Abstract
Liquid-ordered phases are one of two biochemically active membrane states, which until now were thought to be a unique consequence of the interactions between eukaryotic membrane lipids. The formation of a liquid-ordered phase depends crucially on the ordering properties of sterols. However, it is not known whether this capacity exists in organisms that lack sterols, such as bacteria. We show that diplopterol, the simplest bacterial hopanoid, has similar properties and that hopanoids are bacterial “sterol surrogates” with the ability to order saturated lipids and to form a liquid-ordered phase in model membranes. These observations suggest that the evolution of an ordered biochemically active liquid membrane could have evolved before the oxygenation of Earth’s surface and the emergence of sterols.
Keywords: lipid order, polycyclic isoprenoids, bacterial membrane organization, membrane phase evolution, organic geochemistry
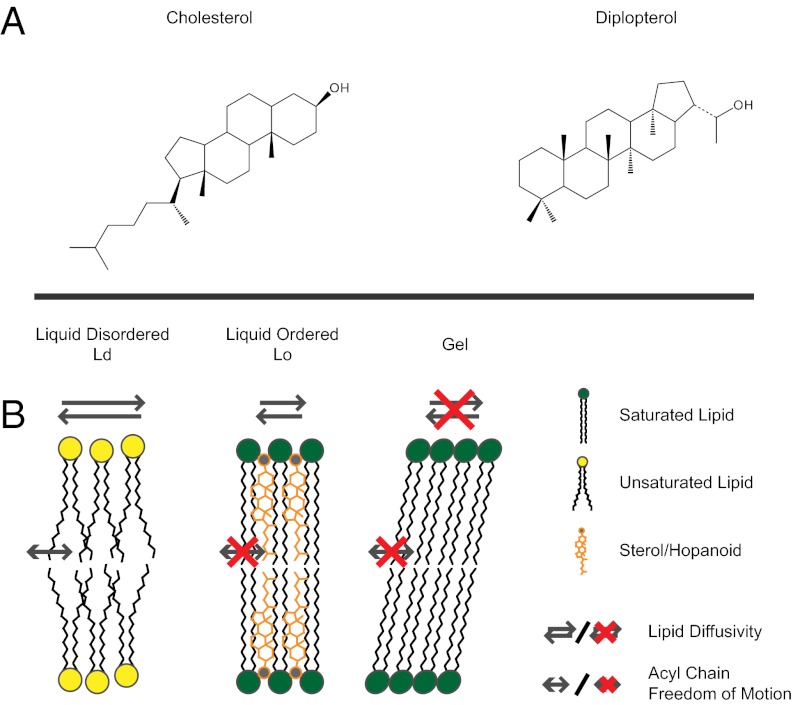
The capacity for sterols to modulate the ordering of lipids forms the basis for a membrane organizing principle in eukaryotes (1). The emergence of sterol-like ordering was likely a critical step in the evolution of biological membranes, allowing cells to control fluidity without compromising membrane integrity and providing a means to compartmentalize membranes into functional domains (2–4). It is not known, however, to what extent such membrane-ordering properties span the domains of life. Prokaryotes generally lack sterols; however, some bacteria produce hopanoids (5, 6), which are structurally similar (Fig. 1A) (7), and their cyclization is catalyzed by related enzymes (8). These similarities inspired the hypothesis that hopanoids are bacterial sterol surrogates (9) and led us to examine whether hopanoids might share the properties of sterols in membranes.
Fig. 1.
Structures of cholesterol and diplopterol (A) and a conceptual cartoon illustrating liquid-disordered (Ld), liquid-ordered (Lo), and gel phase membranes (B).
Results and Discussion
Effects of Cholesterol and Diplopterol on the Phase Behavior and Ordering of Sphingomyelin in Model Membranes.
Sterols and sphingolipids are closely associated in eukaryotic membranes, and the nature of their interactions has been extensively characterized. Therefore, we chose to test whether diplopterol behaves similarly to cholesterol in this well-defined system. Sterols interact with sphingolipids in vitro to form a liquid-ordered (Lo) phase that represents a thermodynamic intermediate between liquid-disordered (Ld) and crystalline gel phases (Fig. 1B) (10). The interactions leading to the formation of a Lo phase derive from the ability of sterols to simultaneously inhibit the formation of the gel phase (by intercalating between sphingolipids and preventing their crystallization) and to order saturated acyl chains. To test whether these properties are also exhibited by hopanoids, we examined the effect of diplopterol on N-stearoyl-d-erythro-sphingosylphosphorylcholine (SM; Fig. S1), a synthetic sphingolipid.
