which suppresses Ca2+ signaling in nonsensory cells of cochlear cultures (17, 21) and causes hearing loss at all tested frequencies when delivered via canalostomy to the inner ear of mouse pups.
The severe hearing loss of Cx30−/− mice (30) and of mice with targeted ablation of Cx26 in the inner ear (31, 32) is associated with substantial cell death affecting hair cells and supporting cells. By contrast, we did not detect morphological abnormalities in the inner ear of adult PIPKIγ+/− mice, in which hair cells, spiral ganglion neurons and nerve fiber counts were indistinguishable from WT controls (SI Appendix, Fig. S1). Indeed, an overall normal functioning of the neural elements that contribute to the generation of synchronized discharges along the auditory nerve and brainstem pathway is required to account for the shape of PIPKIγ+/− ABR waveforms (Fig. 1A); and this is also consistent with the lack of an overt neurologic phenotype in these heterozygous mice, which behave and breed normally. Evidently, complete lack of one of these two connexins, combined with the regulation mechanisms that govern their coordinated expression (27), impacts on viability of inner ear cells more than impairment of Ca2+ signaling due to reduction of PIPKIγ gene dosage. Further understanding how the Ca2+ signaling defects reported here link to hearing loss in these different mouse models may prove crucial to unravel how hearing acquisition proceeds under normal circumstances, as well as to decipher the pathogenic processes underlying human hereditary deafness forms and, eventually, to test prospective therapies.
Materials and Methods
Statistical Analysis.
Means are quoted ± SD, and P values are indicated by letter P. Unless otherwise stated, statistical comparisons of means were made by one-way ANOVA in conjunction with the Tukey range test.
Animal Handling.
Animal handling was approved by the Ethics Committee of Padua University (Comitato Etico di Ateneo per la Sperimentazione Animale; Project 54/2009, Protocol 51731).
ABR Recordings.
ABRs were used to assess hearing threshold of all mice strains used in this study.
Quantitative PCR.
mRNA was extracted from freshly isolated P5 whole cochleae or from apical, middle, and basal turn, using the RNAeasy kit (Qiagen).
Ca2+ Imaging.
Signals were measured as fura-2 emission ratio changes,  , where
, where  is time,
is time,  is emission intensity excited at 360 nm divided by the intensity excited at 380 nm, and
is emission intensity excited at 360 nm divided by the intensity excited at 380 nm, and  indicates baseline ratio.
indicates baseline ratio.
Immunohistochemistry and Confocal Imaging.
Cochlear tissues were prepared according to standard protocols and analyzed using a confocal microscope (TCS SP5; Leica) equipped with an oil immersion objective (either 40× HCX PL APO 1.25 N.A.; Leica).
Further details are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca-Progetti di Rilevante Interesse Nazionale Grant 2009CCZSES (to T.P. and F.M.), Telethon Grant GGP09137 (to F.M.), and National Institutes of Health Grant R37NS036251 (to P.D.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211869109/-/DCSupplemental.
References
- 1.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowska K. One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane. Cell Mol Life Sci. 2010;67:3927–3946. doi: 10.1007/s00018-010-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Paolo G, et al. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, et al. Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation. Proc Natl Acad Sci USA. 2008;105:14064–14069. doi: 10.1073/pnas.0804139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki J, et al. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I alpha. J Exp Med. 2005;201:859–870. doi: 10.1084/jem.20041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci USA. 2007;104:11748–11753. doi: 10.1073/pnas.0700019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly MC, Chen P. Development of form and function in the mammalian cochlea. Curr Opin Neurobiol. 2009;19:395–401. doi: 10.1016/j.conb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mammano F. Ca2+ homeostasis defects and hereditary hearing loss. Biofactors. 2011;37:182–188. doi: 10.1002/biof.150. [DOI] [PubMed] [Google Scholar]
- 11.Müller M, von Hünerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Sobkowicz HM, Bereman B, Rose JE. Organotypic development of the organ of Corti in culture. J Neurocytol. 1975;4:543–572. doi: 10.1007/BF01351537. [DOI] [PubMed] [Google Scholar]
- 13.Lim D, Rueda J. Structural development of the cochlea. In: Romand R, editor. Development of Auditory and Vestibular Systems—2. 1st Ed. New York: Elsevier Science; 1992. pp. 33–58. [Google Scholar]
- 14.Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 15.Mammano F, Bortolozzi M, Ortolano S, Anselmi F. Ca2+ signaling in the inner ear. Physiology (Bethesda) 2007;22:131–144. doi: 10.1152/physiol.00040.2006. [DOI] [PubMed] [Google Scholar]
- 16.Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium. 2007;41:77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Anselmi F, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumder P, et al. ATP-mediated cell-cell signaling in the organ of Corti: The role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 20.Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schütz M, et al. The human deafness-associated connexin 30 T5M mutation causes mild hearing loss and reduces biochemical coupling among cochlear non-sensory cells in knock-in mice. Hum Mol Genet. 2010;19:4759–4773. doi: 10.1093/hmg/ddq402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 23.Mellström B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: Molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- 24.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 25.Prank K, Gabbiani F, Brabant G. Coding efficiency and information rates in transmembrane signaling. Biosystems. 2000;55:15–22. doi: 10.1016/s0303-2647(99)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 27.Ortolano S, et al. Coordinated control of connexin 26 and connexin 30 at the regulatory and functional level in the inner ear. Proc Natl Acad Sci USA. 2008;105:18776–18781. doi: 10.1073/pnas.0800831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 29.Forge A, et al. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 30.Teubner B, et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- 31.Cohen-Salmon M, et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crispino G, et al. BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice. PLoS ONE. 2011;6:e23279. doi: 10.1371/journal.pone.0023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YJ, et al. Critical role of PIP5KIgamma87 in InsP3-mediated Ca(2+) signaling. J Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.