Abstract
Targeting Mycobacterium tuberculosis bacilli in low-oxygen microenvironments, such as caseous granulomas, has been hypothesized to have the potential to shorten therapy for active tuberculosis (TB) and prevent reactivation of latent infection. We previously reported that upon low-dose M. tuberculosis infection, equal proportions of cynomolgus macaques develop active disease or latent infection and that latently infected animals reactivated upon neutralization of TNF. Using this model we now show that chemoprophylaxis of latently infected cynomolgus macaques with 6 mo of isoniazid (INH) effectively prevented anti-TNF antibody-induced reactivation. Similarly, 2-mo treatment of latent animals with a combination of INH and rifampicin (RIF) was highly effective at preventing reactivation disease in this model. Metronidazole (MTZ), which has activity only against anaerobic, nonreplicating bacteria, was as effective as either of these treatments in preventing reactivation of latent infection. Because hypoxic lesions also occur during active TB, we further showed that addition of MTZ to INH/RIF effectively treated animals with active TB within 2 mo. Healing lesions were associated with distinct changes in cellular pathology, with a shift toward increasingly fibrotic and calcified lesions. Our data in the nonhuman primate model of active and latent TB supports targeting bacteria in hypoxic environments for preventing reactivation of latent infection and possibly shortening the duration of therapy in active TB.
Although human infection with Mycobacterium tuberculosis can result in active primary tuberculosis (TB), more typically the outcome is clinically asymptomatic latent infection (1). Latent infection is defined as a positive Tuberculin skin test or IFN-γ release assay and the absence of clinical signs and symptoms of active disease. This definition likely encompasses a wide range of disease outcomes, ranging from subclinical active disease to sterilization of the infecting organism (1); in fact, it may be more appropriate to label “latent” infection as asymptomatic TB. Latently infected immune-competent persons are assumed to have a 10% lifetime risk of reactivation. The risk increases with immune compromise, such as concurrent HIV infection or treatment with immunosuppressive agents, such as TNF-neutralizing drugs (2, 3). An estimated 2 billion people are latently infected with M. tuberculosis, providing a large reservoir for possible reactivation. Because each infectious case of TB can be expected to give rise to 2.6–7.8 new infections, meeting current global control targets requires improved diagnostics and therapies for latent infections (4).
Treatment of active TB for drug-susceptible disease involves standard “short-course” therapy consisting of 2 mo of treatment with isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (ETH), followed by 4 additional months of INH and RIF (http://www.cdc.gov/TB/topic/treatment/default.htm). The length of this standard regimen leads to poor compliance and completion rates. Availability of effective shorter regimens would have a major impact on treatment of tuberculosis worldwide (5).
Latent M. tuberculosis infection is routinely treated with INH for 9 mo (6). In a metaanalysis of 11 clinical trials, prophylactic treatment with INH alone for 6–12 mo reduced the risk of subsequently developing TB by 60% (7). However, clinical hepatitis occurs in up to 3% of persons receiving INH (8). Adherence with this long treatment course of chemotherapy remains a major problem, and shorter options have been tested, although side effects have been noted for these as well (6, 9, 10). INH is primarily effective against replicating bacilli, indicating that M. tuberculosis bacilli are replicating during clinically latent infection, at least sporadically. The protracted INH regimen necessary for efficacy likely reflects periods of nonreplication by subpopulations of M. tuberculosis bacilli within a person with latent infection and suggests that targeting nonreplicating bacilli could effectively shorten therapy.
The histopathologic hallmark of TB is the granuloma, and a spectrum of granuloma types is observed in human tuberculosis. Hypoxia is a key distinguishing feature of certain types of granulomas (11–13). We demonstrated that caseous granulomas, a common type seen in human TB, are hypoxic in various animal models (guinea pigs, rabbits, and nonhuman primates), whereas nonnecrotizing granulomas (primarily composed of macrophages with sparse lymphocytes) are not hypoxic (11). Murine granulomas are less-organized collections of macrophages and lymphocytes without central caseous necrosis and are generally not hypoxic (11, 13). M. tuberculosis grown under low-oxygen conditions is not susceptible to INH (14). Thus, in latent infection and in active disease, bacilli that persist in hypoxic areas cannot be efficiently and rapidly cleared by INH. The apparent clinical utility of INH in prophylactic treatment of latent TB is therefore paradoxical. The other drugs used for the treatment of active TB are also primarily effective against growing organisms, although RIF has some efficacy under lower-oxygen conditions (14), which may contribute to the extended period necessary to cure TB.