Monolayer experiments provide an approach to study the gel-liquid phase transition of SM. Lipids are spread out over an air-water interface to form a monolayer and lateral pressure (measured as surface tension) is measured while the area of the monolayer is decreased. The measurements are depicted as an isothermal plot of pressure versus mean molecular area (MMA; Å2/molecule) of the lipid mixture (Fig. 2A). Lipids such as SM that form a gel phase at physiological temperatures show a characteristic inflection point in the isotherm plot, which reflects a sharp phase transition from liquid to gel phase. This phase transition is eliminated in the presence of cholesterol (Fig. 2A). We observed the same effect of diplopterol on SM (Fig. 2A), demonstrating a shared ability to inhibit gel phase formation.
Fig. 2.
The effects of cholesterol and diplopterol on SM. (A) Monolayers of SM and mixtures containing cholesterol (chol) or diplopterol (dip) were compressed at 25 °C. The condensation effect of chol and dip on SM was calculated as shown to the right of the isotherm traces. (B) The membrane ordering effect (∆GP) of chol or dip on SM was determined by C-laurdan spectroscopy on liposomes labeled with 0.2 mol% C-laurdan and composed of SM, SM/chol (2:1 mol%), and SM/dip (2:1) at 50 °C. The ∆GP was calculated as the difference in GP between liposomes containing pure SM and mixtures of either SM/chol or SM/dip.
To determine whether diplopterol shares an ability with cholesterol to order SM, we measured ordering by 6-dodecanoyl-2-methylcarboxymethylaminonaphthalene (C-laurdan) spectroscopy. C-laurdan is a lipophilic fluorescent probe with a bimodal emission spectrum that shifts in response to the degree of hydration of the membrane. The generalized polarization (GP) index calculated from C-laurdan emission spectra is correlated with lipid order (11). We calculated the ordering effect of cholesterol or diplopterol as the difference in the GP index (∆GP) of liposomal membranes containing pure SM and mixtures containing cholesterol or diplopterol. Measurements were made above the gel-liquid transition temperature of SM to ensure that we were observing an ordering effect on bilayers in a liquid state and not a fluidizing effect on gel phase bilayers. Our results indicate that diplopterol exhibits an ordering effect on SM comparable to the effect of cholesterol (Fig. 2B). This result is further corroborated by monolayer experiments in which the observed MMA of mixtures containing cholesterol or diplopterol with SM was less than the MMA predicted from the sum of the individual components, indicating a energetically favorable condensing interaction (Fig. 2A). Interestingly, compared with cholesterol, diplopterol exhibits a weak ordering effect on lipids containing unsaturated acyl chains (Figs. S2 and S3).
Cholesterol and Diplopterol Interact with Sphingomyelin to Form a Lo Phase in Giant Unilamellar Vesicles.
Having demonstrated a shared ability for diplopterol and cholesterol to inhibit gel phase formation and order SM, we directly assayed whether diplopterol induces the formation of a Lo phase. In model systems composed of synthetic lipids SM and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; Fig. S1), cholesterol induces the formation of two immiscible liquid phases: a Lo enriched in SM and sterol and a Ld enriched in DOPC (12). We investigated the coexistence of Lo and Ld phases in giant unilamellar vesicles (GUVs) by using C-laurdan microscopy. Mixtures containing SM/DOPC and either cholesterol or diplopterol yielded phase-separated GUVs with ordered and disordered phases (Fig. 3 A and B), suggesting that the hopanoid ring structure is capable of inducing phase separation. The relative order (e.g., C-laurdan GP) of GUVs comprised of SM/cholesterol and SM/diplopterol were roughly equal, and within the range characteristic of Lo bilayers (Fig. 3 A and B) (13). By comparison, the GP of DOPC GUVs was negative, indicating a Ld membrane. These observations were confirmed by quantifying the diffusivities of the membranes by fluorescence correlation spectroscopy. The diffusivity of Atto532-labeled sphingomyelin in GUVs containing cholesterol or diplopterol with SM was identical and nearly an order of magnitude slower than for DOPC GUVs (Fig. 3C). These results demonstrate that diplopterol is capable of interacting with SM to form a Lo phase with order and fluidity that are essentially identical to the cholesterol-SM Lo phase.
