Abstract
Reestablishing self-tolerance in autoimmunity is thought to depend on self-reactive regulatory T cells (Tregs). Exploiting these antigen-specific regulators is hampered by the obscure nature of disease-relevant autoantigens. We have uncovered potent disease-suppressive Tregs recognizing Heat Shock Protein (Hsp) 70 self-antigens, enabling selective activity in inflamed tissues. Hsp70 is a major contributor to the MHC class II ligandome. Here we show that a conserved Hsp70 epitope (B29) is present in murine MHC class II and that upon transfer, B29-induced CD4+CD25+Foxp3+ T cells suppress established proteoglycan-induced arthritis in mice. These self-antigen–specific Tregs were activated in vivo, and when using Lymphocyte Activation Gene-3 as a selection marker, as few as 4,000 cells sufficed. Furthermore, depletion of transferred Tregs abrogated disease suppression. Transferred cells exhibited a stable phenotype and were found in joints and draining lymph nodes up to 2 mo after transfer. Given that (i) B29 administration by itself suppressed disease, (ii) our findings were made with wild-type (T-cell receptor nontransgenic) Tregs, and (iii) the B29 human homolog is presented by HLA class II, we are nearing translation of antigen-specific Treg activation as a promising intervention for chronic inflammatory diseases.
Regulatory T cells (Tregs) are a subset of specialized CD4+ helper T cells defined by the expression of the IL-2 receptor α-chain (CD25) (1) and the transcription factor FoxP3 (2). CD4+CD25+FoxP3+ Tregs have the capacity to down-regulate immune responses and are essential for immune homeostasis. Mechanisms by which they regulate include the secretion of anti-inflammatory cytokines such as IL-10 and -35 and the inactivation of antigen-presenting cells through neuropillin-1 (Nrp-1) and Lymphocyte Activation Gene-3 (LAG-3) (3, 4). Although polyclonally expanded populations of Tregs exhibit suppressive activity (5), antigen-specific Tregs appear superior in suppressing autoimmune diseases (6, 7). In addition, the antigen-specific targeting of Tregs may avoid induction of a potentially detrimental systemic immunosuppression. Ideally, effective Treg-mediated suppression would be generated by continuous recognition of ubiquitous self-antigens (8, 9), preferentially expressed during inflammation (10–12). For autoimmune diseases like rheumatoid arthritis (RA), it is unknown which antigens are critical for disease. Interestingly, Heat Shock Proteins (Hsps) are relatively abundantly expressed in tissues such as the inflamed synovium (13–15). We and others have shown that immunization with mycobacterial (Mt) Hsp70 can induce an Hsp-specific T-cell response with anti-inflammatory activity (16–18).
Therefore, we set out to examine the capacity of Hsp70 to serve as a target for antigen-specific Tregs. In this work, we show that immunization or intranasal administration of the Hsp70 epitope B29 induces LAG-3–expressing antigen-specific Tregs that can directly suppress experimental arthritis.
Results
Identification of the Hsp70 Epitope B29.
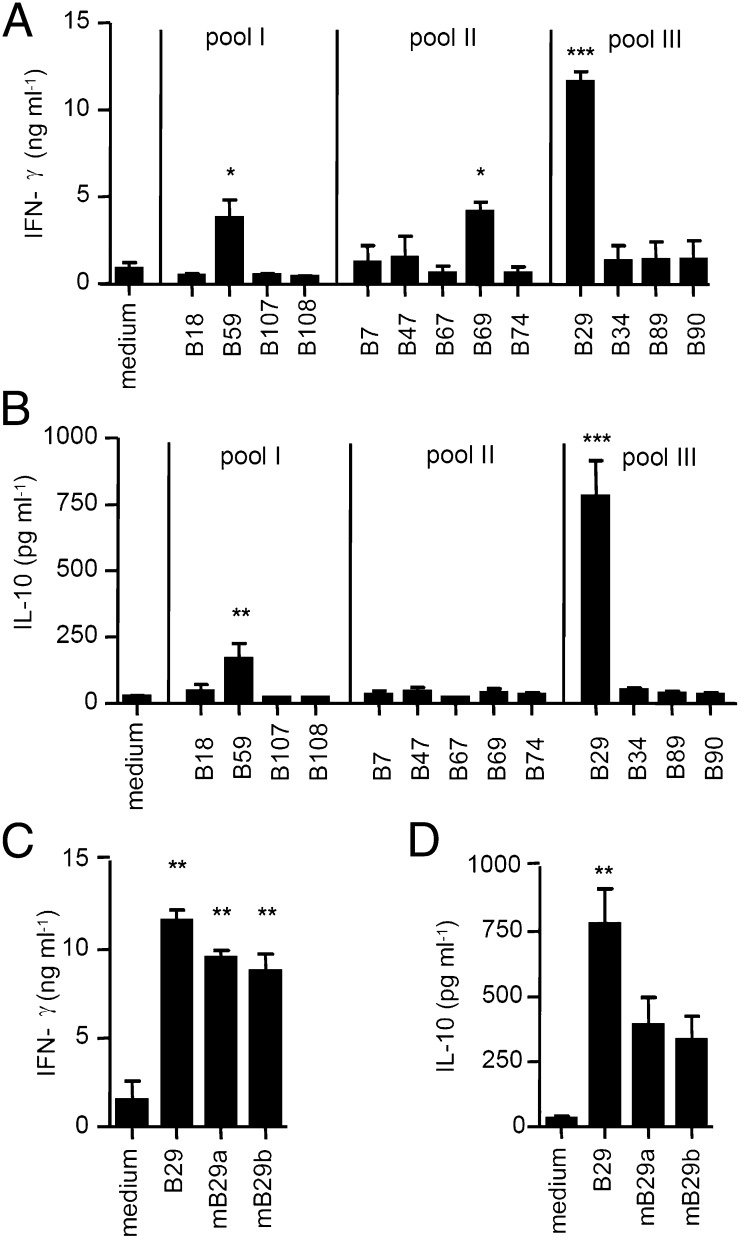
First, we identified Hsp70 T-cell epitopes using Mt Hsp70-immunized BALB/c mice and clustered them according to sequence conservation (Table S1). In subsequent immunizations with pooled Hsp70 T-cell epitopes, we observed a strong response against peptide B29 (Fig. 1 A and B). Cross-reactivity between bacterial- and self-Hsp is proposed to be critical for the regulatory potential of Hsp70 (19). Notably, the sequence of B29 shows strong homology with at least two human/mouse Hsp70 family members (Table S2). B29-induced T cells responded to mB29a or mB29b after in vitro restimulation (Fig. 1 C and D), indicating cross-recognition of mammalian homologs.