Metronidazole (MTZ) is a “tool” compound to address the importance of targeting hypoxic environments in treatment strategies for TB. Metronidazole requires reductive activation by the respiratory chain, which happens aerobically and anaerobically, but under aerobic conditions the single electron-reduced species reacts with molecular oxygen so the reduction is nonproductive (“futile cycling”). If that radical is present under hypoxic conditions it causes DNA damage (15). MTZ can kill M. tuberculosis grown in vitro under hypoxic but not aerobic conditions (14). MTZ was as effective as RIF at reducing bacterial load in rabbits with active TB, which have primarily caseous granulomas (11). MTZ is not effective against M. tuberculosis infection in mouse models or in guinea pigs (12, 16). In human clinical trials, MTZ did not increase sputum conversion rates but did improve clinical signs of disease (17). We hypothesized that a drug that worked under hypoxic conditions, such as MTZ, would affect M. tuberculosis bacilli residing in hypoxic areas, both in active and latent TB, and could shorten therapy by eliminating a population that was refractory to standard drugs. To test this hypothesis, we used a well-characterized nonhuman primate model of active and latent TB that recapitulates the spectrum of disease and granuloma types seen in human TB (18, 19).
Results
Pharmacokinetic Studies and Dose Selection for Treatment.
Concentration time profiles were established for INH, RIF, and MTZ administered by gavage either as single drugs or in combination. Standard pharmacokinetic parameters were calculated from the profiles of five to six animals for each drug (Table 1). A comparison with exposure achieved in humans at clinically used doses is shown. MTZ exposure in macaques after oral administration of 25 mg/kg was within the range of exposure reached in human at the standard dose of 400 mg. For INH and RIF, the dose used produced exposures that were slightly lower than those seen in human subjects receiving standard therapeutic doses. These doses were approved by the facility veterinarian and selected for treatment experiments. Given the relatively short half-life of MTZ (20), a twice-daily regimen was selected in agreement with twice- or thrice-daily administration in human therapy.
Table 1.
Pharmacokinetic parameters of INH, RIF, and MTZ after oral administration to cynomolgous macaques, and comparison with exposure in human subjects at clinically used doses
| Group | INH | RIF | MTZ |
| Cynomolgous macaques | |||
| Dose (mg/kg) | 15 | 20 | 25 |
| Cmax (μg/mL) | 6.5 (3.1) | 4.1 (2.9) | 10.6 (7.8) |
| tmax (h) | 1.2 (0.4) | 5.3 (2.8) | 5.7 (2.0) |
| AUC[0-24] (μg*h/mL) | 13.7 (5.5) | 50.7 (49.5) | 86.6 (44.1) |
| t1/2 (h) | 1.3 (0.6) | n.d.† | 7 to >15 (5.0) |
| Humans subjects at clinically used doses | |||
| Dose (mg/kg) | 5.0 | 10.0 | 7.0 |
| AUC[0-24] (μg*h/mL) | 17 (FA); 30 (SA) [28-29] | 80–120 [32-33] | 80–100 [30-31] |
Values shown are means (SD). AUC[0-24], area under the curve from 0 to 24 h; Cmax, peak concentration in plasma; FA, fast acetylators; n.d., not determined; SA, slow acetylators; tmax, time of peak concentration in plasma after dosing; t1/2, apparent elimination half-life.
†Elimination phase not reached.
Our results show that variability in exposure increased when drugs were given in combination for several days (Table S1). Interestingly, animals with very low INH exposure were found to have significant concentrations of its major metabolite acetyl-INH, suggesting that monkeys can be classified as slow or fast acetylators, similar to humans, where this variability is mediated by polymorphism in the NAT-2 enzyme.
Short-Course MTZ or INH/RIF Is as Effective as 6 mo INH in Preventing Reactivation of Latent Infection.
Cynomolgus macaques infected with a low inoculum (25 cfu) of M. tuberculosis (strain Erdman) develop primary TB and latent infection in approximately equal proportions (18, 19). We developed quantitative outcome measures of disease at necropsy, including gross pathology scoring and determination of bacterial numbers (19), enhancing the use of this animal model for drug efficacy studies. At necropsy, we obtained numerous samples from granulomas, lung lobes, thoracic lymph nodes, and extrapulmonary sites and used quantitative tools we developed and described in detail previously to score gross pathology, bacterial burden, and dissemination (19). These scoring systems distinguish clinically defined active, latent, and reactivated TB in macaques (19, 21). The outbred nature of macaques introduces substantial variability among animals in terms of disease progression and response to therapies, just as in humans, which makes this a challenging but realistic model for testing therapeutics.
Determining efficacy of a drug against latent TB (i.e., prevention of reactivation) is challenging, and human clinical trials require large numbers of subjects with years of follow-up, because the natural reactivation rate is low. In macaques, bacterial numbers are generally quite low during latent infection and only increase upon reactivation (19, 22). Thus, we assumed that simply comparing bacterial numbers in latent monkeys with and without drug treatment would not provide sufficient sensitivity for identifying an efficacious drug against bacilli present in latent animals. Short-term (4–8 wk) anti-TNF antibody treatment caused reactivation in at least 70% of macaques with latent infection (22). Our strategy was to test effectiveness of a drug in preventing TNF neutralization-induced reactivation (Fig. 1A), reasoning that either an effective drug would reduce bacterial load to the point where reactivation does not occur with anti-TNF therapy, or bacterial populations most prone to reactivation would be selectively affected by the drug.