Fig. 3.
Diplopterol forms a liquid ordered phase. (A) Confocal images of GUVs at 22 °C and labeled with 0.2 mol% C-laurdan. The composition (mol %) of GUVs is given below each image, and GP values are depicted by color. (B) Average GP of one- and two-component GUVs and of ordered and disordered domains from three-component GUVs (n = 4). (C) Autocorrelation curves and estimated diffusion times of Atto532 labeled sphingomyelin (0.001 mol%) in SM/Chol, SM/Dip, and DOPC GUVs at 22 °C.
Cholesterol and Diplopterol Modulate the Order and Phase Behavior of Lipid A in Model Membranes.
Thus, we now have evidence that diplopterol and cholesterol share a conserved ability to order saturated lipids and to promote the formation of a Lo phase while preventing the formation of a gel phase. The physiological relevance of this property in bacteria, however, remains unaddressed. The cellular abundance of hopanoids in bacteria varies by nearly two orders of magnitude between organisms (6) and approaches roughly 50% of the lipid content of some bacteria (14). We observed that the ordering effect of diplopterol was apparent at molar concentrations as low as 5% (Fig. S4), indicating that hopanoids could play a role in membrane ordering at physiologically relevant abundances.
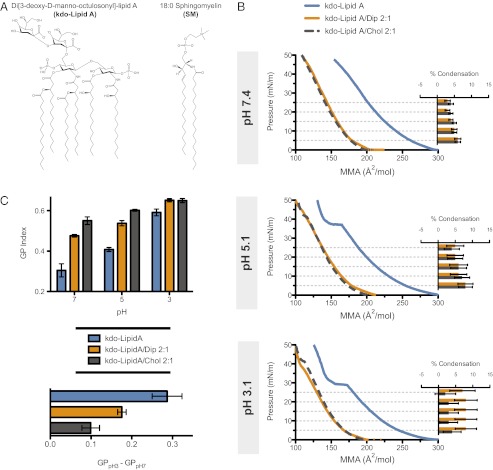
Hopanoids in bacteria have been observed in the outer membranes (15–17), where lipid A is the major component of the extracellular leaflet of the bilayer (18). This lipid bears several structural similarities to SM, including amide-linked saturated acyl chains and hydroxylations (Fig. 4A). This similarity led us to conjecture that diplopterol could also affect lipid A-containing membranes. It was demonstrated that lipid A undergoes a pH-dependent change in order, becoming less fluid at lower pH and approaching a gel state (19). Such extreme pH-induced changes in ordering of lipid A could be detrimental to the integrity and biochemical functionality of the bacterial outer membrane. Indeed, two recent studies showed that mutants of hopanoid-producing bacteria with disrupted hopanoid synthesis exhibited retarded growth at low pH (20, 21). We therefore tested the possibility that hopanoids play a role in modulating the order of lipid A in response to changing pH.
Fig. 4.
Ordering effect of diplopterol (dip) and cholesterol (chol) on kdo-lipid A. (A) comparison of the structures of kdo-lipid A and SM. (B) Isotherms of Lipid A mixtures and calculated condensation effect of chol and dip on kdo-lipid A at 25 °C and pH 7.4, 5.1, and 3.1. (C) The membrane order (GP) of liposomes labeled with 0.2 mol% C-laurdan containing kdo-lipid A, kdo-lipid A/dip, and kdo-lipid A/chol. The pH-induced change in relative membrane order was calculated as the difference in GP (GPpH3 – GPpH7) between pH 3 and 7 for the three mixtures at 25 °C.