Fig. 1.
B29 is a dominant T-cell epitope of Hsp70. Pools containing four or five epitope-peptides were made based on homology between mammalian and bacterial Hsp70 (Table S1). Mice were immunized with one of the pools, and splenocytes were restimulated ex vivo with Hsp70 peptides individually. (A and B) IFN-γ (A) and IL-10 (B) production of spleen cells measured in supernatant after restimulation with medium or bacterial–Hsp70 peptides. Data show the average cytokine production (±SEM) after restimulation with peptides that were present in the immunization pool (n = 3 mice per immunization group). (C and D) IFN-γ (C) and IL-10 (D) production by splenocytes from mice immunized with pool III after restimulation with B29 or mouse homolog peptides mB29a and mB29b. Indicated is the mean cytokine production (±SEM) of n = 3 mice. P values are from an unpaired two-tailed Student t test in which Hsp70 peptides were compared with medium and are representative of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Previous studies have shown that Hsp70-derived peptides are abundantly present in MHC class II molecules (20–22) (Table S3), and length variants of the B29 homologs have been identified frequently in the peptide binding grooves of HLA-DRB*0401 molecules (21, 23–25). In MHC peptide-elution studies, we also found that in BALB/c mice, homologs of B29 are presented with relative abundance in MHC class II molecules of bone marrow (BM)-derived dendritic cells (DCs) (Table S4), indicating that these peptides are naturally presented by MHC class II in our model, thereby allowing for cross-recognition by B29-induced T cells.
B29-Induced Tregs Are Responsive to B29 and Homologs in Vitro.
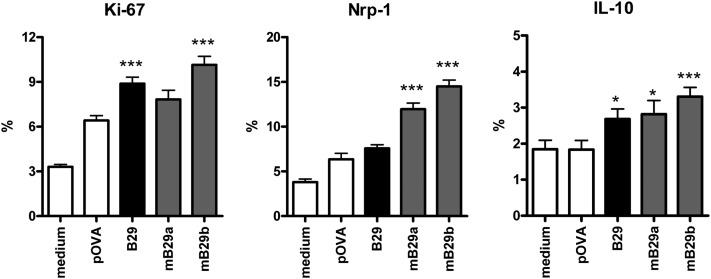
To investigate whether Tregs were included in the B29-induced T cells, we isolated CD4+CD25+ cells from B29 or ovalbumin peptide (pOVA)-immunized mice and analyzed their phenotype and regulatory properties. CD4+CD25+ T cells were suppressive in vitro in a standard suppression assay using anti-CD3 stimulation (Fig. S1A). Phenotypical analysis of CD4+CD25+FoxP3+ T cells from B29-immunized mice showed a slight increase in the percentage of IL-10, IL-35 (p35 subunit), and LAG-3 positive cells (Fig. S1B), which prompted us to use up-regulated Treg markers as a readout for ex vivo-activated B29-specific Tregs. CD4+CD25+FoxP3+ cells from B29-immunized mice showed increased expression of the activation marker Ki-67, as well as up-regulation of Nrp-1 and IL-10 after restimulation with B29 or homolog peptides mB29a and mB29b (Fig. 2). Interestingly, Nrp-1 expression was enhanced only after stimulation with mB29a or mB29b, indicating the differential effects on the induction of individual activation markers in response to self or bacterial epitopes. Tregs from pOVA-immunized mice specifically responded to pOVA, which resulted in increased expression of Ki-67 and Nrp-1 (Fig. S1C). The up-regulation of these markers has been associated with Treg function (3, 4, 11); therefore, these results demonstrate that CD4+CD25+FoxP3+ Tregs from B29-immunized mice can respond to B29 ex vivo and most importantly also to the mouse homologs mB29a and mB29b.
