Abstract
Many parasitic nematodes actively seek out hosts in which to complete their lifecycles. Olfaction is thought to play an important role in the host-seeking process, with parasites following a chemical trail toward host-associated odors. However, little is known about the olfactory cues that attract parasitic nematodes to hosts or the behavioral responses these cues elicit. Moreover, what little is known focuses on easily obtainable laboratory hosts rather than on natural or other ecologically relevant hosts. Here we investigate the olfactory responses of six diverse species of entomopathogenic nematodes (EPNs) to seven ecologically relevant potential invertebrate hosts, including one known natural host and other potential hosts collected from the environment. We show that EPNs respond differentially to the odor blends emitted by live potential hosts as well as to individual host-derived odorants. In addition, we show that EPNs use the universal host cue CO2 as well as host-specific odorants for host location, but the relative importance of CO2 versus host-specific odorants varies for different parasite–host combinations and for different host-seeking behaviors. We also identified host-derived odorants by gas chromatography-mass spectrometry and found that many of these odorants stimulate host-seeking behaviors in a species-specific manner. Taken together, our results demonstrate that parasitic nematodes have evolved specialized olfactory systems that likely contribute to appropriate host selection.
Keywords: entomopathogens, chemosensation, Heterorhabditis, Steinernema
Many parasitic nematodes actively seek out hosts using sensory cues (1). Host seeking is a complex behavior that involves chemosensory, thermosensory, hygrosensory, and mechanosensory cues (1–4). Olfaction is a critical component of host-seeking behavior: Many parasitic nematodes use CO2 and other host volatiles for host location (1, 2, 5–8). However, little is known about how parasites respond to host-derived odors.
Entomopathogenic nematodes (EPNs) are powerful models for the study of odor-driven host-seeking behavior. EPNs comprise a guild—a group of phylogenetically divergent species that exploit the same class of resources in a similar way (9)—that includes the genera Heterorhabditis, Steinernema, and Oscheius (10, 11). EPNs are parasites of insects that infect and kill insect larvae (10, 11). They offer a number of advantages as model systems, including small size, short generation time, and amenability to laboratory culturing and behavioral analysis (12, 13). In addition, they resemble skin-penetrating human-parasitic nematodes in that they actively seek out hosts using olfactory cues (2, 7, 13–16). EPNs also are of interest as biocontrol agents for insect pests and disease vectors and currently are used throughout the world as environmentally safe alternatives to chemical insecticides. The three genera of EPNs are phylogenetically distant but have highly similar lifestyles as a result of convergent evolution to insect parasitism (17).
EPNs are thought to engage in host-seeking behavior only during a particular life stage called the “infective juvenile” (IJ), a developmentally arrested third larval stage analogous to the dauer stage of some free-living worms (18). After long-range host location, IJs are thought to use short-range sensory cues for host recognition (19). IJs then infect the host either by entering through natural orifices or by penetrating through the insect cuticle (20). Following infection, IJs release a bacterial endosymbiont into the insect host and resume development (21–23). The bacteria proliferate inside the insect, producing an arsenal of secondary metabolites that lead to rapid insect death and digestion of insect tissues. The nematodes feed on the multiplying bacteria and the liberated nutrients of broken-down insect tissues. They reproduce in the cadaver until resources are depleted, at which time new IJs form and disperse in search of new hosts (24).
EPNs use a wide range of host-seeking strategies. Some are “cruisers” that actively seek out hosts, whereas others are “ambushers” that remain stationary and infect passing hosts. However, these strategies represent endpoints along a continuum, and many species are “intermediates” that are capable of using both cruising and ambushing strategies for host location (25, 26). In addition, some EPNs of the genus Steinernema exhibit jumping, a rare behavior among soft-bodied, limbless organisms (27, 28). Among EPNs, jumping is a highly specialized ambushing behavior in which the IJ propels itself into the air (13, 27, 29). Jumping is thought to be a short-range host-seeking strategy that facilitates attachment to the host when the host is in close proximity (27, 30, 31). In general, cruisers are most effective at infecting stationary hosts, whereas ambushers are most effective at infecting fast-moving hosts (32). Previous studies have demonstrated that EPNs are attracted to CO2 as well as to a number of other odorants (13–15, 33–35). However, little is known about how EPNs respond to host odors or how olfactory responses contribute to differences in host-seeking strategy.
