Abstract
Interleukin-10 (IL-10) is an important factor involved in T-cell dysfunction during persistent viral infection. Although several factors can negatively regulate T-cell activity, targeting of the IL-10 pathway alone is sufficient to regenerate T-cell activity and increase viral control. How IL-10 mediates these effects is unclear. Here, we investigated the cellular source of IL-10 necessary for establishing T-cell exhaustion and viral persistence, using IL-10 reporter mice (VertX), cell-type–specific IL-10 and IL-10 receptor deletion mice, and bone marrow chimeric mice. During establishment of viral persistence, the cellular subset with the most prevalent expression of IL-10 was CD8α−CD4+ dendritic cells (DCs), which produced IL-10 with increasing kinetics until 9 d postinfection. After this time point, DCs exhibited a modest decline in percentage of IL-10+ cells whereas B cells and CD4+ T cells increased minimally. Further analysis of the DC population demonstrated that IL-10 was primarily expressed in infected DCs. These DCs were a notable source of IL-10 as mutant mice with a DC-specific deletion of IL-10 had significantly decreased serum levels. Interestingly, viral infection was not directly causative of IL-10 expression; rather, IL-10 production appeared to be linked to type I IFN signaling. Our findings further illuminate the contribution of DCs to the production of IL-10 and to viral persistence.
Keywords: lymphocytic choriomeningitis virus, il10 conditional knockout
Effective T-cell responses are essential for the control and clearance of viral infection (1). However, during persistent viral infections, T cells experience T-cell exhaustion, a serialized loss of effector activity that includes proliferation, cytokine expression, and cell killing, resulting in dampened antiviral immune response and abortive control of viral infection (2). Interleukin-10, an anti-inflammatory cytokine, is an important factor contributing to T-cell exhaustion in a murine model of chronic infection (3). During persistent infection with the clone 13 (CL-13) strain of lymphocytic choriomeningitis virus (LCMV), interleukin-10 (IL-10) is significantly up-regulated (3, 4) and is critical to establishing T-cell exhaustion and viral persistence, as deletion of the il10 gene or early blockade of the IL-10 receptor (IL-10R) is sufficient to prevent both events (3). Once viral persistence is initiated, IL-10 is also necessary for the maintenance of T-cell exhaustion. Antibody blockade of IL-10 signaling in the chronic phase resurrects the function of exhausted T cells, leading to a significant decrease in viral titers (3, 5). The results of these studies are encouraging for developing treatments of chronic viral infections because they indicate that T-cell exhaustion, a hallmark of chronic viral infection, is reversible when the correct pathway is targeted. Further, examination of human chronic infections has identified increased levels of IL-10 in HIV (6), hepatitis B virus (7), and hepatitis C virus (8) infection. Thus, IL-10 and its signaling pathway are potential therapeutic targets, both before and after the establishment of viral persistence, to improve T-cell responses and abolish viral infection.
Although IL-10 is essential to the development of viral persistence and T-cell exhaustion, the specific mechanisms remain unclear. During Cl-13 infection, il10 mRNA is up-regulated in several cell types but predominantly within dendritic cells (DCs), indicating that DCs may be important for IL-10 production (3). However, other in vitro studies report that CD4+ T cells are the important source of IL-10 (4). Thus, the biologically important source(s) of IL-10 is controversial. This issue is further complicated by the fact that IL-10 can be secreted by a wide variety of immune cells and is differentially regulated (9), suggesting that the source of IL-10 is a major determinant of its effect, on the basis of factors such as mechanism of IL-10 regulation, proximity to target cells, and IL-10R expression.
DCs are essential to the initiation of the immune response and are critical in determining its quality and quantity. There are three main subsets of DCs in the spleen: CD8α+, CD8α−CD4+, and CD8α−CD4− (10). The latter two subsets are traditionally categorized together as CD8α− DCs. Both CD8α− and CD8α+ DCs present antigen on MHC II and MHC I; however, CD8α+ DCs have the additional ability of cross-presentation, whereby extracellular antigen is taken up and presented on MHC I to CD8+ T cells (11). The antigen-presenting abilities of both DC subsets can be manipulated by IL-10 or CL-13. IL-10 prevents DC up-regulation of stimulatory molecules, including MHC I, MHC II, CD80, and CD86 (12, 13), thereby impairing the ability of DCs to generate T-cell responses. Similarly, during LCMV CL-13 infection, DCs exhibit reduced cell-surface expression of MHC and costimulatory molecules (14).
To elucidate the mechanism of IL-10, we investigated the cellular source(s) of IL-10 relevant to establishing persistent LCMV infection. We demonstrate a high frequency of IL-10+ DCs throughout the commencement of viral persistence whereas the frequency of other IL-10+ subsets minimally increased during the initial 9 d postinfection (dpi). Further analysis of infection in conditional knockout mice pinpoints DCs as the major contributor to IL-10 production at the onset of persistence. These IL-10+ DCs are a specific subset of CD8α−CD4+ DCs to which CL-13 infection is restricted. Moreover, virus infection is not the direct cause of IL-10 expression; rather, IL-10 production is associated with type I IFN signaling.
Results
IL-10 Is Secreted Primarily by DCs.