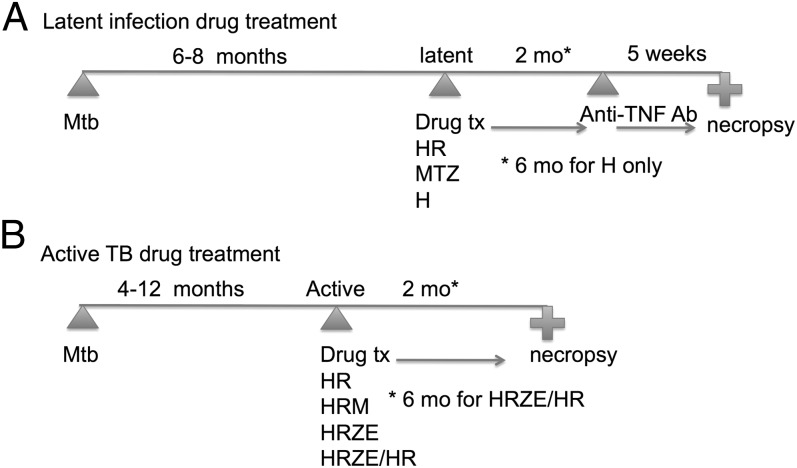
Fig. 1.
Study design for drug treatment studies in M. tuberculosis-infected macaques. (A) Drug treatment of latently infected monkeys, with subsequent anti-TNF antibody (adalimumab) treatment to induce reactivation, with necropsy. (B) Drug treatment of active tuberculosis monkeys, with necropsy. H, INH; R, RIF; Z, PZA; E, ETH; M, MTZ.
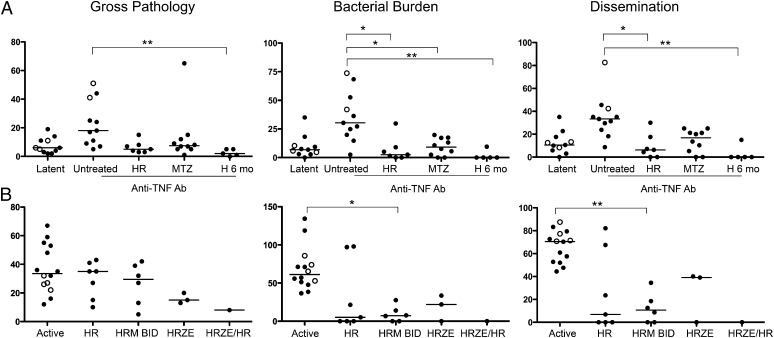
Anti-TNF antibody treatment caused reactivation in 9 of 11 latently infected control animals, according to gross pathology, bacterial burden, and bacterial dissemination (Fig. 2A). We used these quantitative measures at necropsy, rather than more subtle and ambiguous clinical signs, to determine whether drug-treated animals reactivated the latent infection after anti-TNF treatment for 5 wk. Similar to human clinical trials, INH for 6 mo resulted in monkeys that showed no signs of reactivation disease (Fig. 2A). Four of the five INH-treated animals seemed to be completely sterilized by this treatment (no bacilli were cultured from numerous samples at necropsy). To further validate this model against existing human clinical data, we treated seven latently infected macaques with INH and RIF in combination once daily, for 2 mo. INH plus RIF treatment significantly reduced reactivation of latent infection (Fig. 2A).
Fig. 2.
Effective drug treatment prevents reactivation of latent infection and reduces bacterial numbers and spread of infection in active disease. Gross pathology, bacterial load (cfu score), and extent of involvement and dissemination (% positive samples) were quantified from each monkey (represented by a single point on each graph) at necropsy. (A) Latent monkeys were necropsied as controls, and monkeys were either treated with drugs (INH/RIF daily or MTZ twice daily for 2 mo, or INH daily for 6 mo) or untreated, and then administered anti-TNF antibody (adalimumab) for 5 wk, then necropsied. Approximately 80% of latent monkeys reactivated, whereas INH/RIF or MTZ for 2 mo or INH for 6 mo prevented reactivation in response to anti-TNF antibody treatment. (B) Active TB monkeys were either untreated, treated with INH/RIF, INH/RIF/MTZ, INH/RIF/PZA/ETH for 2 mo, or INH/RIF/PZA/ETH for 2 mo followed by INH/RIF for 4 mo (only one monkey and not used in the statistical analysis). Lines in each group represent the median. Each circle represents an individual animal. Open circles reflect historical control animals, whereas closed circles reflect animals infected at the same time as the treatment studies. Statistics: Kruskal-Wallis with Dunn’s multiple comparisons performed when Kruskal-Wallis P < 0.05; *P < 0.05; **P < 0.01; ***P < 0.001. All other groups were not significant in the analysis. E, ETH; H, INH; M, MTZ; R, RIF; Z, PZA.