To determine whether diplopterol or cholesterol could influence the pH-induced phase transition of lipid A, we examined their effect on synthetic Di[3-deoxy-d-manno-octulosonyl]-lipid A (kdo-lipid A; Fig. 4A) in monolayers at neutral and acidic pH. At pH 7.4, kdo-lipid A yielded a monotonically increasing change in surface pressure with decreasing molecular area, demonstrating that it is in a fluid state with no phase transition occurring (Fig. 4B). As predicted, at pH 5 and 3, we observed the emergence of a phase transition, indentified by a “shelf” in the isotherms (Fig. 4B). The addition of diplopterol or cholesterol eliminated this phase transition. Additionally, both of these lipids exhibited a condensing effect on kdo-lipid A (Fig. 4B), consistent with our previous results demonstrating their ability to condense SM. Together, these observations indicate that both diplopterol and cholesterol are capable of simultaneously condensing lipid A and inhibiting its gel phase formation, thus buffering the effects of low pH on lipid A.
Finally, we measured the order of kdo-lipid A–containing membranes by using C-laurdan spectroscopy. At pH 7, liposomes comprised of kdo-lipid A alone were less ordered than with either diplopterol or cholesterol, and values of the latter two mixtures were within the range expected for Lo membranes (Fig. 4C). Thus, in terms of order, lipid A/diplopterol membranes at neutral pH are analogous to SM/cholesterol membranes. At pH 5.1 and 3.1, the GP of pure lipid A in comparison with pH 7 increased to ∼0.4 and 0.6, respectively, indicating increasing membrane order. However, the lipid A mixtures with diplopterol and cholesterol exhibited a smaller change in the GP. These results again demonstrate that diplopterol and cholesterol have the ability to moderate pH-induced changes in ordering.
This capability of hopanoids could play a key role in modulating the ordering of the outer membrane of hopanoid-producing bacteria in environments with variable pH and could explain the prominence of hopanoids in bacteria that live in acidic environments (5, 6, 22), environments that experience large pH shifts such as soils (23, 24), and the rarity of hopanoids in pH-buffered environments such as the oceans (25–27). It may also explain the previously mentioned sensitivity of hopanoid deficient mutants to low pH (20, 21).
Concluding Comments.
Here, we demonstrate that the hopanoid ring structure, like the sterol ring structure, is capable of interacting with saturated lipids to form a Lo phase and to modulate the order of lipid A. The similarities between hopanoids and sterols were first considered more than 30 y ago by pioneers in the field (7), and they were subsequently dubbed as “bacterial sterol surrogates” (9). However, the significance of the Lo phase as a unique product of lipid ordering and the biological implications of the Lo phase for membrane organization were not known at that time. The ordering properties of hopanoids in some bacteria could potentially confer the ability to subcompartmentalize their membranes into functional domains (28). Evidence for lipid-dependent functional domains has been reported for Bacillus subtilis (29), and there is evidence for lateral membrane heterogeneity in Gloeobacter violaceus (30), a hopanoid-producing cyanobacterium. Interestingly, it has been suggested that the averaged order of eukaryotic plasma membranes and bacterial inner membranes lacking hopanoids converge (31). These studies imply that bacteria that lack hopanoids may use alternate mechanisms for achieving lateral heterogeneity and modulating membrane order.
Because the biosynthesis of hopanoids does not require molecular oxygen (32–35), our results demonstrate that the capacity to order membranes could have preceded the emergence of free oxygen on Earth’s surface. Furthermore, the shared ability of hopanoids and sterols to mediate Lo phase formation suggests that this property might be a conserved feature of all membrane polycyclic isoprenoids. This possibility prompts the need to extend our observations to other hopanoids (such as the bacteriohopanepolyols) and other cyclic lipids including tetrahymanol. If ordering and the promotion of coexisting liquid phases are conserved properties of these lipids, the invention of isoprenoidal cylcase enzymes could mark an important event in the evolution of biological complexity: the evolution of a second biochemically active liquid membrane phase and the ability to regulate membrane order by decoupling lipid lateral diffusivity from acyl chain freedom of motion.
Materials and Methods
Materials.
