Abstract
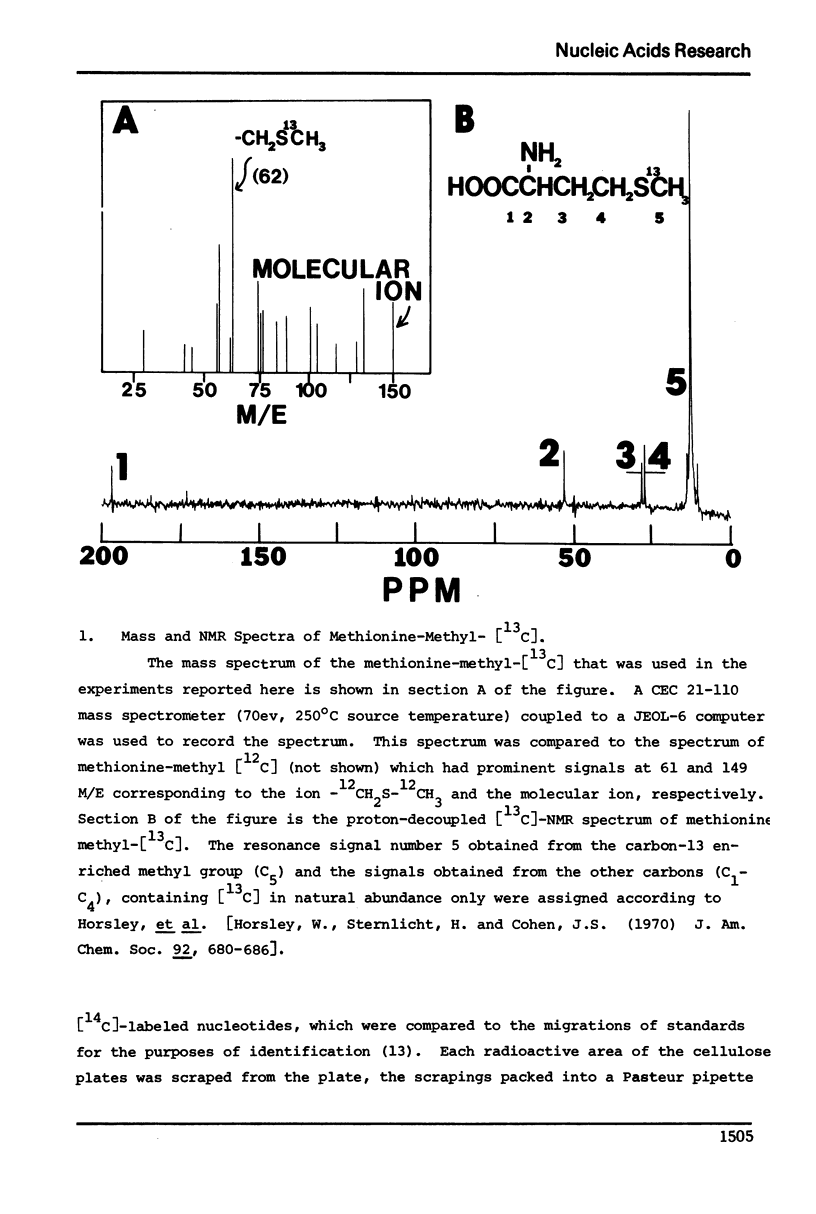
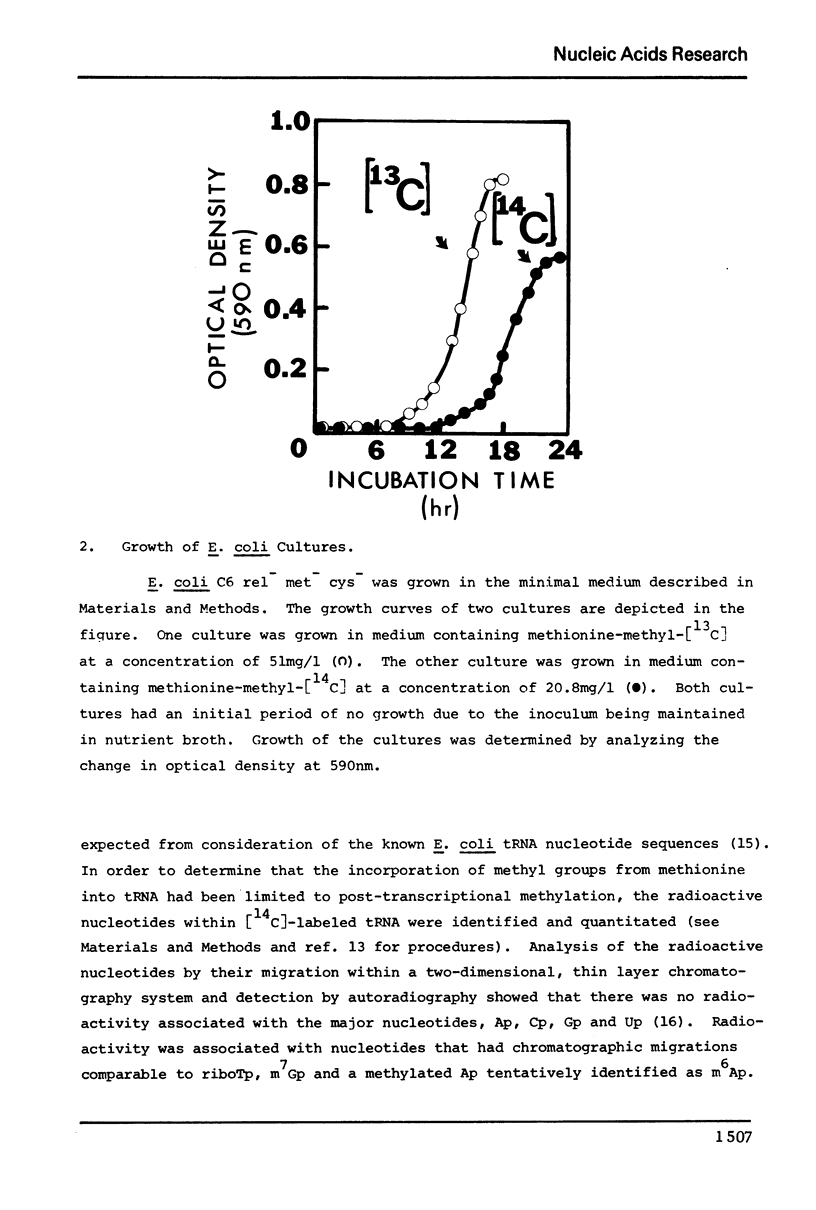
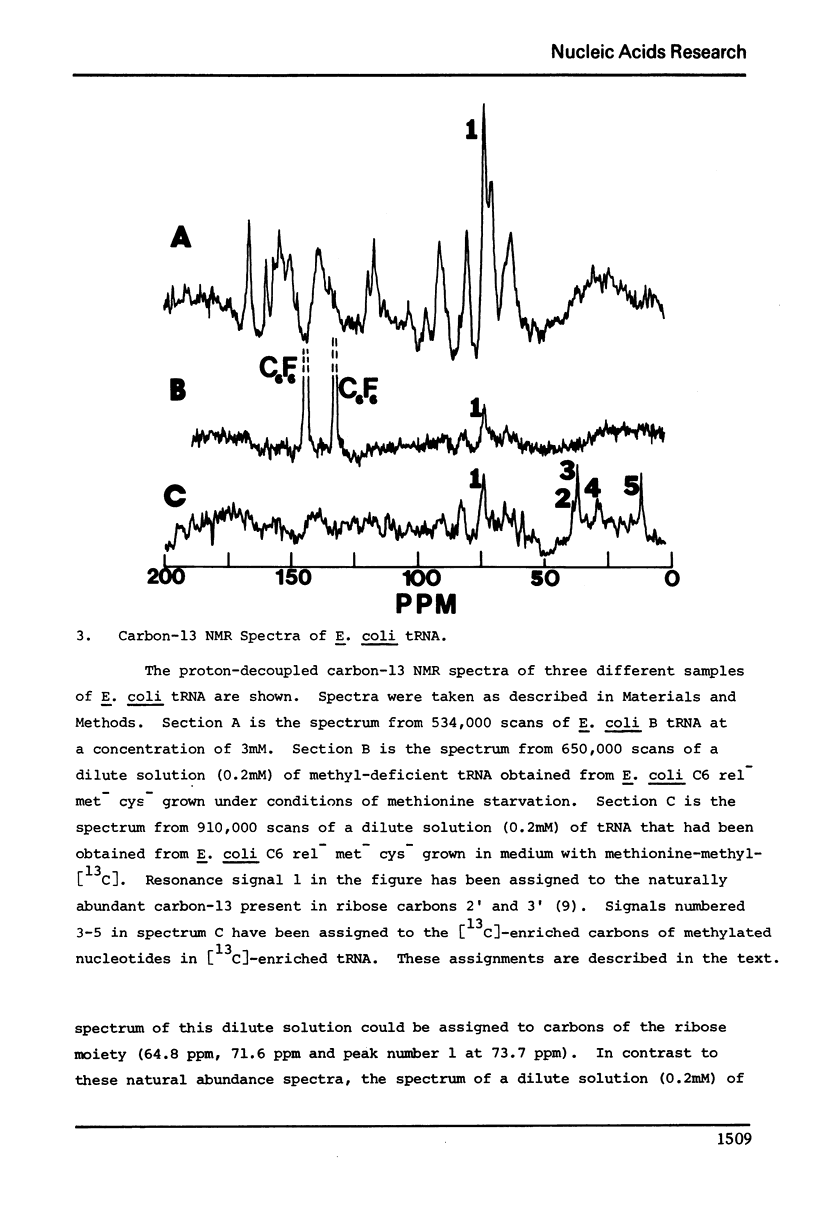
The enrichment of tRNA at specific sites with carbon-13 has been accomplished in vivo using a mutant of Escherichia coli. A relaxed strain of E. coli auxotrophic for methionine was grown in a specifically defined medium supplemented with either [14C] or [13C]-methyl labeled methionine. Cells were collected at the end of the log-phase of growth and tRNA was extracted. Analysis of the radioactivity of the [14C]-labeled tRNA established an incorporation ratio of three labeled carbons per tRNA molecule. Incorporation of the [14C]-label in vivo was confined to the methylation of nucleotides as determined by thin layer chromatography of nucleotides resulting from a ribonuclease digestion of [14C]-labeled tRNA. The carbon-13 NMR spectrum of [13C]-enriched tRNA indicated a similar degree of incorporation into the methylated nucleotides by the substantial enhancement of [13C]-methyl NMR signals only. Assignment of signals has been made for the methyl groups of ribothymidine and N7-methylguanosine in E. coli tRNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Agris P. F., Söll D., Seno T. Biological function of 2-thiouridine in Escherichia coli glutamic acid transfer ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4331–4337. doi: 10.1021/bi00746a005. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. Nucleotide sequence of 5S-ribosomal RNA from Escherichia coli. Nature. 