We previously demonstrated that caseous lesions from monkeys with active TB were positive for pimonidazole staining (11), which marks areas of hypoxia. Latent monkeys have caseous as well as fibrocalcific granulomas, and granulomas from a latent monkey were positive for pimonidazole staining (Fig. S1), indicating hypoxic areas of the granulomas. To determine whether a drug that was only effective against bacteria in hypoxic environments could prevent reactivation, we treated 10 latently infected animals with MTZ twice daily for 2 mo (Fig. 1A). MTZ proved to be as effective as INH/RIF in preventing anti-TNF–induced reactivation, with cfu scores in the same range as latent untreated and INH-treated monkeys (Fig. 2A). In both the INH/RIF and MTZ groups, two monkeys were apparently sterilized by the treatment, which is a lower frequency of sterilization than the 6-mo INH regimen. None of the anti-TNF–treated control monkeys were sterile (i.e., M. tuberculosis was grown from the tissues of all control monkeys). These data indicate that a short-term treatment with MTZ alone substantially reduces reactivation from latent infection as well as INH for 6 mo or short-course combination INH/RIF treatment.
Metronidazole with INH/RIF Is Effective Against Active TB.
Active TB in the primate model presents, as in humans, as a spectrum of disease ranging from minimal to fulminant (18, 19, 23). For this study we used previously published criteria to determine that monkeys had active pulmonary TB before initiation of treatment, including change in appetite, weight loss, X-ray findings, cough, positive M. tuberculosis growth from bronchoalveolar lavage (BAL) or gastric aspirate, or elevated erythrocyte sedimentation rate (19). There was historical evidence for the efficacy of combination therapy in nonhuman primates (24). To facilitate regimen comparisons we treated four active TB animals with the standard human “intensive phase,” a four-drug regimen consisting of INH/RIF/PZA/ETH for 2 mo. Three animals were necropsied, and one animal was treated for an additional 4 mo of INH/RIF to mimic a full-length human standard chemotherapy regimen (Fig. 1B). Standard 6-mo therapy can completely cure active TB in primates (Fig. 2B). We reasoned that INH/RIF/PZA/ETH for 2 mo was a good control for other short-term (2 mo) treatments; this regimen reduced bacterial burden compared with active TB monkeys (Fig. 2B). INH/RIF for 2 mo reduced bacterial burden in five of seven monkeys, with three of these monkeys being apparently sterilized (Fig. 2B). However, given the variability in outcome with INH/RIF, whereby the monkeys with high bacterial burdens could be interpreted as slow responders or treatment failures, the scores were not statistically significantly different compared with the active control group.
Our hypothesis was that adding MTZ to the INH/RIF treatment (Fig. 1B) would increase overall efficacy by killing bacteria in hypoxic microenvironments where INH and RIF were less effective. Addition of MTZ twice daily to the INH/RIF daily treatment regimen seemed to effectively treat all animals and significantly reduced bacterial burden and dissemination compared with controls (Fig. 2B). However, there was not a significant difference in cfu score between INH/RIF and INH/RIF/MTZ.
We separated the bacterial burden data (cfu score) of thoracic lymph nodes from that of lung lesions to determine whether MTZ had a more pronounced effect on one of these compartments. Both INH/RIF and INH/RIF/MTZ were more effective against lung lesions than against infected thoracic lymph nodes (Fig. S2). Both were effective regimens at reducing extrapulmonary bacterial burden: 71.4% of active controls had growth of M. tuberculosis from extrapulmonary sites (usually liver or spleen), compared with 28.5% with INH/RIF and 33.3% with INH/RIF/MTZ.
An outcome measure used in humans to assess efficacy of treatment is negative sputum conversion for M. tuberculosis growth. We used M. tuberculosis growth from BAL samples and gastric aspirates as a surrogate for sputum. Many, but not all, monkeys with active TB have positive cultures from either of these sites; all untreated controls in this study were positive for the 2-mo study period (Fig. S3). All monkeys treated with INH/RIF/MTZ converted from positive to negative cultures, compared with only 30% in the INH/RIF group after 8 wk of treatment (Fig. S3). In untreated control monkeys, there was a significant reduction in weight over 2 mo before necropsy (Fig. S4). In contrast, the weights in all treated groups were unchanged over the 2-mo treatment period. These data support that there was improvement in microbiologic and clinical parameters after a short course of treatment.
IFN-γ Responses in the Airways During Treatment Do Not Predict Efficacy of Therapy.
We previously established that IFN-γ responses from T cells obtained by BAL were significantly higher in monkeys with active TB compared with latent TB both early in infection and at necropsy (19). Thus, we tracked IFN-γ responses in BAL cells during treatment to determine whether changes in this parameter could predict efficacy of treatment. Given the monkey-to-monkey variability, we assessed whether there were differences in IFN-γ production before and after treatment (before anti-TNF therapy) within both latent and active TB treatment groups, but we observed no significant differences (Fig. S5). Thus, simply following mycobacteria-specific IFN-γ production is unlikely to be a useful surrogate for successful drug treatment, even when the airways are sampled.