Fig. 2.
B29 immunization primes for a recall response to B29 and its homologs in CD4+CD25+FoxP3+ T cells. Splenocytes from B29-immunized mice were restimulated in the presence of pOVA (control peptide), B29, or homologous mB29a or mB29b peptides and stained for Ki-67 (Left), Nrp-1 (Center), and IL-10 (Right). Percentage of positive cells (±SEM) is shown within the CD4+CD25+FoxP3+ population. P values are from an unpaired two-tailed Student t test in which Hsp70 peptides were compared with pOVA. *P < 0.05; ***P < 0.001. Data are mean of 5–10 animals per group and are representative of two independent experiments.
Adoptive Transfer with B29-Induced Tregs Suppresses Arthritis.
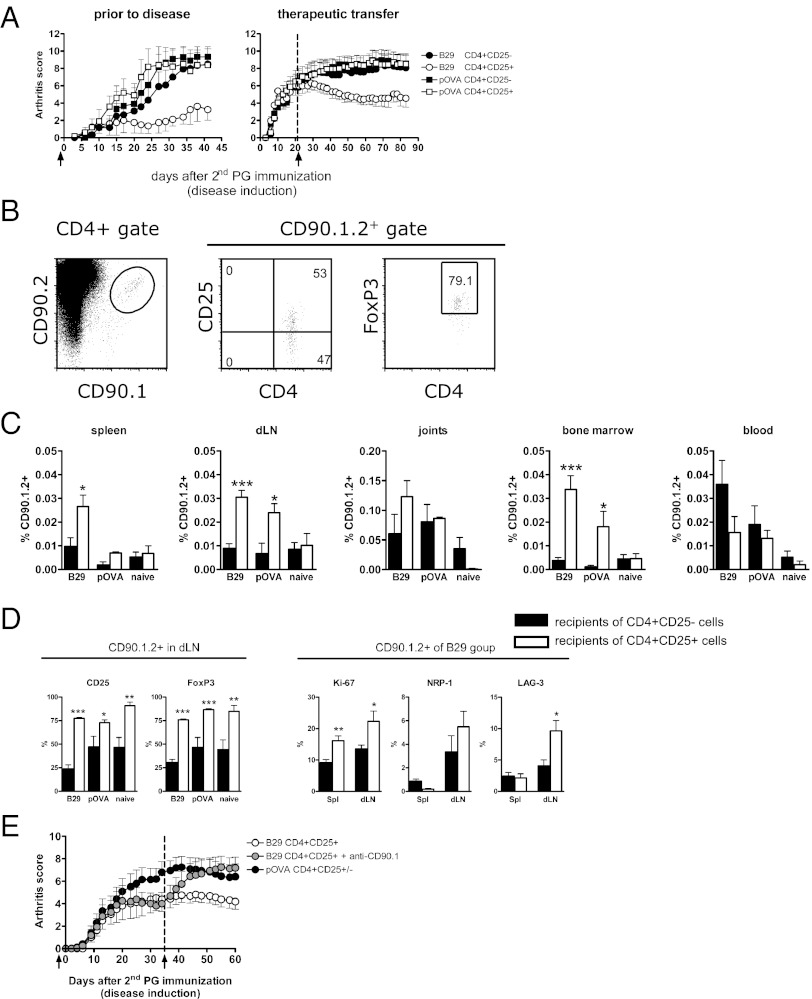
We next examined whether B29-induced cells were functionally activated in vivo after adoptive transfer. CD4+CD25+ Tregs from B29 or pOVA-immunized donor mice were isolated and injected into recipients with proteoglycan (PG)-induced arthritis (PGIA), an experimental mouse model for RA (26). Adoptive transfer with B29-specific Tregs suppressed disease both prophylactically and therapeutically (Fig. 3A), indicating an active suppressive function of the transferred Tregs in response to endogenously presented Hsp70 peptides. Tregs from pOVA-immunized donors were not suppressive, presumably due to the absence of their cognate antigen (ovalbumin) in vivo. It has been shown that in vitro preactivation of polyclonal Tregs was essential for suppressing experimental arthritis upon adoptive transfer (27). Notably, however, the transferred B29-specific Tregs were not in vitro preactivated, yet demonstrated very effective suppression in vivo. Because Tregs must be activated via their T-cell receptor (TCR) to become suppressive (7, 28), we may infer in vivo antigen recognition by the B29-specific Tregs. To examine whether i.v.-administered cells act locally at the site of inflammation and remain phenotypically stable over time, we transferred CD90.1.2+ congenic Tregs from B29-immunized, pOVA-immunized, or naïve donor mice into CD90.2+ recipients with PGIA. These injected cells were distinguished from host cells by flow cytometry (Fig. 3B) and were found in various organs at least up to 50 d after transfer (Fig. 3C). Compared with transferred CD25− T cells, the CD25+ B29-specific Tregs were relatively enriched in most tissues (although not significantly in the joints), whereas the opposite was found in the blood. Of all tissues examined, the joints contained the highest frequency of CD25+ B29-specific Tregs. Additionally, most transferred CD4+CD25+ Tregs remained CD25+ and FoxP3+ until the end of the experiment (Fig. 3D), irrespective of surviving cell numbers or donor mouse, with the highest survival potential seen with cells from B29-immunized donors (Fig. 3C). Moreover, the transferred CD90.1.2+ Tregs from B29-immunized donors revealed enhanced Ki-67, Nrp-1, and LAG-3 expression in the draining lymph nodes (dLN) of the joints (Fig. 3D), indicating local activation. These experiments demonstrate that transferred Tregs can migrate to sites of inflammation, and this Treg population remains present for up to 50 d while maintaining an activated phenotype. To further investigate whether the B29-specific Tregs suppressed disease by direct regulation or through induction of infectious tolerance (29), transferred CD90.1.2+ cells were depleted with anti-CD90.1 antibody. At day 35, when Treg-mediated suppression of disease was established, depletion of B29-specific Tregs abrogated disease suppression (Fig. 3E). Overall, these data reveal that Tregs from B29-immunized mice are potent suppressors of inflammation and are directly involved in disease suppression.