1967 Aug 12;215(5102):735–736. doi: 10.1038/215735a0. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Titchener E. B., Cline A. L. Sulfur-deficient transfer ribonucleic acid in a cysteine-requiring, "relaxed" mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1322–1327. doi: 10.1128/jb.100.3.1322-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L. S., Ts'o P. O., von der Haar F., Sprinzl M., Cramer F. NMR study on the methyl and methylene proton resonances of tRNA Phe yeast. Biochem Biophys Res Commun. 1974 Jul 10;59(1):22–29. doi: 10.1016/s0006-291x(74)80168-3. [DOI] [PubMed] [Google Scholar]
- Kearns D. R., Lightfoot D. R., Wong K. L., Wong Y. P., Reid B. R., Cary L., Shulman R. G. High-resolution NMR investigation of base pairing structure of transfer RNA. Ann N Y Acad Sci. 1973 Dec 31;222:324–336. doi: 10.1111/j.1749-6632.1973.tb15271.x. [DOI] [PubMed] [Google Scholar]
- Kearns D. R., Wong K. L., Wong Y. P. Effect of the removal of the Y base on the conformation of yeast tRNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3843–3846. doi: 10.1073/pnas.70.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R., Wong Y. P., Chang S. H., Hawkins E. Investigation of the structure of native and denatured conformations of tRNALeu3 by high-resolution nuclear magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4736–4746. doi: 10.1021/bi00720a009. [DOI] [PubMed] [Google Scholar]