Drug Treatment Induced Changes in Granuloma Structure.
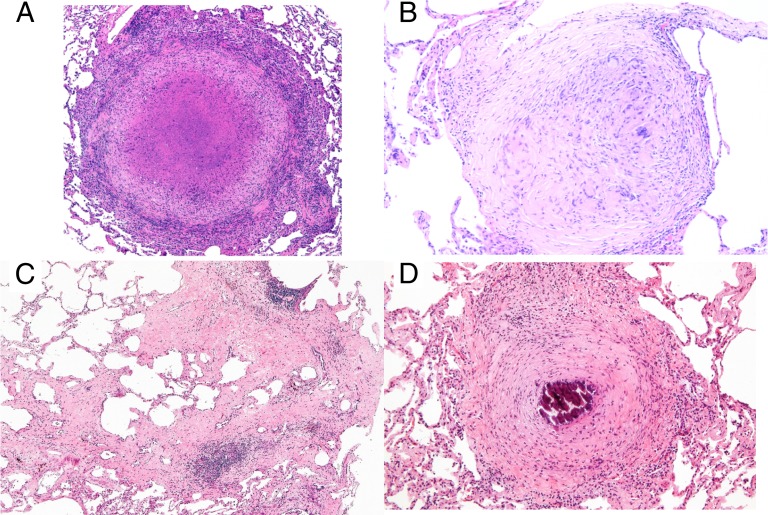
We previously characterized the histopathologic spectrum of granulomas types seen in active and latently infected monkeys (18, 19). Like humans, monkeys with active TB have caseous granulomas and nonnecrotizing granulomas (solid cellular granulomas composed of epithelioid macrophages with sparse lymphocytes), occasionally mineralized granulomas, as well as TB pneumonia (25). The granulomas from the untreated active monkeys in this study were primarily caseous (Fig. 3A), with nonnecrotizing or suppurative (neutrophilic) granulomas or TB pneumonia also present. In contrast, monkeys after 2 mo of treatment had a much smaller proportion of caseous granulomas, with more internally fibrotic (“healing”) granulomas (Fig. 3B), interstitial fibrosis without much remaining granuloma structure (Fig. 3C), and fibrocalcific (mineralized with peripheral fibrosis) (Fig. 3D) granulomas. In some cases, a continuum could be observed in granulomas undergoing central fibrosis and subsequently evolving into completely fibrotic or fibrocalcific granulomas. The fibrotic process was often accompanied by lymphocytic aggregates, which may migrate around and/or into healing lesions in response to released mycobacterial antigens.
Fig. 3.
Tuberculous granulomas in the lung evolve through the course of drug treatment. (A) Representative caseous granuloma from an untreated active control monkey, with an eosinophilic necrotic center surrounded by epithelioid macrophages and peripherally located lymphocytes. (B) “Healing” granuloma characterized by a central area containing residual epithelioid macrophages with prominent features of evolving fibroplasia (“fibrous transformation”). (C) Poorly circumscribed areas of interstitial fibrosis with and without prominent areas of cellularity in lungs of a monkey after drug treatment. (D) Representative fibrocalcific granuloma with a central area of mineralization surrounded by prominent peripheral fibrosis. H&E stain. (Magnification: A, C, and D, 5×; B, 10×.)
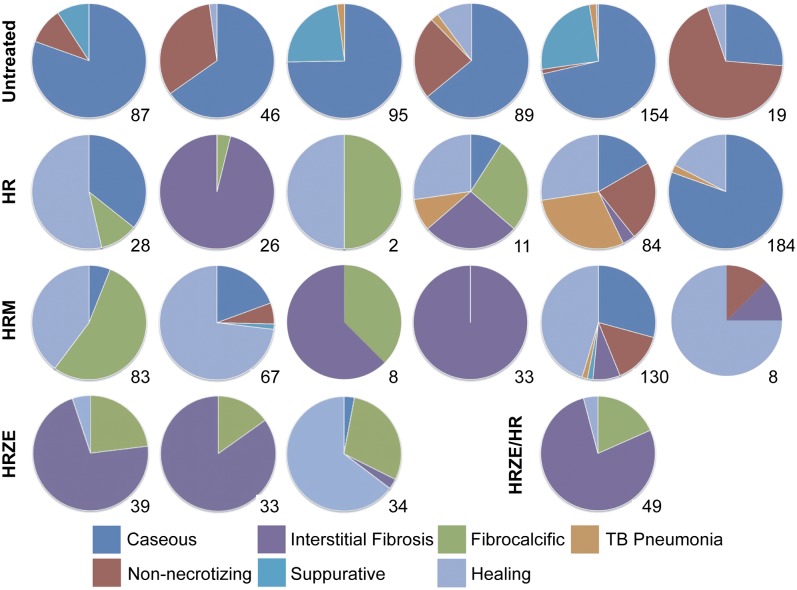
All histological sections (specifically dissected granulomas as well as several random samples from each lung lobe) from each animal with active disease (untreated and treated) were quantified with respect to granuloma type and pathology. The proportion of granuloma types in the lung is represented in a pie chart for each animal, as well as the total number of granulomas observed in all lung sections (Fig. 4). The active controls were dominated by caseous, suppurative, or nonnecrotizing granulomas; effective drug therapy resulted in reduction in these types of lesions, with a substantial increase in “healing” lesions, particularly those showing interstitial fibrosis and fibrocalcification (Fig. 4).