Fig. 3.
Adoptively transferred B29-induced Tregs are activated and long-lived in vivo, resulting in disease suppression. (A) Mean arthritis scores (±SEM) are depicted for recipients with PGIA receiving either CD4+CD25+ or CD4+CD25− cells from B29- or pOVA-immunized donors. (Left) Prophylactic treatment. One day before the second PG immunization mice received 3 × 105 cells i.v. (n = 4–6 per group). (Right) Therapeutic treatment. Three weeks after the second PG immunization 1 × 106 cells were transferred i.v. (arrow) (n = 9–10 per group). Data are representative of three to four independent experiments. (B) Flow cytometric staining for CD25 and FoxP3 of CD90.1.2+ cells within the splenic CD4+ T-cell population 50 d after prophylactic transfer. (C) Distribution of CD90.1.2+ cells within CD4+ cells from naïve, B29-, and pOVA-immunized donor mice 50 d after transfer in (from left) spleen, dLN (pooled axillary, brachial, and popliteal), joints, BM, and blood (n = 9–10 per group). (D) Characterization of CD90.1.2+ cells that were located in dLN of recipients that had received CD4+CD25− (filled bars) or CD4+CD25+ cells (open bars) from donors as described in C. (Left) The CD90.1.2+ cells were stained for CD25 and FoxP3. (Right) The CD90.1.2+ cells from B29-immunized donors present in spleen, and dLN of recipients were further characterized for their expression of Ki-67, Nrp-1, or LAG-3. In C and D, P values are from an unpaired two-tailed Student t test in which CD4+CD25− cells were compared with CD4+CD25+ cells. *P < 0.05; **P <0.01; ***P < 0.001. The data are representative of four independent experiments (n = 6–9 per group). (E) Five weeks after disease induction, animals from the B29 CD4+CD25+ group were randomized, and half were given anti-CD90.1 to deplete CD90.1.2+ cells. Mean arthritis scores (±SEM) are depicted. (n = 8–10 per group). The data shown are representative of two independent experiments.
Selection for FoxP3 or LAG-3 Defines B29-Induced Tregs.
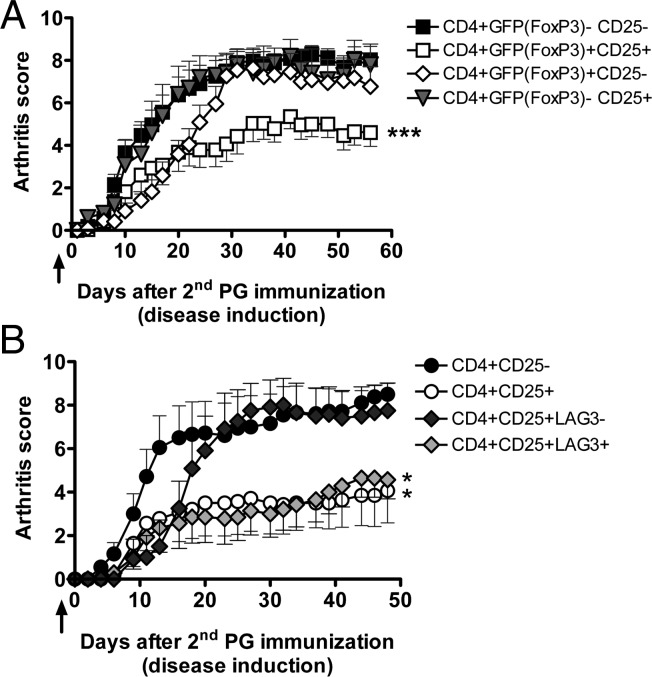
Although 90% of the transferred CD25+ cells expressed FoxP3 (Fig. S2), we cannot exclude that the B29-specific T cells were activated T cells without FoxP3 expression. To examine this possibility, we used a FoxP3–green fluorescent protein (GFP) reporter mouse, which allowed us to isolate CD4+CD25+FoxP3+ cells by the expression of CD25 and GFP (30). Only adoptive transfer of CD4+CD25+GFP+ cells from B29-immunized donors resulted in suppression of disease induction (Fig. 4A). As expected, the CD4+CD25+GFP+ T cells from pOVA-immunized mice were not capable of suppressing disease (Fig. S2). Thus, these data confirm that B29 immunization induces a population of CD4+CD25+FoxP3+ Tregs that suppress induction of experimental arthritis upon transfer. Because LAG-3 was slightly up-regulated on CD4+CD25+FoxP3+ Tregs after immunization with B29 (Fig. S1) and was enriched on CD90.1.2+ Tregs 50 d after transfer (Fig. 3D), we hypothesized that LAG-3 was expressed on B29-specific Tregs. To test this hypothesis, CD4+CD25+LAG-3+ or CD4+CD25+LAG-3− cells from B29-immunized donors were transferred to mice followed by disease induction. Animals receiving as few as 4,000 CD4+CD25+LAG-3+ Tregs had significantly lower arthritis scores than recipients of CD4+CD25− T cells (Fig. 4B). Interestingly, transferring 300,000 LAG-3− Tregs did not suppress disease. The suppression of disease obtained with transferring 4,000 LAG-3+ Tregs was similar to the suppression with 300,000 CD4+CD25+ Tregs not specifically sorted for LAG-3, indicating that the suppressive B29-specific Tregs are present in the LAG-3+ population.