SM, kdo-lipid A, DPPC, POPC, DPPC, DPPG, POPG, DOPG, and cholesterol were purchased from Avanti Polar Lipids. Diplopterol was purchased from Chiron. Atto532 labeled sphingomyelin was purchased from AttoTech. C-laurdan was a gift from B. R. Cho (Department of Chemistry and Center for Electro- and Photo-Responsive Molecules, Korea University, Seoul, Korea). Stock concentrations of lipids were measured by phosphate assay. Cholesterol and diplopterol were weighed out on a precision scale and solubilized in a known volume of chloroform/methanol (2:1).
Monolayers.
Monolayers were prepared as described (36). Briefly, chloroform/methanol (2:1) solutions of pure lipids and mixtures were prepared at 0.5 mg/mL lipid concentrations. Monolayers were prepared by injecting 10–20 µL of lipid solution onto an aqueous subphase maintained at 25 °C by a built-in water jacket supplied by a temperature controlled circulating water bath. The subphase was comprised of 150 mM NaCl, 3.3 mM sodium citrate, 3.3 mM sodium phosphate, 3.3 mM glycine, and 0.1 mM EDTA (EDTA), with pH titrated to 7.4, 5.1, or 3.1 by HCl or NaOH. Isotherms were recorded by using a 70 cm2 teflon Langmuir trough fitted with a motorized compression barrier equipped with pressure sensor and Wilhelmy plate (Nima Technnology).
The MMAs for each mixture were estimated from the averages of isotherms from three monolayers that were prepared independently. The theoretical mean area per molecule (lipid) for each mixture was calculated as follows:
where Ai = MMA of the mixture, X1, X2 = the mole fraction of lipid 1 and 2, and A1,A2 = the MMAs of lipid 1 and 2 at surface pressures 5, 10, 15, 20, and 25 mN/m. The percent change in molecular area (condensation effect) was calculated as follows:
where c = % condensation, Ao = the observed MMA at 5, 10, 15, 20, and 25 mN/m, and Ai = the theoretical MMA of the two lipids.
Preparation of Liposomes and C-Laurdan Spectroscopy.
Lipids in 2:1 chloroform/methanol were mixed and dried under vacuum for 4 h. Lipids were then hydrated in HBS (50 mM Hepes, 150 mM NaCl, and 0.2 mM EDTA at pH 7.2) at 68 °C for 20 min. The resulting liposomes were subjected to 10 freeze-thaw cycles, followed by sonication for 5 min to promote formation of unilamellar membranes. Bilayer formation was confirmed in unlabeled liposomes by the presence an emission peak at 425 nm (λex = 385 nm) (37). Liposome preparations containing 200 μM lipid were stained with 100 nM C-laurdan and incubated at room temperature for 20 min to equilibrate. Liposomes of each mixture were independently mixed and prepared in triplicate. Spectra were recorded with 1-nm resolution on a Fluoromax-3 fluorescence spectrometer (Horriba) with temperature maintained at either 25 or 50 °C by a temperature controlled circulating water bath. Excitation of C-laurdan was 385 nm. Spectra were recorded from triplicate preparations of each mixture and averaged. The GP values for C-laurdan were calculated from two emission bands 400–460 nm (Ch1) and 470–530 nm (Ch2) according to equation 1 from Parassassi et al. (11):
Preparation of GUVs.
GUVs were prepared according to Bacia et al. (12). Briefly, lipids and fluorescent probes were mixed in chloroform/methanol 2:1 to achieve 1 mg/mL lipid concentration. Five microliters of lipid mixture was deposited on two platinum electrodes and dried under vacuum for 1 h. Dried lipids were electroswelled in 300 mM sucrose at 2 V and 10 Hz for 2 h followed by 2 Hz for 30 min. Electroswelling was performed at 68 °C. After cooling, GUVs for C-laurdan microscopy were stained with 100 nM C-laurdan and incubated at room temperature for 20 min to equilibrate.
Two-Photon Fluorescence Microscopy.
C-laurdan spectra were measured on GUVs by two-photon confocal fluorescence microscopy by using the same instrumentation and methodology as described by Kaiser et al. (13). Images were recorded on a Bio-Rad two-photon setup with a Mira 2000 two-photon laser by using a ×60WI objective (NA 1.2). C-laurdan was excited at 800 nm, and the mission was captured by using 425/50 (Ch1-low λ) and 525/70 (Ch1-high λ) filters. Microscopy was performed at 22 °C. Image processing and analysis were performed with Matlab (Mathworks) as described by Kaiser et al. (13). For each GUV, three areas were chosen per phase and averaged. Measurements from four GUVs were averaged and SDs calculated.