Here, we show that EPNs respond differently to different potential hosts and host-derived odorants and that olfactory responses differ even for closely related EPNs. We also identify host-derived odorants that stimulate host-seeking behaviors in a species-specific manner. Our results suggest that parasitic nematodes have specialized olfactory systems that contribute to differences in host preference and host-seeking strategy among species.
Results
We examined the odor-evoked host-seeking behaviors of six different EPNs in response to seven potential invertebrate hosts. The EPNs—Heterorhabditis bacteriophora, Steinernema carpocapsae, Steinernema scapterisci, Steinernema riobrave, Steinernema glaseri, and Oscheius carolinensis—were chosen on the basis of both phylogenetic and behavioral diversity (Fig. S1). These species vary greatly in their host-seeking strategies: H. bacteriophora and S. glaseri are cruisers, S. carpocapsae and S. scapterisci are ambushers, and S. riobrave employs an intermediate host-seeking strategy. In addition, S. carpocapsae, S. scapterisci, and S. riobrave display jumping as well as chemotaxis behavior. The host-seeking behavior of O. carolinensis, a recently discovered EPN and the closest known EPN relative of C. elegans (21), has not yet been characterized.
These six EPN species also were chosen because of their differing host ranges. H. bacteriophora and S. carpocapsae are thought to have very broad host ranges, with S. carpocapsae capable of infecting more than 250 different species of insects from 13 orders under laboratory conditions (36, 37). By contrast, S. scapterisci is an orthopteran specialist with a much narrower host range than most EPNs; its only known natural host is the mole cricket (38–40). S. glaseri has a somewhat broader host range; it is capable of infecting insects in several orders but is thought to prey primarily on sedentary subterranean larvae, such as those of beetles (36, 41, 42). S. riobrave has not been tested as thoroughly, but it is presumed to have a fairly broad host range, and it has been used successfully as a biocontrol agent against both lepidopteran and coleopteran hosts (43, 44). The host range of O. carolinensis has not yet been tested (45). Little is known about the natural hosts of EPNs. Of the six EPN species used in this study, natural hosts are known for H. bacteriophora, S. carpocapsae, S. scapterisci, and S. glaseri and are Heliothis punctigera (Lepidoptera: Noctuidae) (46), Cydia pomonella (Lepidoptera: Nocteuidae) (47), Scapteriscus vicinus and Scapteriscus borellii (Orthoptera: Gryllotalpidae) (38, 48), and Popillia japonica (Coleoptera: Scarabaeidae) (49), respectively. Whether these species represent true natural hosts or merely opportunistic hosts remains unclear except for S. scapterisci, which has been used successfully for decades to control invasive species of mole crickets (40).
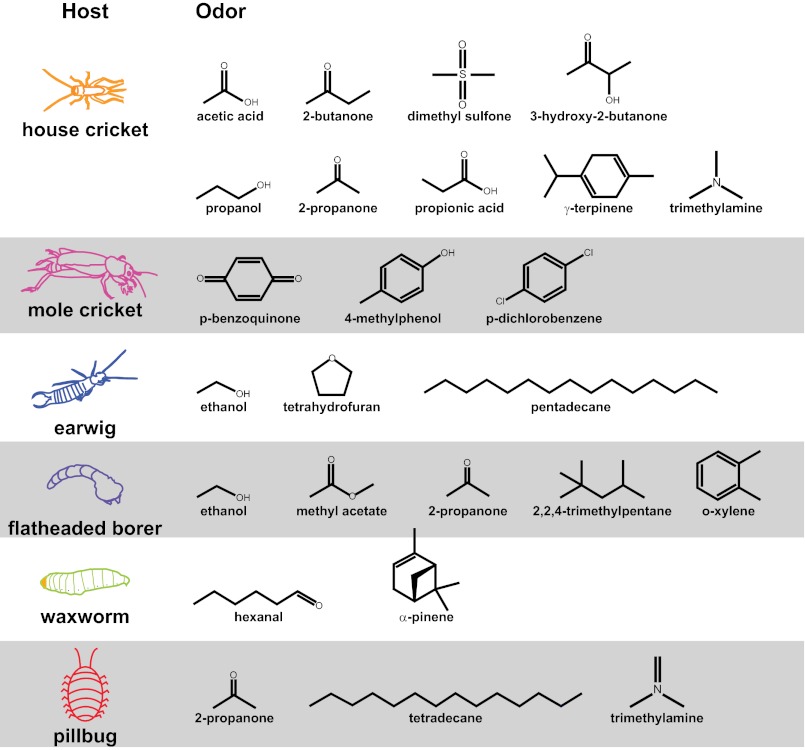
The seven potential invertebrate hosts—the mole cricket Scapteriscus borellii, the house cricket Acheta domesticus, the earwig Euborellia femoralis, the waxworm Galleria mellonella, the flatheaded borer Chrysobothris mali, the pillbug Armadillidium vulgare, and the slug Lehmannia valentiana—also were chosen based on their phylogenetic and ecological diversity (Fig. 1A). Mole crickets are the only known natural host for S. scapterisci (40), and house crickets are related to mole crickets and can serve as laboratory hosts for both S. scapterisci and S. carpocapsae (50). Earwigs were chosen because some earwig species are thought to be preferred natural hosts for S. carpocapsae (37). Waxworms were selected because they are a common laboratory host for EPNs and typically are used as bait when collecting EPNs from soil; thus, many described EPNs are attracted to waxworms, even in complex soil environments (42, 51). However, waxworms are damaging residents of beehives and are not likely to encounter soil-dwelling EPNs under natural conditions. Similarly, larval flatheaded borers are not likely to be encountered by EPNs, because they develop under the bark in the phloem of host plants (52). They represent nonnatural but potential hosts of EPNs, ones that EPNs have not evolved to find or infect. In contrast, pillbugs and slugs are noninsects that are similar in size to many potential insect hosts of EPNs and often are in the same or overlapping communities with EPNs. Pillbugs belong to the same phylum as insects (Arthropoda) but to a different order (Isopoda); slugs belong to a different phylum (Mollusca) and are much more distantly related to insects. Both pillbugs and slugs have been explored as potential alternative hosts for EPNs and found to be nonhosts or dead-end hosts for several EPNs (53–57); however, the potential for EPNs to use isopods and gastropods as alternative or reservoir hosts when insects are scarce has not been explored fully, and whether EPNs display any behavioral preference for isopods and gastropods had not yet been tested. Mole crickets, earwigs, flatheaded borers, pillbugs, and slugs were collected from their natural habitats in the greater Los Angeles area and were tested within a few weeks of collection (Fig. S2).