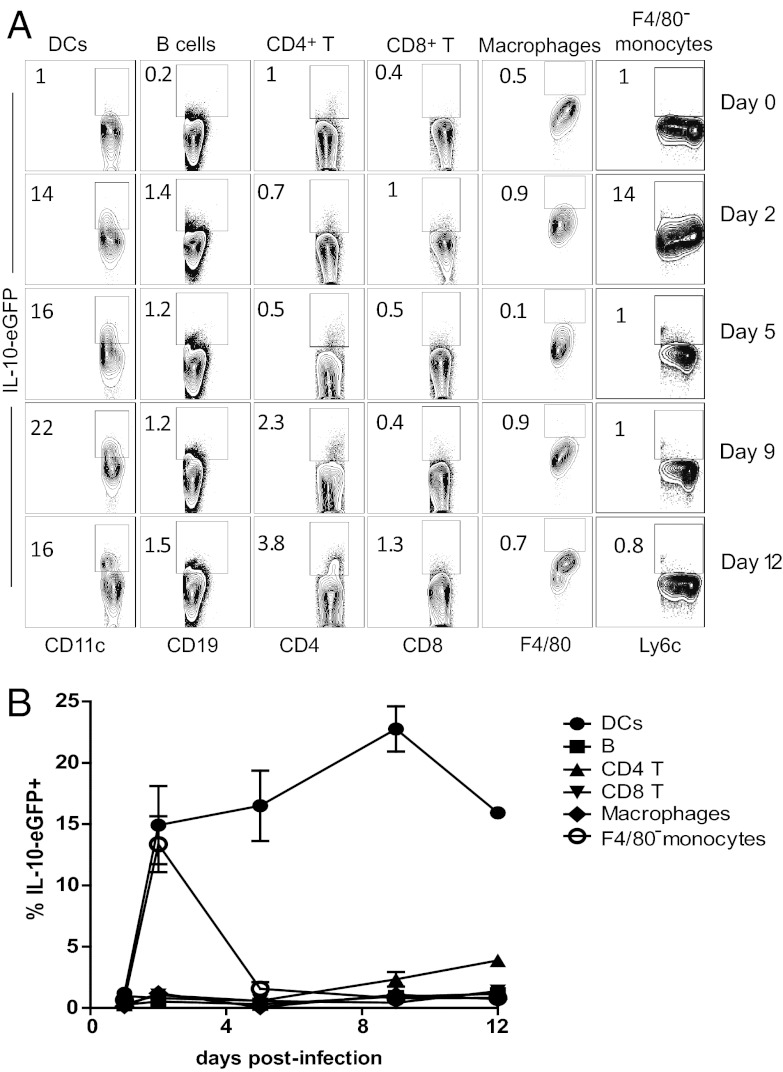
To examine the development of IL-10 and characteristics of cells that produce IL-10 during the induction of CL-13 persistence, we used a reporter mouse in which the il10 gene drives expression of enhanced green fluorescent protein (eGFP) (VertX) (15), thereby producing IL-10+ cells identifiable by green fluorescence. In the murine host, CL-13 peaks at 7 dpi and establishes persistence characterized by the initiation of T-cell exhaustion by 9 dpi. Therefore, to examine IL-10 leading up to the establishment of viral persistence and T-cell exhaustion, VertX mice were infected with 2 × 106 plaque-forming units (pfu) of CL-13 and examined at 0, 2, 5, 9, and 12 dpi for lymphocyte expression of IL-10. Analysis revealed that at all time points tested, a significant proportion of DCs (CD45+CD3−CD19−NK1.1−CD11chi) produced IL-10 (Fig. 1 A and B). IL-10+ DCs were present early by 2 dpi (14.9 ± 0.1%), increased to 22.8 ± 1.8% at 9 dpi when T-cell exhaustion is established, and declined to 15.9 ± 0.4% by 12 dpi (Fig. 1B). Comparatively, F4/80− monocytes [CD45+CD3−CD19−NK1.1−CD11b+Ly6c+Ly6g−F4/80−; splenic monocytes (16)] exhibited brief production at 2dpi, whereas macrophages (CD45+CD3−CD19−NK1.1−CD11b+Ly6c+Ly6g−F4/80+) exhibited a negligible increase over background fluorescence at any time point (Fig. 1 A and B and Fig. S1). IL-10 was produced to a minimal extent in B cells (CD45+CD3−CD19+CD11c−), CD4+ T cells (CD45+CD19−CD3+CD8−CD4+), and CD8+ T cells (CD45+CD19−CD3+CD4−CD8+) at 9 dpi or later. At 12 dpi, their levels minimally increased to 1.2 ± 0.1% of B cells and 3.9 ± 0.4% of CD4+ T cells, levels that were at least fourfold lower than those of DCs. Together, these data identify DCs as a population relevant to IL-10 production during the establishment of persistence.
Fig. 1.
IL-10 expression in lymphocytes during CL-13 infection. IL-10-eGFP (VertX) reporter mice were infected with 2 × 106 pfu of CL-13 and lymphocyte populations were analyzed for IL-10 expression over the first 12 d of infection. (A) Flow plots depict IL-10–eGFP expression by cellular subset. The numbers are the mean frequency of IL-10+ cells in that population. (B) Summary of the frequencies of IL-10+ cellular subsets. Error bars show ±SD. Data are representative of three independent experiments using two to three mice per time point.
DC IL-10 Expression Is Restricted to a Virally Infected CD4+ CD8α− Subset.
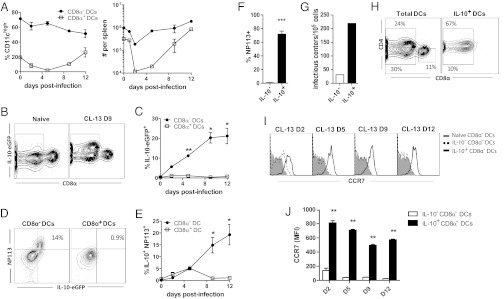
Next, we investigated the DC subsets that express IL-10 during CL-13 infection. Analysis of IL-10+ DCs revealed IL-10 expression was restricted to CD8α− DCs (Fig. 2 B and C) and that IL-10 expression coincided with expression of LCMV nucleoprotein (NP) (Fig. 2 D–F). The presence of LCMV NP was indicative of infectious virus because IL-10+ DCs gave sevenfold more infectious centers than IL-10− DCs when assessed in an infectious center assay at 9 dpi (Fig. 2G). Although CD8α− DCs are more prevalent than CD8α+ (Fig. 2A), this result was unexpected because CD8α+ DCs are thought to be important for the Th1 response (17) and, presumably, would be a more likely target for viral infection. Further study revealed that colocalization resided mainly in the CD4+ subset of CD8α− DCs (Fig. 2H).