Fig. 4.
FoxP3 or LAG-3 selected B29-induced Tregs are suppressive in low numbers. (A) Mean arthritis scores (±SEM) of recipients with PGIA after adoptive transfer. One day before the second PG immunization, animals received 3 × 105 CD4+ cells i.v. from B29-immunized FoxP3–GFP reporter donor mice (arrow). Donor CD4+ cells were selected on expression of CD25 and/or GFP (FoxP3). [CD4+GFP(FoxP3)−CD25−, n = 16; CD4+GFP(FoxP3)+CD25+, n = 14; CD4+GFP(FoxP3)+CD25−, n = 11; CD4+GFP(FoxP3)−CD25+, n = 5]. The data shown are combined from two independent experiments. (B) Acceptors received CD4+ cells selected on expression of CD25 and/or LAG-3 .i.v. from B29-immunized donor mice (arrow). Mean arthritis scores (±SEM) of recipients are depicted. P values are from a two-way ANOVA followed by Bonferroni post hoc comparison. *P < 0.05; ***P < 0.001. (3 × 105 CD4+CD25−, n = 9; 3 × 105 CD4+CD25+, n = 7; 3 × 105 CD4+CD25+LAG-3−, n = 6; 4 × 103 CD4+CD25+LAG-3+, n = 7).
Intranasal Administration of B29 Ameliorates Arthritis.
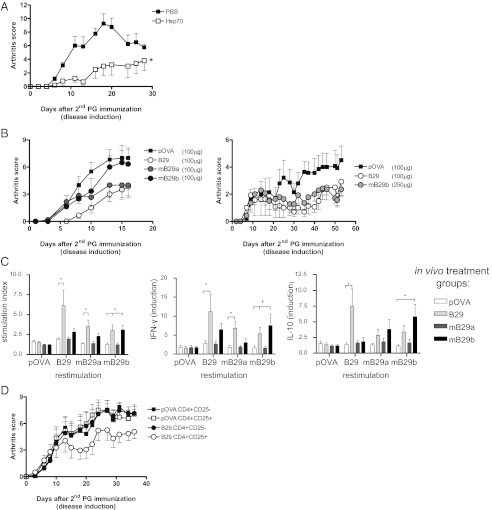
Finally, we wanted to test the direct immunosuppressive activity of the B29 peptide. We monitored disease development upon nasal administration (31, 32), also because of successful disease suppression using the whole Hsp70 protein administered intranasally (Fig. 5A). Nasal treatment with B29 or its homologs diminished disease after administration (Fig. 5B), although the mB29b homolog was only effective at higher concentrations. At the end of the experiment, restimulated splenocytes taken from treated animals showed peptide-specific and cross-reactive T-cell responses as demonstrated by proliferation and cytokine production (Fig. 5C), indicating a continued presence of B29-specific T cells. To confirm that intranasally administered B29 induced disease-suppressing Tregs, we transferred CD4+CD25+ Tregs from B29-treated mice into arthritic mice. Recipients of B29-induced Tregs had lower arthritic scores than recipients of Tregs from pOVA-treated donors (Fig. 5D). These data show that B29 also induces Tregs when nasally administered.
Fig. 5.
Nasal application of Hsp70 or its peptides suppresses experimental arthritis. (A) Mean arthritis scores (±SEM) after intranasal Mt Hsp70 administration. n = 5 mice per treatment group. (B) Mean arthritis scores (±SEM) after B29, mB29a, mB29b, or control pOVA given intranasally (n = 6 mice per group). (C) Proliferation and cytokine production of splenocytes from intranasally treated animals as described in B. Proliferation in the presence of B29, mB29a, mB29b, or pOVA is shown. Peptide-specific IFN-γ and IL-10 production are presented as cytokine production in the presence of peptide relative to background production. Results are expressed as mean stimulation index (±SEM; n = 6 per group). Data are representative of two independent experiments. (D) Mean arthritis scores (±SEM) of recipients with PGIA after adoptive transfer. Donor mice were treated as in B: 5 × 105 CD4+CD25+ or CD4+CD25− cells from spleen were transferred i.v. 1 d before disease. For A and C, P values were obtained with a two-way ANOVA followed by Bonferroni post hoc comparison. *P < 0.05.