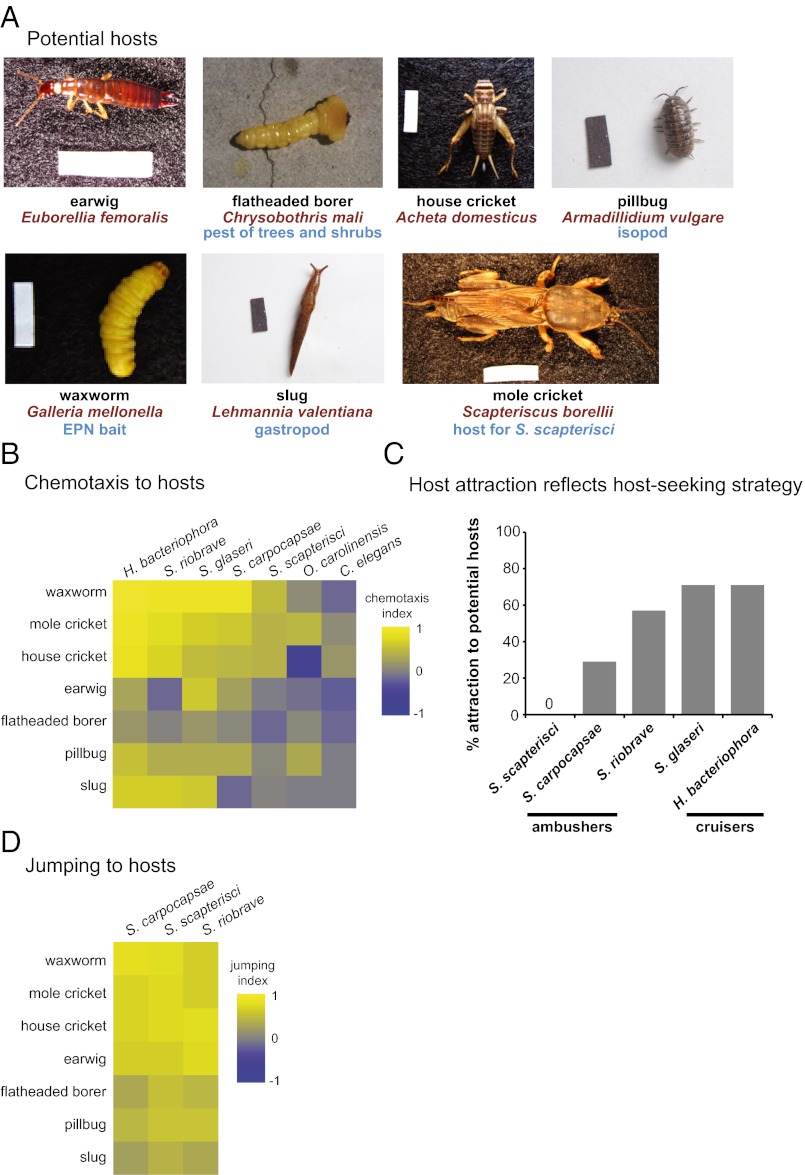
Fig. 1.
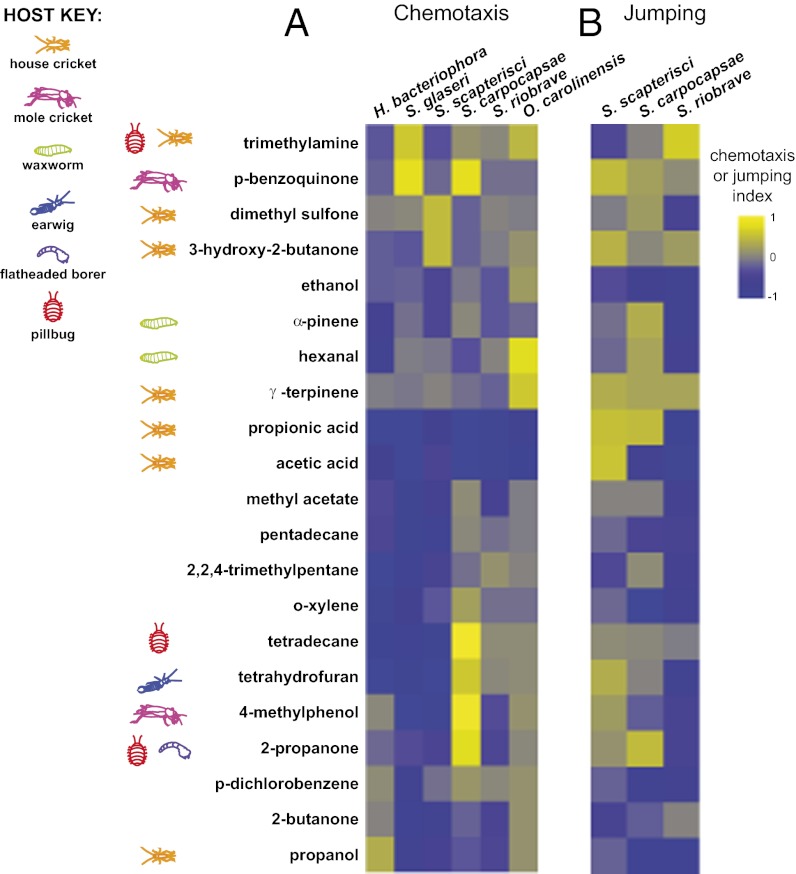
EPNs respond differently to different potential hosts. (A) Potential invertebrate hosts tested. Mole crickets, earwigs, flatheaded borers, pillbugs, and slugs were collected from the greater Los Angeles area. Waxworms and house crickets were purchased commercially. (Scale bars: 1 cm × 2.5 mm.) (B) Chemotaxis of EPN IJs and C. elegans dauers to volatiles released by live potential hosts. The order of both the nematodes and the hosts in the heatmap was determined by hierarchical cluster analysis (Ward’s method). EPNs respond differently to different hosts (P < 0.0001), different hosts evoke different overall responses from EPNs (P < 0.0001), and different EPNs show different odor–response profiles (P < 0.0001; two-factor ANOVA with replication, with a Bonferroni posttest). n = 6–30 trials for each EPN-host combination. Mean, n, and SEM values for each assay are given in Dataset S1; P values for each posttest are given in Datasets S2 and S3. (C) Chemotaxis behavior reflects host-seeking strategy, so that cruisers display more overall attraction to hosts than do ambushers. The y-axis indicates the percentage of hosts that were strongly attractive (as defined by a chemotaxis index of ≥0.5). S. scapterisci and S. carpocapsae are cruisers, S. glaseri and H. bacteriophora are ambushers, and S. riobrave employs both cruising and ambushing strategies for host seeking. The responses of the ambushers S. scapterisci and S. carpocapsae cluster separately from the responses of the cruisers S. glaseri and H. bacteriophora and the ambusher/cruiser S. riobrave by k-means cluster analysis and hierarchical cluster analysis (Ward’s method, cophenetic correlation = 0.85). (D) Jumping of EPNs in response to volatiles released by live potential hosts. The order of the nematodes in the heatmap was determined by hierarchical cluster analysis (Ward’s method); the order of the hosts is the same as in B. EPNs respond differently to different hosts (P < 0.0001), and different hosts evoke different overall responses from EPNs (P < 0.0001) (two-factor ANOVA with replication, with a Bonferroni