Fluorescence Correlation Spectroscopy (FCS).
FCS experiments were carried out as described (38, 39). Briefly, the FCS focal spot was placed either on the top or the bottom of the GUVs. The maximum intensity was found by moving the focal volume up and down slightly, which ensures that membrane is at the center of the focal spot. Atto532-labeled sphingomyelin (AttoTech) was used as the membrane fluorescent probe. A 543-nm laser was used to excite the fluorescent lipid analog, and band pass 560–615 was used to collect the emission. Measurements were done at least on five different vesicles. Diffusion times were obtained by fitting the autocorrelation curves with a 2D one-component diffusion model:
 |
Supplementary Material
Acknowledgments
We thank Ilya Levental and Michal Grzybek for their invaluable support in the laboratory and for critical feedback on the manuscript. We also thank Michal Surma, Alexander Bradley, Robert Ernst, and Unal Coskun for critical feedback and helpful discussions. Dr. B. R. Cho kindly provided the C-laurdan used in this study. This work was supported by the Alexander von Humboldt Foundation, the National Science Foundation [International Research Fellowship Program (IRFP) 1064754], German Research Foundation (DFG) “Schwerpunktprogramm 1175” (SI459/2-1), DFG “Transregio 83” (TRR83 TP02), European Science Foundation (ESF) “LIPIDPROD” (SI459/3-1), German Federal Ministry of Education and Research (BMBF) “ForMaT” (03FO1212), and the Klaus Tschira Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212141109/-/DCSupplemental.
References
- 1.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Xu XL, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. (Translated from English) Biochemistry-Us. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 3.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 4.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson A, Flood Page SR, Jorgenson TL, Fischer WW, Higgins MB. Novel hopanoid cyclases from the environment. Environ Microbiol. 2007;9:2175–2188. doi: 10.1111/j.1462-2920.2007.01331.x. [DOI] [PubMed] [Google Scholar]
- 6.Rohmer M, Bouviernave P, Ourisson G. Distribution of hopanoid triterpenes in prokaryotes. J Gen Microbiol. 1984;130:1137–1150. [Google Scholar]
- 7.Rohmer M, Bouvier P, Ourisson G. Molecular evolution of biomembranes: Structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci USA. 1979;76:847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendt KU, Poralla K, Schulz GE. Structure and function of a squalene cyclase. Science. 1997;277:1811–1815. doi: 10.1126/science.277.5333.1811. [DOI] [PubMed] [Google Scholar]
- 9.Ourisson G, Rohmer M, Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- 10.Ipsen JH, Karlström G, Mouritsen OG, Wennerström H, Zuckermann MJ. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 11.Parasassi T, De Stasio G, d’Ubaldo A, Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990;57:1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc Natl Acad Sci USA. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser HJ, et al. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci USA. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans MAF, Neuss B, Sahm H. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J Bacteriol. 1991;173:5592–5595. doi: 10.1128/jb.173.17.5592-5595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughty DM, Hunter RC, Summons RE, Newman DK. 2-Methylhopanoids are maximally produced in akinetes of Nostoc punctiforme: Geobiological implications. Geobiology. 2009;7:524–532. doi: 10.1111/j.1472-4669.2009.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgens UJ, Simonin P, Rohmer M. Localization and distribution of hopanoids in membrane systems of the cyanobacterium Synechocystis Pcc-6714. FEMS Microbiol Lett. 1992;92:285–288. doi: 10.1016/0378-1097(92)90723-2. [DOI] [PubMed] [Google Scholar]
- 17.Hancock IC, Williams KM. The outer-membrane of methylobacterium-organophilum. J Gen Microbiol. 1986;132:599–610. [Google Scholar]
- 18.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandenburg K, Seydel U. Investigation into the fluidity of lipopolysaccharide and free lipid A membrane systems by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Eur J Biochem. 1990;191:229–236. doi: 10.1111/j.1432-1033.1990.tb19114.x. [DOI] [PubMed] [Google Scholar]