Fig. 2.
IL-10 and LCMV NP localize to CD4+CD8α− DCs. VertX mice were infected with 2 × 106 pfu CL-13 and the DC compartment was analyzed at 0 2, 5, 9, and 12 dpi (n = 3 per time point). (A) The frequency (Left) and population size (Right) of CD8α− vs. CD8α+ DCs within the spleen during CL-13 infection. (B and C) IL-10 expression in DC subsets. (B) Representative flow plots show IL-10 and CD8α expression at 9 dpi. (C) The frequency of IL-10 expression in CD8α− vs. CD8α+ DCs during the first 12 d of infection. (D and E) Coexpression of IL-10 and LCMV NP in CD8α− vs. CD8α+ DCs. (D) Representative flow plots depict IL-10 and LCMV NP expression. (E) Frequency of IL-10 and LCMV NP coexpression in CD8α− vs. CD8α+ DCs. (F) Frequency of LCMV NP in IL-10− vs. IL-10+ CD8α− DCs at 9 dpi. (G) Infectious virus in sorted IL-10+ and IL-10− DCs from 9 dpi was evaluated by infectious center assay. (H) Flow plots portray expression of CD4 and CD8α on the total DC population or on IL-10+ DCs at 9 dpi. (I) Histograms contrast staining for CCR7 expression in IL-10− and IL-10+ CD8α− DCs in infected mice in comparison with DCs from naive mice. (J) The bar graph summarizes the mean fluorescent intensity of CCR7 staining at the same time points. *P < 0.01; **P < 0.001; ***P < 0.0001. All values are graphed as mean ± SD and all data are representative of three independent experiments.
The homing of DCs to and within lymphoid tissue is controlled by the chemokine receptor CCR7 (18), which spatially coordinates the interaction of DCs and lymphocytes (19). To assess whether IL-10+ DCs remained in the spleen, we assessed cell-surface expression of CCR7. Intriguingly, IL-10+ CD8α− DCs expressed significantly higher levels of CCR7 than IL-10− CD8α− DCs that resembled naive CD8α− DCs (Fig. 2 I and J). Because CCR7 ligands are expressed mainly in the T-cell zones, CCR7 expression suggests that these virally infected IL-10+CD8α− DCs remain within these high-traffic areas where they can influence the immune response.
DCs Are an Important Source of IL-10 During CL-13 Infection.
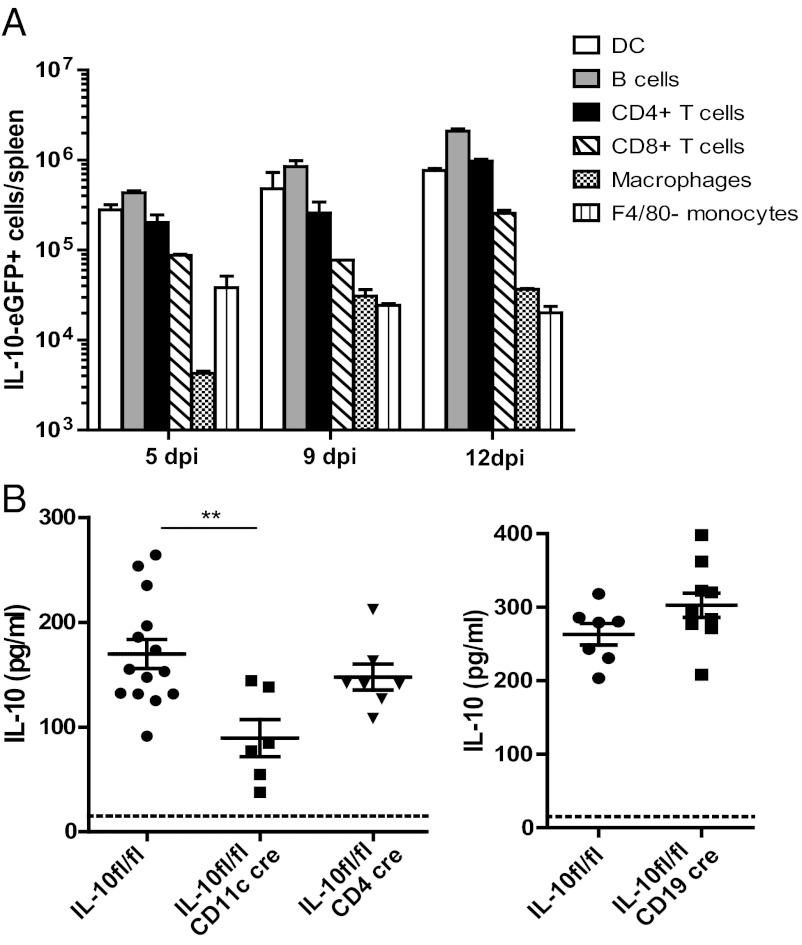
Serum IL-10 levels exhibit a short spike at 1 dpi, increase after 5 dpi, and plateau around 10 dpi, where they remain until viral titers decrease (20). Although the DC population contains the highest proportion of IL-10–producing cells, the number of IL-10+ DCs in the spleen is less than that of IL-10+ B cells and only slightly greater than that of IL-10+ CD4+ T cells at 5 and 9 dpi (Fig. 3A). To examine which cell types are responsible for the high IL-10 levels during establishment of persistence, we used il10 conditional knockout mice. Mice harboring a floxed IL-10 gene (IL-10fl/fl) were crossed to mice expressing Cre under the CD11c (15), CD19 (21), or CD4 (22) promoter to delete il10 from DCs, B cells, or T cells, respectively (23). At 10 dpi, we observed significant decreases in serum IL-10 levels in IL-10fl/fl CD11c cre but not in IL-10fl/fl CD19 cre or IL-10fl/fl CD4 cre mice (Fig. 3B). IL-10 production in B and CD4+ T cells in IL-10fl/fl CD11c cre mice did not differ from that in controls (Fig. S2), indicating that IL-10 production in those cells is independent of production by DCs. Thus, DCs produce a substantial amount of IL-10, and loss of IL-10 from this compartment cannot be compensated by T- or B-cell populations.