- Komoroski R. A., Allerhand A. Natural-abundance carbon-13 Fourier-transform nuclear magnetic resonance spectra and spin lattice relaxation times of unfractionated yeast transfer-FNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1804–1808. doi: 10.1073/pnas.69.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoroski R. A., Allerhand A. Observation of resonances from some minor bases in the natural-abundance carbon-13 nuclear magnetic resonance spectrum of unfractionated yeast transfer ribonucleic acid. Evidence for fast internal motion of the dihydrouracil rings. Biochemistry. 1974 Jan 15;13(2):369–372. doi: 10.1021/bi00699a023. [DOI] [PubMed] [Google Scholar]
- Kreishman G. P., Miller J. P., Dea P., Hussain Z., Wilson L. A., Schweizer M. P. 300 MHz PMR studies on the conformation of the hexanucleotide, 2'OMeGpApApUpApPsi, from the anticodon loop of torula yeast tRNAphe. Biochem Biophys Res Commun. 1974 May 7;58(1):27–34. doi: 10.1016/0006-291x(74)90886-9. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Schweizer M. P. Effects of dilute HCl on yeast tRNAPhe and E. coli tRNA1fMet. Nucleic Acids Res. 1974 Feb;1(2):183–192. doi: 10.1093/nar/1.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns T. W., Podratz K. C., Katzman P. A. A method for determination of the methylated constituents of transfer ribonucleic acid. Biochemistry. 1974 Oct 8;13(21):4409–4416. doi: 10.1021/bi00718a026. [DOI] [PubMed] [Google Scholar]
- Wong K. L., Kearns D. R. Investigation of the base-pairing structure of the anticodon hairpin from E. coli initiator tRNA by high-resolution nmr. Biopolymers. 1974;13(2):371–380. doi: 10.1002/bip.1974.360130212. [DOI] [PubMed] [Google Scholar]