Discussion
The induction or activation of Tregs is considered a promising approach in the treatment of inflammatory diseases (32). Tregs are selected centrally or in the periphery on the basis of TCR specificity for self-antigens. It has been shown that the antigen-specific Tregs have a superior potential to down-modulate inflammation (6, 7). It may seem attractive to use autoantigens in the case of autoimmune diseases to induce or activate disease antigen-specific Tregs. In the case of complex diseases such as RA, this approach may be difficult given the absence of identified critical self-antigens. Moreover, it has been argued that disease suppression is unlikely to result from the activities of a relatively small number of autoantigen-specific Tregs present in polyclonal T-cell populations (11). A more likely scenario would be control by the induction of antigen-specific Tregs, activated through MHC class II presentation of ubiquitous self-peptides (11). Here we show that Hsp70 can be a source of such ubiquitous self-peptides, especially when cells experience stress. Multiple peptides of various Hsp70 family members have been found in various MHC class II molecules (21, 23–25). A contributing reason for this finding is that Hsp70 is one of the molecular components of the process of so-called chaperone-mediated autophagy (33). Under stress, cells exploit autophagy for the economic use of proteins and amino acids. In the case of cellular stress imposed by nutrient deprivation, human cells were found to have uploaded their MHC class II molecules with fragments of Hsp70 (21). Interestingly, in the latter case, the B29 self-homologs were found to be present among the of HLA-DR eluted peptides. Recently, autophagy was suggested to exert an anti-inflammatory role by controlling inflammasomes, because autophagy was found to accompany inflammasome activation and to temper inflammation by eliminating active inflammasomes (34). Therefore, it is possible that stress-induced autophagy has anti-inflammatory effects at multiple levels, varying from inhibition of inflammasome activity to production of stress protein-specific Tregs. Apart from appearing in MHC class II, endogenous Hsp70-derived peptides have been identified in MHC class I molecules (22). Indeed, it has been shown that IL-10 producing, CD8+ Tregs specific for BiP, a member of the Hsp70 family, could be isolated from normal individuals (35). In such a case, CD4+ Tregs and CD8+ Tregs specific for the same antigen might regulate synergistically.
Interestingly, as a result of our T-cell epitope mapping, we identified the B29 epitope from bacterial Hsp70 to exhibit prominent immunogenicity, despite being highly conserved and herewith closely resembling its mammalian homologs mB29a and mB29b. Subsequent intranasal administration or parenteral immunization with B29 was found to induce cross-reactive T cells that recognized the mB29a and mB29b self-homologs. In adoptive transfer studies to characterize B29-induced Tregs, we found that relatively low numbers of CD4+CD25+ cells from B29-immunized donors were able to suppress arthritis. The suppressive potential of B29-induced Tregs was further demonstrated by the fact that therapeutic transfer arrested established arthritis. Upon transfer, donor cells were found in the dLN and the joints and had an activated phenotype. Relatively few CD4+CD25+ transferred cells were located in the peripheral blood (compared with CD4+CD25− cells), showing that these cells actively homed to the site of inflammation.
We saw that LAG-3 was up-regulated after B29 immunization (Fig. S1B), indicating LAG-3 being a marker for activation. It has been shown that Tregs are capable of delivering inhibitory signals via LAG-3 to MHC class II-presenting cells (4, 11). We were able to isolate LAG-3+ B29-induced Tregs, and, using adoptive transfer, only a few cells were needed to suppress disease. Therefore, it seems that the B29-specific Tregs use LAG-3 as a mechanism to suppress inflammation.
When intranasally administering B29, or its mammalian homologs mB29a and mB29b, to suppress inflammation, we observed a superior disease-suppressive quality of B29 in comparison with the mammalian homologs. It is therefore possible that B29 is an example of a natural epitope acting as a relatively strongly agonistic mimetope or Altered Peptide Ligand, endowing B29 with its Treg-inducing potential (36, 37). For therapeutic application, we propose applying the B29 peptide under subimmunogenic (36) conditions—for instance during anti-TNF treatment—to yield a maximum induction or conversion of T cells into antigen-specific Tregs. Alternatively, B29 may be used for maintaining tolerance subsequent to, or after discontinuation of, anti-TNF treatment (38, 39).
In conclusion, our data show that the administration of Hsp70 peptide B29 elicits potent primary antigen-specific Tregs, which suppress disease in a mouse model of RA. The transferred antigen-specific Treg population was long lived in vivo, expressed FoxP3 or LAG-3, and its direct presence was required for suppression. All together, Hsp70-specific Tregs may well exemplify the great potential of antigen-specific immune interventions in autoimmune diseases, even without knowledge of critical disease-specific autoantigens.
Materials and Methods
Mice.
Female BALB/c mice, aged 16–26 wk (retired breeders; for PGIA) or aged 8–12 wk (all other experiments) were purchased from Charles River. CD90.1 and FoxP3-GFP BALB/c mice were purchased from Jackson and bred in-house. CD90.1 mice were crossed with CD90.2 mice (Charles River). CD90.1.2 offspring were used for transfer experiments. Animals were kept under standard conditions at the animal facility, and all experiments were approved by the Animal Experiment Committee of Utrecht University.