Fig. 3.
Cell-specific deletion of IL-10. (A) Analysis of the number of IL-10+ cells per spleen by lymphocyte population. (B) Serum IL-10 concentration (pg/mL) was determined by ELISA in il10 conditional knockout mice. Significant reduction of IL-10 levels occurred only in mice with il10 deleted from the DC population. **P = 0.002. Data are representative of at least two independent experiments.
IL-10 Does Not Affect DC Expression of T-Cell Stimulatory Ligands.
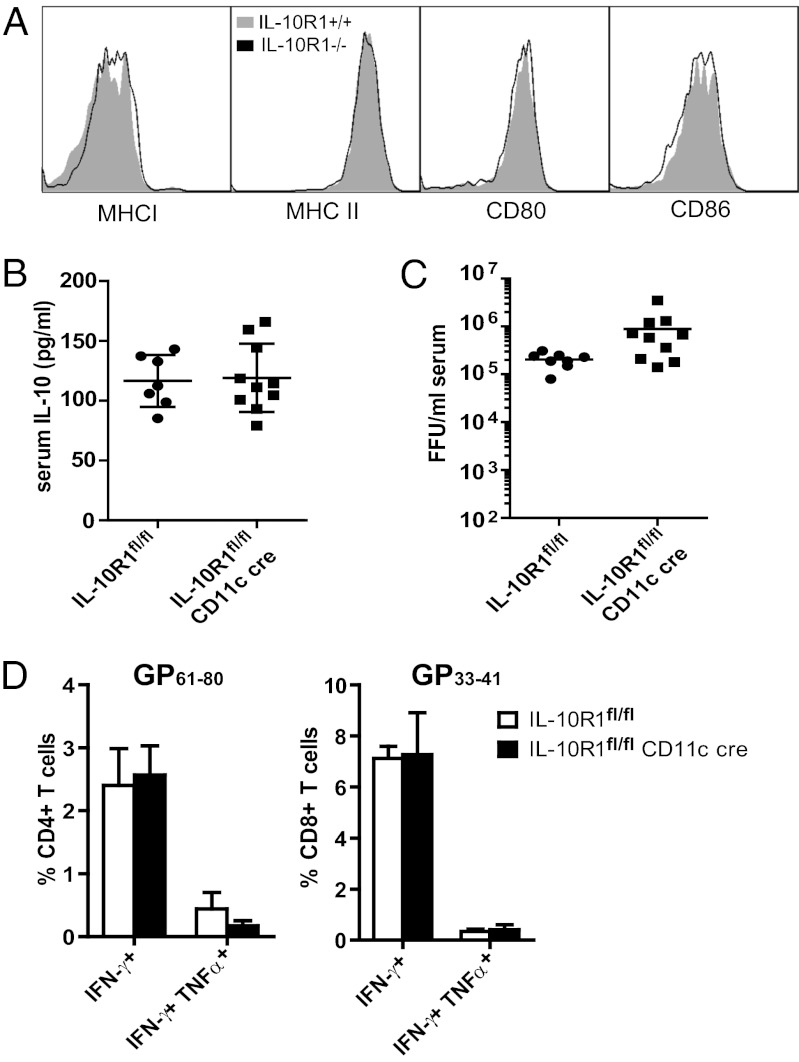
IL-10 signaling is known to inhibit expression of MHC and costimulatory molecules (12, 13), and during CL-13 infection, DCs display a reduced capacity to present antigen due to reduced expression of these same molecules (14, 24). Therefore, we investigated whether IL-10 altered DC interactions with T cells during CL-13 infection. IL-10+ DCs have higher levels of cell-surface IL-10R (Fig. S3), which suggested that IL-10 may be regulating DC function in an autocrine manner. To assess whether IL-10 directly represses DC function, we compared wild-type DCs to DCs in which the gene for the binding portion of the IL-10R (IL-10R1) was deleted. We generated and infected mixed bone marrow chimeras in which irradiated IL-10R1+/+ mice (Ly5.1) were reconstituted with both IL-10R1+/+ and IL-10R1−/− bone marrow to compare both cell types under the same conditions. At 10 dpi, IL-10R1−/− DCs did not differ from IL-10R+/+ DCs in the expression of MHC I and MHC II or costimulatory molecules CD80 and CD86 (Fig. 4A), indicating that expression of molecules necessary for antigen stimulation is unaffected by IL-10 signaling.
Fig. 4.
Deletion of IL-10R in DCs does not alter T-cell responses. (A) Representative histograms show MHC I, MHC II, CD80, and CD86 expression on IL-10R1−/− and WT CD8α− DCs from mixed bone marrow chimeric mice (n = 4) 10 d after infection. (B–D) At 10 dpi, IL-10R1fl/fl CD11c cre and control IL-10R1fl/fl mice were assessed for IL-10 concentration in the serum (pg/mL) as quantified by ELISA (B) and serum viral titers [focus-forming units (FFU)/mL] (C). Cytokine production (D) was evaluated for CD4+ T cells (Left) or CD8+ T cells (Right) after ex vivo stimulation with the indicated peptide. The bar graphs indicate the mean ± SD of at least five mice per group. Data are representative of two independent experiments.