- 20.Welander PV, et al. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J Bacteriol. 2009;191:6145–6156. doi: 10.1128/JB.00460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmerk CL, Bernards MA, Valvano MA. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J Bacteriol. 2011;193:6712–6723. doi: 10.1128/JB.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DS, et al. Community genomic analysis of an extremely acidophilic sulfur-oxidizing biofilm. ISME J. 2012;6:158–170. doi: 10.1038/ismej.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke MP, Talbot HM, Farrimond P. Bacterial populations recorded in bacteriohopanepolyol distributions in soils from Northern England. Org Geochem. 2008;39:1347–1358. [Google Scholar]
- 24.Xu YP, Cooke MP, Talbot HM, Simpson MJ. Bacteriohopanepolyol signatures of bacterial populations in Western Canadian soils. Org Geochem. 2009;40:79–86. [Google Scholar]
- 25.Saenz JP, Eglinton TI, Summons RE. Abundance and structural diversity of bacteriohopanepolyols in suspended particulate matter along a river to ocean transect. Org Geochem. 2011;42:774–780. [Google Scholar]
- 26.Pearson A, Rusch DB. Distribution of microbial terpenoid lipid cyclases in the global ocean metagenome. ISME J. 2009;3:352–363. doi: 10.1038/ismej.2008.116. [DOI] [PubMed] [Google Scholar]
- 27.Saenz JP, Wakeham SG, Eglinton TI, Summons RE. New constraints on the provenance of hopanoids in the marine geologic record: Bacteriohopanepolyols in marine suboxic and anoxic environments. Org Geochem. 2011;42:1351–1362. [Google Scholar]
- 28.Saenz JP. Hopanoid enrichment in a detergent resistant membrane fraction of Crocosphaera watsonii: Implications for bacterial lipid raft formation. Org Geochem. 2010;41:853–856. [Google Scholar]
- 29.López D, Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–1902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rexroth S, et al. The plasma membrane of the cyanobacterium Gloeobacter violaceus contains segregated bioenergetic domains. Plant Cell. 2011;23:2379–2390. doi: 10.1105/tpc.111.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser HJ, et al. Molecular convergence of bacterial and eukaryotic surface order. J Biol Chem. 2011;286:40631–40637. doi: 10.1074/jbc.M111.276444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neunlist S, Holst O, Rohmer M. Prokaryotic triterpenoids - the hopanoids of the purple non-sulfur bacterium Rhodomicrobium vannielii: An aminotriol and its aminoacyl derivatives, N-tryptophanyl and N-ornithinyl aminotriol. Eur J Biochem. 1985;147:561–568. [PubMed] [Google Scholar]
- 33.Fischer WW, Summons RE, Pearson A. Targeted genomic detection of biosynthetic pathways: Anaerobic production of hopanoid biomarkers by a common sedimentary microbe. Geobiology. 2005;3:33–40. [Google Scholar]
- 34.Härtner T, Straub KL, Kannenberg EL. Occurrence of hopanoid lipids in anaerobic Geobacter species. FEMS Microbiol Lett. 2005;243:59–64. doi: 10.1016/j.femsle.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Sinninghe Damsté JS, et al. The occurrence of hopanoids in planctomycetes: Implications for the sedimentary biomarker record. Org Geochem. 2004;35:561–566. [Google Scholar]
- 36.Grzybek M, Kubiak J, Łach A, Przybyło M, Sikorski AF. A raft-associated species of phosphatidylethanolamine interacts with cholesterol comparably to sphingomyelin. A Langmuir-Blodgett monolayer study. PLoS ONE. 2009;4:e5053. doi: 10.1371/journal.pone.0005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sezgin E, et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- 38.Kahya N, Scherfeld D, Schwille P. Differential lipid packing abilities and dynamics in giant unilamellar vesicles composed of short-chain saturated glycerol-phospholipids, sphingomyelin and cholesterol. Chem Phys Lipids. 2005;135:169–180. doi: 10.1016/j.chemphyslip.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Sezgin E, et al. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochimica Et Biophysica Acta-Biomembranes. 2012;1818:1777–1784. doi: 10.1016/j.bbamem.2012.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.