Antigens, Peptides, and Antibodies.
Recombinant Mt Hsp70 (LIONEX Diagnostics & Therapeutics GmbH) contained <2.1 equivalent units (EU) of LPS per mg. Peptides for epitope mapping were prepared by automated simultaneous multiple peptide synthesis (SMPS). The SMPS setup was developed by using a standard autosampler (Gilson Medical Electronics). Briefly, standard Fmoc chemistry with in situ benzotriazole-1-yl-oxy-trispyrrolidinophosphonium hexafluorophosphate/N-methyl maleimide activation of the amino acids in a fivefold molar excess with respect to 2 micromol reactive equivalents per peptide on the PAL–PEG–PS resin (Perseptive Biosystems) was used. Peptides were obtained as C-terminal amides after cleavage with 90–95% (vol/vol) TFA/scavenger cocktails. Peptides were analyzed by reversed-phase HPLC and checked via electrospray mass spectrometry on an liquid chromatography quadrupole (LCQ) ion-trap mass spectrometer (Thermoquest). Purity of the peptides ranged between 50% and 90%. All other peptides were obtained from GenScript (including pOVA323–339). Peptides were analyzed by reversed-phase HPLC and checked via electrospray mass spectrometry on an LCQ ion-trap mass spectrometer. For flow cytometry, antibodies were purchased from BD Bioscience [CD25-APC/-PE (PC61), Ki-67-PE (B56), IL-10-PE (JES5-16)], eBioscience [CD4-FITC/-eFlour450 (RM4-5), FoxP3-APC/-eFlour450/-PE/-PerCPCy5.5 (FJK-16), LAG-3-PE (eBioC9), CD90.1-FITC (HIS51), CD90.2-APC (53-2.1)], R&D Systems [Nrp-1-APC and IL-35(p35)-APC], and Biolegend [Helios-Alexa647 (22F6)]. For CD90.1 depletion, 250 μg of anti-CD90.1 (HIS51 BD Bioscience) was given i.p.
Identification of Dominant T-Cell Epitopes of Mt Hsp70.
Mice were immunized on days 0 and 14 by i.p injection of 100 μg of recombinant Mt Hsp70 with 2 mg of adjuvant dimethyl dioctadecyl ammonium bromide (DDA) (Sigma). At day 28, mice spleen and lymph node (LN) cells were isolated and restimulated with a panel of 123 overlapping 15-mer peptides covering the complete sequence of Hsp70, followed by analysis of peptide-specific proliferation. Next, pools of identified dominant peptides were used for immunization. These pools were composed of four or five peptides, based on their degree of sequence identity with mouse Hsp70; Pool I contained nonconserved peptides, pool II partially conserved peptides, and pool III highly conserved peptides. Immunization with 100 μL i.p. and 100 μL s.c. in the neck was done at a concentration of 125 μg/mL of each peptide with 2 mg of DDA on days 0 and 14, followed by analysis of peptide-specific cytokine production on day 28. IFN-γ and IL-10 production upon immunization or PGIA induction was measured in culture supernatants of stimulated (20 μg/mL peptide) spleen or LN cells after 72 h by ELISA according to manufacturer protocol (BD).
MHC Peptide Elution.
Cells were isolated from BM and cultured with GM-CSF to yield BM-derived DC (40). MHC class II from cultured DC was isolated and identification of the MHC class II presented epitopes was performed as described (41). The BioWorks 3.3.1 SP1 application tool (Thermo Scientific) was used to search the mass spectral data against a subset protein database containing the mouse taxonomy (UniProt/Swiss-Prot protein database; http://www.ebi.ac.uk).
Nasal Administration of Hsp70 and Peptides.
Mice were treated on days −7, −5, and −3 before the first PG immunization with Mt Hsp70 (30 μg) or peptides (100 or 250 μg) in 10 μL of PBS via intranasal application, whereas control mice received 10 μL of PBS alone or pOVA (100 μg). Proliferation and cytokine production of splenocytes was determined 28 d after disease induction. Proliferation was determined 72 h later by measuring 3H-thymidine incorporation. Peptide-specific IFN-γ and IL-10 production in culture supernatants was measured by ELISA according to manufacturer’s instructions.
Ex Vivo Restimulation and Phenotypic Analysis Tregs.
Mice were injected i.p and s.c. with 100 μg of peptide with 2 mg of DDA. Splenocytes were harvested 10 d after immunization and directly analyzed or were cultured for 24 h in the presence of 10 μg/mL peptide followed by FACS analysis. Intracellular IL-10 and p35 expression was determined by flow cytometry after phorbol 12-myristate 13-acetate/Ionomycin stimulation.
Induction of PGIA.
PG was isolated from human articular cartilage as described (26). Arthritis was induced with by two i.p. injections with 250–300 μg of PG protein with 2 mg of DDA with an interval of 21 d. Subsequently, mice were randomized among experimental groups, and arthritis scores were determined in a blinded fashion by at least two independent investigators using a visual scoring system based on swelling and redness of paws as described (26).
Adoptive Transfer.