Fig. 4.
Evolution of pathology in response to drug therapy in monkeys with active TB. Each pie chart represents one monkey in the control or treatment groups. Each lesion type is represented by a color (see legend). The numbers in the right lower corner under each pie represent the number of lesions observed by microscopic histopathology in all sections for that animal.
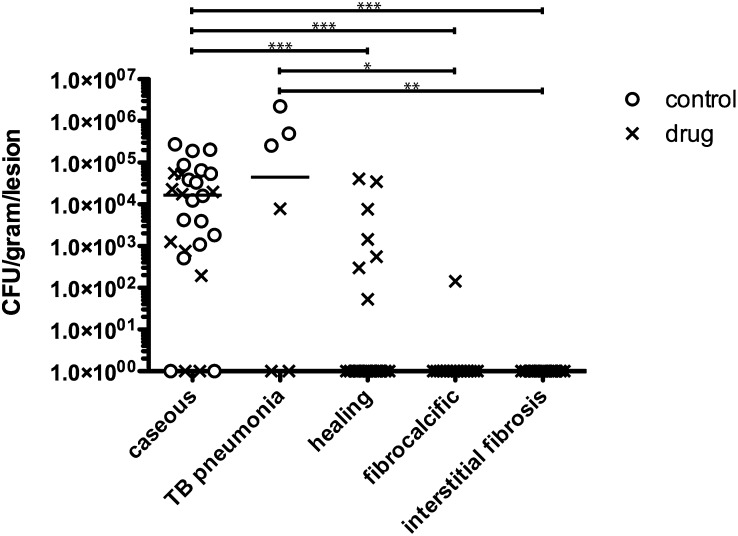
We assessed which types of lesions were associated with bacterial growth in controls and after drug treatment. Matched lesions where both bacterial numbers (cfu/g) and histology were available revealed that bacterial growth was significantly higher in caseous granulomas or areas of TB pneumonia compared with fibrocalcific granulomas or interstitial fibrosis (Fig. 5). “Healing” granulomas were those that appeared caseous with apparent evolution of internal fibrosis (i.e., an intermediate between caseous and totally fibrotic granulomas). “Healing” granulomas had an intermediate bacterial burden (significantly lower than caseous granulomas), which suggests a transitional state during treatment (Fig. 5). In INH/RIF- and INH/RIF/MTZ-treated animals, most remaining bacilli were found in caseous granulomas and occasionally in “healing” granulomas (Fig. 5).
Fig. 5.
Caseous lesions have significantly more bacilli than fibrocalcific, healing, or interstitial fibrosis lesions. Lesions with both histology and cfu data available from active TB controls (open circles) and drug-treated animals (Xs) were matched and compared across pathologic categories. Each point represents a lesion. Remaining bacilli in the drug-treated monkeys were predominantly in caseous granulomas, with some in healing granulomas. Each line represents the median number of lesions among both treated and controls within that lesion type (lines not seen reflect a median of zero). Statistics: Kruskal-Wallis with Dunn’s multiple comparisons; *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
M. tuberculosis exists in different microenvironments among various granuloma types and perhaps even within the same granuloma, depending upon the physical location of individual bacilli. This suggests that rapid clearance of M. tuberculosis in patients may require combinations of drugs optimized for each microenvironment. The relatively poor activity of the existing front-line agents against hypoxic organisms suggests that this environmental variable may provide the key to shortening the duration of chemotherapy. The presence of hypoxic regions in caseous granulomas (11) suggests that treatment with a drug that has efficacy under low-oxygen conditions may improve therapy by eliminating one particular subpopulation of M. tuberculosis bacilli. Here, we used a nonhuman primate model that recapitulates both the spectrum of granulomas and disease types (active to latent) seen in humans to test whether MTZ could improve short-course therapy against TB.
In the macaque model, 2 mo of MTZ alone was as effective as 2 mo of INH/RIF at preventing anti-TNF antibody-induced reactivation. Bacterial numbers were very low in both groups of treated animals after prophylactic treatment. Although bacterial numbers were also low in the latent (untreated) group, 80% of untreated animals in this study reactivated the infection after TNF neutralization, similar to our previously reported data (22). Thus, short-term drug treatment reduced bacterial load or altered granuloma structure and prevented reactivation, even without sterilizing all of the animals. As with humans, INH for 6 mo was also effective at preventing reactivation and sterilized four of five monkeys.