To test whether IL-10 signaling on DCs affected T-cell responses in a biologically relevant manner, we tested T-cell responses in mice with a DC-specific deletion of IL-10R1. IL-10R1 conditional knockout mice (25) expressing cre under the control of the CD11c promoter (IL-10R1fl/fl CD11c cre), resulting in IL-10R1 deletion on DCs, were infected with CL-13. At 10 dpi, IL-10 levels in IL-10R1fl/fl CD11c cre mice were similar to those in IL-10R1fl/fl controls (Fig. 4B). However, DCs in IL-10R1fl/fl CD11c cre mice failed to generate improved LCMV-specific CD4+ or CD8+ T-cell responses (Fig. 4D). There were no significant differences in the frequency of IFN-γ+ or IFN-γ+ TNF-α+ CD4+ T cells or CD8+ T cells after stimulation with the respective immunodominant CD4 epitope (GP61–88) or CD8 epitope (GP33–41). Serum viral titers were marginally higher than in control mice (Fig. 4C), indicating no increase in viral control in the absence of IL-10 signaling on DCs. Therefore, removal of IL-10 signaling on DCs does not improve the ability of DCs to support T-cell responses in a biologically significant manner.
Type I IFN but Not CL-13 Infection Induces IL-10.
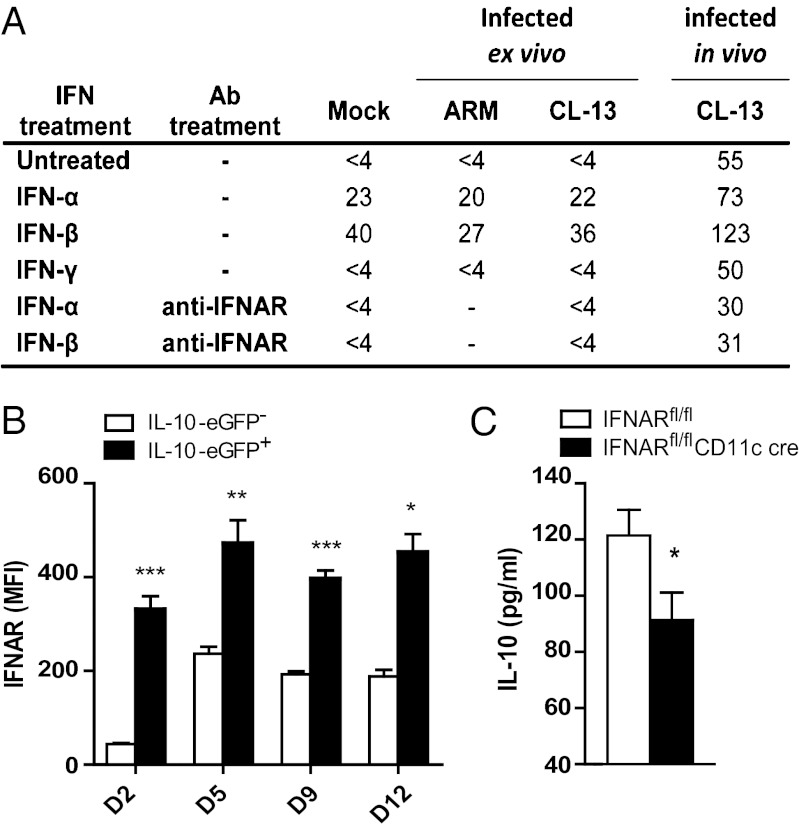
The infected status of IL-10+ DCs suggested the possibility that CL-13 induces cellular production of IL-10 as a strategy to evade clearance. To test this idea, we isolated splenic DCs from naive C57BL/6 mice and infected them ex vivo. DCs infected with LCMV Armstrong 53b (causes acute infection in vivo) or CL-13 at a multiplicity of infection (MOI) of 4 did not secrete detectable levels of IL-10 by 24 h postinfection (Fig. 5A). Comparatively, DCs isolated from CL-13–infected mice secreted high levels of IL-10. When we exposed the splenic DCs to type I IFN but not to type II IFN, DCs produced IL-10 in the presence or absence of virus. Both IFN-α and IFN-β elicited IL-10, with higher levels elicited by IFN-β. When the type I IFN receptor (IFNAR) was blocked using a monoclonal anti-IFNAR antibody, IL-10 was no longer detectable from naive or ex vivo DCs treated with type I IFN and was reduced in DCs from CL-13–infected mice, suggesting that type I IFN is involved in stimulating IL-10 production in DCs. When we compared the expression of the type I IFN receptor on IL-10+ vs. IL-10− CD8α− DCs, the IL-10+ cells expressed higher levels of the receptor (Fig. 5B), suggesting that these IL-10+ cells are more sensitive to IFNAR signaling. These results were complemented by experiments in IFNAR conditional knockout mice expressing cre under the control of the CD11c promoter (IFNARfl/fl CD11c cre). These mice displayed significantly lower levels of serum IL-10 at 9 dpi than control mice (Fig. 5C). Thus, LCMV infection of DCs alone is not sufficient to solicit IL-10, and other factors, particularly type I IFN, are necessary to trigger IL-10.
Fig. 5.