Donor mice were treated with Hsp70 or peptides as indicated above. CD4+CD25+/−, GFP+/−, or LAG-3+/− cells were isolated from spleen 10 d after the last peptide administration. Preenrichment for CD4+ cells was performed by using Dynal bead isolation (Invitrogen) followed by FACS sort (Influx; BD) with purities up to 96%. Cells were transferred via i.v. injection in 100 μL of PBS.
In Vitro Suppression Assay.
Cells were isolated 10 d after immunization. Irradiated splenocytes were used as APC (2 × 105 cells) and cultured with CD4+CD25− cells in a ratio of 2:1. CD4+CD25+ were added in various concentrations. Cells were cultured for 72 h in the presence of 5 μg/mL soluble anti-CD3, and 3H-thymidine was added for an additional 18 h.
Statistical Analysis.
All data are presented as mean ± SEM. Statistical analysis was carried out using Prism software (Graphpad Software). Comparisons between two groups were done by using the Student t test (unpaired, two-tailed). Multigroup comparisons were done by using one-way ANOVA followed by a Bonferroni post hoc test. P < 0.05 with a 95% confidence interval was considered significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Acknowledgments
We thank C. Keijzer, B. Margry, and R. Spiering for technical assistance; K. Malone for providing scientific writing services; G. Arkesteijn for sorting; and workers at the animal facility for animal care. This work was supported by Innovation Oriented Programme in Genomics Project Grants IGE3018 and IGE07004, European Union Grant Seventh Framework Programme TOLERAGE: HEALTH-F4-2008-202156, and the Dutch Arthritis Association.
Footnotes
Conflict of interest statement: W.v.E. has equity in Trajectum Pharma BV.
†This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206803109/-/DCSupplemental.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright GP, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa H, et al. Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med. 2005;201:681–686. doi: 10.1084/jem.20041959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Cohen IR. Real and artificial immune systems: Computing the state of the body. Nat Rev Immunol. 2007;7:569–574. doi: 10.1038/nri2102. [DOI] [PubMed] [Google Scholar]
- 13.Schett G, et al. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest. 1998;102:302–311. doi: 10.1172/JCI2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boog CJ, et al. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schett G, Tohidast-Akrad M, Steiner G, Smolen J. The stressed synovium. Arthritis Res. 2001;3:80–86. doi: 10.1186/ar144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieten L, et al. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS ONE. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: How immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, et al. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- 19.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 20.Chicz RM, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dengjel J, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 23.Muntasell A, et al. Dissection of the HLA-DR4 peptide repertoire in endocrine epithelial cells: Strong influence of invariant chain and HLA-DM expression on the nature of ligands. J Immunol. 2004;173:1085–1093. doi: 10.4049/jimmunol.173.2.1085. [DOI] [PubMed] [Google Scholar]
- 24.Halder T, et al. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238–3244. [PubMed] [Google Scholar]
- 25.Sanjeevi CB, Lybrand TP, Stevanovic S, Rammensee HG. Molecular modeling of eluted peptides from DQ6 molecules (DQB1*0602 and DQB1*0604) negatively and positively associated with type 1 diabetes. Ann N Y Acad Sci. 2002;958:317–320. doi: 10.1111/j.1749-6632.2002.tb02995.x. [DOI] [PubMed] [Google Scholar]
- 26.Hanyecz A, et al. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–1676. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 27.Kelchtermans H, et al. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 28.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 29.Kendal AR, Waldmann H. Infectious tolerance: Therapeutic potential. Curr Opin Immunol. 2010;22:560–565. doi: 10.1016/j.coi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Unger WW, et al. Early events in peripheral regulatory T cell induction via the nasal mucosa. J Immunol. 2003;171:4592–4603. doi: 10.4049/jimmunol.171.9.4592. [DOI] [PubMed] [Google Scholar]
- 32.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 33.Crotzer VL, Blum JS. Autophagy and intracellular surveillance: Modulating MHC class II antigen presentation with stress. Proc Natl Acad Sci USA. 2005;102:7779–7780. doi: 10.1073/pnas.0503088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodman-Smith MD, Corrigall VM, Kemeny DM, Panayi GS. BiP, a putative autoantigen in rheumatoid arthritis, stimulates IL-10-producing CD8-positive T cells from normal individuals. Rheumatology (Oxford) 2003;42:637–644. doi: 10.1093/rheumatology/keg204. [DOI] [PubMed] [Google Scholar]
- 36.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaguchi S, Powrie F, Ransohoff RM. Re-establishing immunological self-tolerance in autoimmune disease. Nat Med. 2012;18:54–58. doi: 10.1038/nm.2622. [DOI] [PubMed] [Google Scholar]
- 38.Roord ST, et al. Modulation of T cell function by combination of epitope specific and low dose anticytokine therapy controls autoimmune arthritis. PLoS ONE. 2006;1:e87. doi: 10.1371/journal.pone.0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eden W, Lisse J, Prakken B, Albani S. Biologics and postbiologics: Novel immunotherapeutics for the induction and maintenance of remission. Drug Discov Today. 2010;15:71–77. doi: 10.1016/j.drudis.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 41.Meiring HD, van der Heeft E, ten Hove GJ, de Jong APJM. Nanoscale LC-MS(n): Technical design and applications to peptide and protein analysis. J Sep Sci. 2002;25:557–568. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.