Hypoxic lesions are also found in active TB. Although four-drug combination therapy is used in the first 2 mo of treatment, successful treatment requires an additional 4 mo of INH/RIF. Although INH/RIF was effective in most of the active TB monkeys, not all animals responded to the treatment. We speculate that the types of granulomas predominating during disease may influence the effectiveness of a therapy and contribute to the variability in outcome of short-course treatment; in fact there is an overlap of granuloma types between active and latent infection, and similar drugs may work in both situations to shorten therapy. Although the addition of MTZ twice daily to the INH/RIF 2-mo regimen was effective in all animals and provided a significant reduction in cfu score compared with untreated controls, there was not a statistically significant difference between INH/RIF- and INH/RIF/MTZ-treated groups. It is possible with greatly increased sample sizes that we could observe a significant difference between short-term INH/RIF treatment and INH/RIF+MTZ, but this remains a speculation.
MTZ was shown to be ineffective in murine models of TB (16), most likely because mouse granulomas are quite different from human granulomas, lacking caseous necrosis and hypoxic environments (11, 13). MTZ alone was also ineffective against TB in guinea pigs, even though this animal can develop caseous (and hypoxic) granulomas (12). In guinea pigs, the presence of multiple, nonnecrotizing lesions is likely responsible for progression of disease in MTZ-treated animals. However, the authors stated that there were significant toxicity and tolerability issues in the guinea pigs, especially with combination therapy, which was only carried out for 2 wk, and achieving adequate exposure of MTZ was problematic. In contrast, rabbits have primarily caseous granulomas in active TB, and MTZ treatment significantly reduced bacterial numbers in this animal model (11). However, none of these models provide the opportunity to assess drug treatments in the context of latent infection, nor do they develop the full spectrum of granulomas types as seen in humans.
The present study also provided an important opportunity to identify the characteristics of healing lesions in lungs of primates during drug treatment, data that are difficult if not impossible to obtain during successful treatment of human TB. Drug-treated animals display predominantly fibrocalcific or fibrotic lesions, including interstitial fibrosis or scarring, which were associated with few to no bacteria, whereas caseous lesions and tuberculous pneumonia in active controls had high bacterial numbers. The transitional category of “healing” granulomas had intermediate numbers of bacilli, reflecting those granulomas in the process of conversion from caseous to fibrotic granulomas or scars. The combined histologic and bacterial data support that targeting bacilli within caseous granulomas is necessary to improve treatment. Lymphocytic infiltrates were often observed in areas of evolving fibrosis, suggesting that drugs may result in increased antigenic availability. How the lymphocytic infiltrates contribute to fibrosis is not known but may involve increased cytokine production or the induction of inflammatory or regulatory macrophages. Further investigation of these processes may provide clues as to the mechanisms of fibrosis and persistent lung damage even after treatment of TB. The pathological changes in treated animals may be specific to the drug combination used and may even involve the evolution of pathology that ultimately results in a slowing of sterilization.
This study provides data that a drug targeting hypoxic regions in the host in latent and active TB can be effective in treatment of this disease, and it confirms the importance of this microenvironment in lesions during both latent infection and active TB. These results also provide a stringent and realistic model for testing drug effectiveness, in active TB and in latent infection. MTZ is simply a proof of principle drug here. Whether MTZ will be useful in humans has yet to be proven (ClinicalTrials.gov Identifier: NCT00425113), but our data suggest that development of drugs targeting M. tuberculosis within hypoxic environments should be a priority.
Materials and Methods
Additional detailed methods are in SI Materials and Methods.
Animals and Infections.
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. The animals were housed and maintained in accordance with standards established in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Cynomolgus macaques were infected via bronchoscope with 25 cfu of M. tuberculosis Erdman strain (18, 19, 26). Using our published criteria, monkeys were determined to have active or latent TB by 6–8 mo after infection (18, 19) and were randomized to treatment or nontreatment groups.
In Vivo Pharmacokinetic Studies.
Groups of three animals received a single dose of INH at 15 mg/kg, RIF at 20 mg/kg, or MTZ at 25 mg/kg, administered by gavage. A group of six animals received daily doses of INH 15 mg/kg and RIF 20 mg/kg, along with MTZ twice daily at 25 mg/kg for 4 consecutive days. Serum samples were collected for determination of drug levels; details on analysis of samples are provided in SI Materials and Methods.
Treatment.
Treatment is outlined in Fig. 1. Monkeys with latent M. tuberculosis infection were either untreated (n = 22), treated with INH and RIF for 2 mo (n = 7), or INH for 6 mo (n = 5) orally once daily. MTZ was administered in two oral daily doses of 25 mg/kg (n = 10). Anti-TNF antibody (adalimumab) was administered to all antibiotic-treated latent monkeys and 11 of 22 untreated latent controls for 5 wk to induce reactivation.