Expression of the type I IFN receptor is linked to IL-10 expression. (A) IL-10 concentration (pg/mL) in DC supernatant 24 h after infection with LCMV and/or incubation with type I IFN. DCs are from spleens of naive mice infected at an MOI of 4, after which IFN or antibody was added or DCs were isolated from spleens of mice infected with 2 × 106 pfu CL-13 at 9 dpi. Data are representative of at least two independent experiments. (B) Cell-surface expression of IFNAR was analyzed on IL-10+ and IL-10− CD8α− DCs in VertX mice infected with 2 × 106 pfu CL-13 (n = 3 per time point). Graph depicts mean fluorescent intensity of IFNAR staining at the indicated days postinfection. (C) IFNARfl/fl CD11c cre mice were infected with 2 × 106 pfu CL-13 (n = 4 mice per group) and serum IL-10 concentration (pg/mL) was measured at 9 dpi by ELISA. *P < 0.05; **P < 0.001; ***P < 0.0001.
Discussion
Here, we characterize the main cellular source of IL-10 production during persistent CL-13 infection. Investigating several cell types known to make IL-10 during the initiation of IL-10–mediated T-cell exhaustion, we made several unique observations. First, IL-10–producing DCs are detectable at the essential time points during the acute phase of CL-13 infection up through the time that T-cell exhaustion is initiated. IL-10+ DCs increase in frequency shortly after infection, peak soon after the establishment of persistence and T-cell exhaustion, and then moderately decline. In contrast, IL-10+ B cells, T cells, and macrophages were present only at low frequencies, with B and CD4+ T-cell expression of IL-10 modestly increasing only after 9 dpi. F4/80− monocytes exhibited brief IL-10 production only at 2 dpi. The timing of the kinetics of IL-10+ DCs strongly suggests that DCs are involved in establishing IL-10–mediated persistence. Within the DC population, we find that IL-10 production is restricted to CD8α− DCs, predominantly the CD4+ subtype, and these cells express significantly higher levels of CCR7 than their IL-10− counterparts. Because CCR7 is necessary for migration of DCs to T-cell areas in secondary lymphoid tissue (18), CCR7 expression on IL-10+ DCs increases the likelihood that these cells remain in T-cell zones where they can exert influence on both resident and migrating cells.
Our observation that macrophages do not express IL-10 conflicts with a recent publication by Wilson et al. (26) in which IL-10 was observed in a high percentage of macrophages. Although the same strain of mice was used to detect IL-10, our study differs in the markers used to identify macrophages (CD11b+Ly6c+Ly6g−F4/80+ vs. F4/80+) and in the site of i.v. infection (tail vs. orbital). Wilson et al. did not specify the age of their mice, however, this and other factors may be different as well. In our analysis, we observed no increase in IL-10–eGFP fluorescence in macrophages from infected mice over those from naive and non-GFP mice (Fig. 1 and Fig. S1). It is worth noting that mature macrophages have high autofluorescence that may mask the eGFP signal in this population.
Our data show that an identifying characteristic of IL-10 production in DCs is the presence of LCMV NP, signifying that infection is predominantly limited to IL-10+ cells. Although our results ascertain that CD8α−CD4+ DCs are relevant to viral infection and IL-10 production, little is known about the specific role of this DC subset. CD8α− CD4+ DCs can secrete IL-10 (27), potentially identifying them as having a more inhibitory role. More broadly, a recognized difference between CD8α− and CD8α+ DCs is their ability to cross-present antigens. CD8α− DCs are less able to take up and present exogenous antigen (11) than CD8α+ DCs, which would presumably make this former subset a more likely viral target. However, our data indicate the opposite case with minimal infection in CD8α+ DCs. A more likely explanation is the differential expression of toll-like receptor 7 (TLR7), which senses single-stranded RNA. TLR7 is expressed on CD8α− but not CD8α+ DCs (28), and activation of TLR7 is necessary for the eventual clearance of CL-13 infection (29). Consequently, infection of CD8α− DCs may be a viral strategy to interfere with the cascade of events that stem from TLR7 signaling that favor the host by leading to viral clearance.
The association of LCMV NP and IL-10 production in CD8α− DCs suggested the possibility that CL-13 directly induced IL-10 expression, yet IL-10 could not be detected in splenic DC cultures infected with either CL-13 or ARM ex vivo. However, when DC cultures were treated with type I IFN, they produced detectable levels of IL-10, regardless of the presence or absence of virus. Similarly, DCs from CL-13–infected mice increased their IL-10 production when exposed to type I IFN, whereas blockade of the IFN receptor reduced IL-10 production beyond that of untreated DCs, suggesting the involvement of type I IFN in IL-10 expression. This idea was supported by the decrease in serum IL-10 levels observed in conditional knockout mice in which IFNAR was deleted from the DC compartment. In nonviral systems, type I IFN can instigate IL-10 production in various cell types (30, 31), including murine DCs (32), likely through the common use of the transcription factor Stat3 in both type I IFN and IL-10 signaling pathways (33, 34). Throughout CL-13 infection, DCs are exposed to type I IFN as its gene expression is present in the spleen throughout infection (35). However, why would infected DCs make IL-10 in a type I IFN-dependent manner when all DCs would be exposed to IFN? One possible explanation is that infected DCs are more sensitive to type I IFN, as IL-10+ CD8α− DCs (predominantly infected) exhibited higher expression levels of IFNAR than their IL-10− counterparts. Other factors beyond type I IFN signaling are likely necessary to generate high levels of IL-10 production because type I IFN-elicited IL-10 levels in vitro were low compared with those in DCs from CL-13 infected mice.