Monkeys with active TB were treated with INH and RIF once daily (n = 7); INH and RIF once daily plus MTZ twice daily (n = 6); or INH, RIF, PZA, and ETH (n = 3) once daily for 2 mo, with 15 untreated active controls. One monkey was treated with INH/RIF/PZA/ETH for 2 mo and INH/RIF for the next 4 mo to mimic standard therapy in humans.
Necropsy.
Gross pathology scores were obtained using our published scoring system (19), which takes into account TB-specific disease in all lung lobes, lymph nodes, and extrapulmonary sites. Bacterial numbers were determined on numerous samples from each monkey, including most granulomas and several random samples from each lung lobe, thoracic and several peripheral lymph nodes, liver, spleen, and any other involved organs. Bacterial numbers are reported as a cfu score and as the percentage of tissues from each monkey that grew M. tuberculosis (% positive), as previously described (19) and in SI Materials and Methods.
Statistical Analysis.
Pair-wise comparisons were performed using Wilcoxin paired t test (nonnormally distributed data) analysis. Analysis of more than two groups was performed using ANOVA with Bonferroni post hoc analysis for normally distributed data and Kruskal-Wallis analysis with Dunn’s multiple comparison for nonnormally distributed data. Statistical analysis was performed with Prism (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
We thank Mark Rodgers, Lekneitah Smith, Catherine Cochran, Kang Min Low, and Carolyn Bigbee for technical assistance; Melanie O’Malley, Jennifer Kerr, Daniel Fillmore, and Jaime Tomko for veterinary technician expertise; Matthew Bigbee for assistance with figures; Charles Scanga; and the Grand Challenge 11 consortium members, particularly Douglas Young, for invaluable advice and discussion on research design and interpretation and for comments on the manuscript. BEI Resources, National Institute of Allergy and Infectious Diseases Contract HHSN266200400091C, provided Culture Filtrate Protein. This study was funded by the Bill and Melinda Gates Foundation Grand Challenge 11 “Drugs for Latent TB”; the Otis Foundation (P.L.L.); and, in part, by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.J.R. is a guest editor invited by the Editorial Board.
See Commentary on page 13890.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121497109/-/DCSupplemental.
References
- 1.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 3.Keane J. Tumor necrosis factor blockers and reactivation of latent tuberculosis. Clin Infect Dis. 2004;39:300–302. doi: 10.1086/421499. [DOI] [PubMed] [Google Scholar]
- 4.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface. 2008;5:653–662. doi: 10.1098/rsif.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JL, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180:558–563. doi: 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung CC, Rieder HL, Lange C, Yew WW. Treatment of latent infection with Mycobacterium tuberculosis: Update 2010. Eur Respir J. 2011;37:690–711. doi: 10.1183/09031936.00079310. [DOI] [PubMed] [Google Scholar]
- 7.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;(2):CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: A 7-year survey from a public health tuberculosis clinic. JAMA. 1999;281:1014–1018. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Global Tuberculosis Control WHO Report 2010. Geneva: WHO; 2010. [Google Scholar]
- 10.Sterling TR, et al. TB Trials Consortium PREVENT TB Study Team Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 11.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoff DR, et al. Metronidazole lacks antibacterial activity in guinea pigs infected with Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2008;52:4137–4140. doi: 10.1128/AAC.00196-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MC, et al. Characterization of the tuberculous granuloma in murine and human lungs: Cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 14.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sander P, et al. Mycobacterium bovis BCG recA deletion mutant shows increased susceptibility to DNA-damaging agents but wild-type survival in a mouse infection model. Infect Immun. 2001;69:3562–3568. doi: 10.1128/IAI.69.6.3562-3568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks JV, Furney SK, Orme IM. Metronidazole therapy in mice infected with tuberculosis. Antimicrob Agents Chemother. 1999;43:1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai CR, et al. Role of metronidazole in improving response and specific drug sensitivity in advanced pulmonary tuberculosis. J Assoc Physicians India. 1989;37:694–697. [PubMed] [Google Scholar]
- 18.Capuano SV, 3rd, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PL, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36:353–373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Diedrich CR, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin PL, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh GP, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 24.Wolf RH, Gibson SV, Watson EA, Baskin GB. Multidrug chemotherapy of tuberculosis in rhesus monkeys. Lab Anim Sci. 1988;38:25–33. [PubMed] [Google Scholar]
- 25.Flynn JL, Klein E. Pulmonary tuberculosis in monkeys. In: Leong FJ, Dartois V, Dick T, editors. A Color Atlas of Comparative Pulmonary Tuberculosis Histopathology. New York: Taylor & Francis; 2011. pp. 83–106. [Google Scholar]
- 26.Lin PL, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74:3790–3803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.