When we examined the contribution of each cell type to IL-10 levels using an il10 conditional knockout system, IL-10 decreased significantly only when the gene was deleted from CD11c+ cells. This result indicates that DCs are an important source of IL-10 early in establishment of persistence and T-cell exhaustion whereas B and T cells are not. The decrease in serum IL-10 in IL-10fl/fl CD11c cre mice was likely not due to a concomitant decrease in production by B cells and CD4+ T cells as the prevalence of IL-10+ cells in either population was unaltered. Although most IL-10+ DCs express CD4, the CD4+ DC population (∼25% of the DCs) in IL-10fl/fl CD4 cre mice does not appear to be affected (23), likely due to lower expression levels of CD4. The high levels of IL-10 in IL-10fl/fl CD4 cre mice at 10 dpi indicate that T cells are not an important source of IL-10 during early CL-13 infection and contradict a previous report that concluded that DCs polarize CD4+ T cells to produce IL-10 and in turn these CD4+ T cells amplify the IL-10 cascade (4). The most likely explanation for the difference between results is our use of an in vivo assay vs. their use of an in vitro assay. Whereas our results indicate that IL-10–secreting T and B cells play a minimal role in establishment of persistent infection, the number of IL-10+ cells in these subsets increased after initiation of exhaustion at 12 dpi, suggesting that non-DC cells could play a role in maintenance, rather than establishment, of T-cell exhaustion.
Finally, we note that IL-10 signaling on DCs does not affect their antigen-presenting capacity. Although IL-10 signaling can inhibit cell-surface expression of T-cell stimulatory molecules and DCs exhibit such a decrease during CL-13 infection, our data reveal that IL-10 is not responsible for this phenomenon during CL-13 infection. Deletion of IL-10 signaling does not alter DC expression of MHC I, MHC II, CD80, or CD86, signifying that IL-10 does not overtly influence DC function during CL-13 infection. This result was supported by the lack of improved T-cell responses in mice with DCs deficient in IL-10R1. Furthermore, these mice displayed slightly higher viral titers, indicating that removal of IL-10 signaling on DCs does not improve viral control. Although IL-10 signaling on other cells may mask improvement of DC function, this explanation still supports the conclusion that IL-10 signaling on DCs is nonvital in this system. More importantly, these data suggest that IL-10 is being produced by DCs not for autocrine signaling or suppressing other DCs but rather to target another cell type.
In summary, IL-10 is an important factor in T-cell exhaustion and viral persistence that provides a potential target for resurrecting T-cell responses. Our data show that virally infected DCs are a biologically important source of IL-10 in this system, further elucidating the mechanism of this cytokine in the establishment of viral persistence.
Materials and Methods
Mice and Virus.
C57BL/6 mice were obtained from the Rodent Breeding Colony at The Scripps Research Institute. The IL-10–eGFP reporter (VertX) mice (15) were kindly provided by Christopher Karp (University of Cincinnati, Cincinnati). IL-10fl/fl CD4 cre and IL-10fl/fl CD19 cre mice (15, 23) were generously provided by Axel Roers (Technical University of Dresden, Dresden, Germany) and IL-10fl/fl CD11c cre mice were generated by breeding B6.Cg-Tg(Itgax-cre)1-1Reiz/J mice (The Jackson Laboratories) onto the IL-10fl/fl background. Similarly, IL-10R1fl/fl CD11c cre mice were generated by breeding B6.Cg-Tg(Itgax-cre)1-1Reiz/J mice (The Jackson Laboratories) onto the IL-10R1fl/fbackground (25) [provided by Werner Müller (University of Manchester, Manchester, UK)]. IFNARfl/fl CD11c cre mice (36) were kindly provided by Robert Schreiber (Washington University, St. Louis). Mice were genotyped as described in refs. 15, 23, 25, and 36.
All mice were housed at The Scripps Research Institute under specific pathogen-free conditions. Mouse handling conformed to requirements of the National Institutes of Health and The Scripps Research Institute Animal Research Committee. Mice (6–8 wk old) were infected i.v. (tail vein) with 2 × 106 pfu LCMV CL-13. Viral stocks were prepared and titered as previously described (37).
IL-10 ELISA.
IL-10 was measured using the Quantikine IL-10 ELISA kit (R&D Systems) according to manufacturer’s instructions after overnight incubation of the sample at 4 °C.
Flow Cytometry and Intracellular Cytokine Analysis.
To analyze IL-10 production, VertX mice were infected with CL-13 on staggered days to harvest spleens from all time points on the same day. Spleens were harvested, digested for 30 min with Collagenase D (1 mg/mL) and Dnase I (100 μg/mL) in RPMI with 5% (vol/vol) FBS, and processed into single-cell suspension through a 70-μm cell strainer (BD Biosciences). Erythrocytes were lysed using 0.83% ammonium chloride. Splenocytes were stained with subset-specific antibodies for DCs (CD45+CD3−CD19−NK1.1−CD11chi), CD4+ T cells (CD45+CD19−NK1.1−CD3+CD4+CD8−), CD8+ T cells (CD45+CD19−NK1.1−CD3+CD4−CD8+), B cells (CD45+CD3−NK1.1−CD11c−CD19+), macrophages (CD3−CD19−NK1.1−CD11b+Ly6C+Ly6G−F4/80+), and monocytes (CD3−CD19−NK1.1−CD11b+Ly6C+Ly6G−F4/80−) and other cell surface markers including CCR7 and IFNAR. For detection of LCMV antigen, cells were fixed, permeabilized, and stained with Alexa Fluor 647-conjugated anti-LCMV NP monoclonal antibody (NP113) (24). Splenocytes from naive mice and non-GFP C57BL/6 mice (VertX experiments) were used as gating controls.
To assess T-cell responses, splenocytes were stimulated for 5 h with 5 μg/mL of MHC class II-restricted LCMV GP61–80 or 2 μg/mL of MHC class I-restricted LCMV NP396–404 or GP33–41 peptide in the presence of 50 units/mL recombinant murine IL-2 (R&D Systems) and 5 μg/mL brefeldin A (Sigma). Cells were stained for surface expression of CD3, CD4, and CD8. Cells were fixed, permeabilized, and stained with antibodies to TNF-α, IFN-γ, and IL-2 (eBioscience). To enumerate IL-10–producing cells in IL10fl/fl CD11c cre mice, splenocytes were incubated with brefeldin A before staining for extracellular markers and intracellular IL-10. All cell analysis was performed on an LSR II flow cytometer (Becton Dickinson).
Infectious Center Assay.
Spleens were harvested from VertX mice at 9 dpi, processed and stained as above, and sorted by IL-10–eGFP expression on a FACSAria cell sorter (Becton Dickinson). Purity after sorting was >95%. Sorted cells were titered in an infectious center assay as previously described (38).
Bone Marrow Chimeric Mice.
Bone marrow was isolated from the femur, tibia, and humerus bones of IL-10R1−/− and B6.SJL-Ptprca Pep3b/BoyJ (Ly5.1) mice, T cells were removed by magnetic separation (Easysep CD90.2 positive selection kit; Stemcell), and the remaining cells were mixed in a 1:1 ratio. Recipient mice were irradiated (1,000 rads), given 5 × 106 bone marrow cells intravenously, and kept on antibiotic feed for 4 wk. After 6 wk, IL-10R1−/− and Ly5.1 populations had reconstituted at fairly equivalent levels. All mice were infected with LCMV CL-13 at 7–8 wk after irradiation. Anti-LCMV T-cell responses were analyzed at 10 dpi as described above.
In Vitro Infection of DCs.
C57BL/6 mice were injected with 10 μg of plasmid encoding the secreted portion of human Fms-like tyrosine kinase receptor-3 ligand (pUMVC3-hFLex; kindly provided by B. Nayak, The Novartis Foundation, La Jolla, CA) to expand the DC population in vivo (39). One week after injection, splenocytes were harvested as described above (without erythrocyte lysis). For DCs from infected mice, spleens were collected from mice infected with CL-13 9 d prior (not treated with pUMVC3-hFLex). DCs were magnetically isolated (Easysep pan-DC negative enrichment kit; Stemcell) and infected at an MOI of 4 for 1 h at 37 °C. Subsequently, DCs were plated at 1 × 106 cells per well in 125 μL of media (RPMI, 10% FBS, 1% l-glutamine, 1% penicillin/streptomycin, Hepes, nonessential amino acids, sodium pyruvate, and 55 μM 2-mercaptoethanol). Type I or type II IFN was added at 100 units/mL. Supernatant was collected at 24 hpi to test for IL-10 by ELISA.
Statistical Analysis.
Student’s t tests were performed using Graphpad Prism software.
Supplementary Material
Acknowledgments
We thank Axel Roers for IL-10 floxed mice, Werner Müller for IL-10R1–deficient and floxed mice, Christopher Karp for the IL-10–eGFP (VertX) mice, and Robert Schreiber for IFNAR floxed mice. We also thank Brian Sullivan for manuscript editing and technical assistance. This work was supported by funding from the National Institutes of Health (AI019484 and T32 HL007195). This is manuscript no. 21794 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211910109/-/DCSupplemental.
References
- 1.Khanolkar A, Fuller MJ, Zajac AJ. T cell responses to viral infections: Lessons from lymphocytic choriomeningitis virus. Immunol Res. 2002;26:309–321. doi: 10.1385/IR:26:1-3:309. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockman MA, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkaya H, et al. Circulating IL-2, IL-10 and TNF-alpha in chronic hepatitis B: Their relations to HBeAg status and the activity of liver disease. Hepatogastroenterology. 2000;47:1675–1679. [PubMed] [Google Scholar]
- 8.Reiser M, et al. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol. 1997;26:471–478. doi: 10.1016/s0168-8278(97)80409-6. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 11.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 12.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 13.Chan A, Baird M, Mercer AA, Fleming SB. Maturation and function of human dendritic cells are inhibited by orf virus-encoded interleukin-10. J Gen Virol. 2006;87:3177–3181. doi: 10.1099/vir.0.82238-0. [DOI] [PubMed] [Google Scholar]
- 14.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-López R, et al. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez-Sánchez N, Riol-Blanco L, Rodríguez-Fernández JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176:5153–5159. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 19.Förster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci USA. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 23.Roers A, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevilla N, et al. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pils MC, et al. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol. 2010;40:443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- 26.Wilson EB, et al. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2012;11:481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legge KL, et al. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards AD, et al. Toll-like receptor expression in murine DC subsets: Lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 29.Walsh KB, et al. Toll-like receptor 7 is required for effective adaptive immune responses that prevent persistent virus infection. Cell Host Microbe. 2012;11:643–653. doi: 10.1016/j.chom.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: Involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Yuan S, Cheng G, Guo B. Type I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammation. PLoS ONE. 2011;6:e28432. doi: 10.1371/journal.pone.0028432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarasinghe R, et al. Induction of an anti-inflammatory cytokine, IL-10, in dendritic cells after toll-like receptor signaling. J Interferon Cytokine Res. 2006;26(12):893–900. doi: 10.1089/jir.2006.26.893. [DOI] [PubMed] [Google Scholar]
- 33.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler-Heitbrock L, et al. IFN-alpha induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J Immunol. 2003;171:285–290. doi: 10.4049/jimmunol.171.1.285. [DOI] [PubMed] [Google Scholar]
- 35.Hahm B, Trifilo MJ, Zuniga EI, Oldstone MB. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed R, King CC, Oldstone MB. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987;61:1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nayak BP, Sailaja G, Jabbar AM. Augmenting the immunogenicity of DNA vaccines: Role of plasmid-encoded Flt-3 ligand, as a molecular adjuvant in genetic vaccination. Virology. 2006;348:277–288. doi: 10.1016/j.virol.2006.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.