posttest). However, different EPNs do not show significantly different odor–response profiles (two-factor ANOVA with replication). n = 2–13 trials for each EPN–host combination. Mean, n, and SEM values for each assay are given in Dataset S1; P values for each posttest are given in Datasets S4 and S5. In B and D, response magnitudes are color-coded so that a chemotaxis index or jumping index of +1 is yellow, −1 is blue, and 0 is gray.
EPNs Respond Differently to Different Host Odors.
We examined EPN responses to odors emitted from live hosts using both chemotaxis and jumping assays (13). We found that all six EPNs responded significantly more to some potential hosts than others, and some potential hosts were significantly more attractive overall than others (Fig. 1B and Datasets S1–S3). In addition, odor–response profiles differ for the different EPNs, so that some hosts are more attractive to some EPNs than to others (Fig. 1B and Datasets S1–S3). Overall, we found that host attraction reflects host-seeking strategy such that cruisers showed more robust attraction to live hosts than ambushers in our chemotaxis assay (Fig. 1C). Thus, the host-seeking behavior of EPNs likely reflects their ability to respond differentially to odors emitted by different potential hosts. For comparison, we also examined the responses of Caenorhabditis elegans dauers to the potential host odors; the Hawaii strain was used for this comparison because it most closely resembles wild C. elegans strains (58). We found that all the invertebrate odors were neutral or repulsive (chemotaxis index < 0.2) for C. elegans dauers (Fig. 1B and Dataset S1). Thus, the host attraction we observe is specific to the EPNs.
Jumping behavior in response to potential hosts also varied for different EPNs and different hosts (Fig. 1D and Datasets S1, S4, and S5). EPNs showed significantly higher rates of jumping in response to some potential hosts than to others, and some potential hosts evoked significantly higher rates of jumping overall than others (Fig. 1D and Datasets S1, S4, and S5). However, the three jumping EPN species did not show species-specific jumping profiles: The relative responses elicited by the different potential hosts did not vary significantly across species (Fig. 1D and Datasets S1, S4, and S5). These results suggest that chemotaxis behavior may display more species specificity than jumping behavior.
EPNs Vary in Their Virulence Toward Potential Hosts.
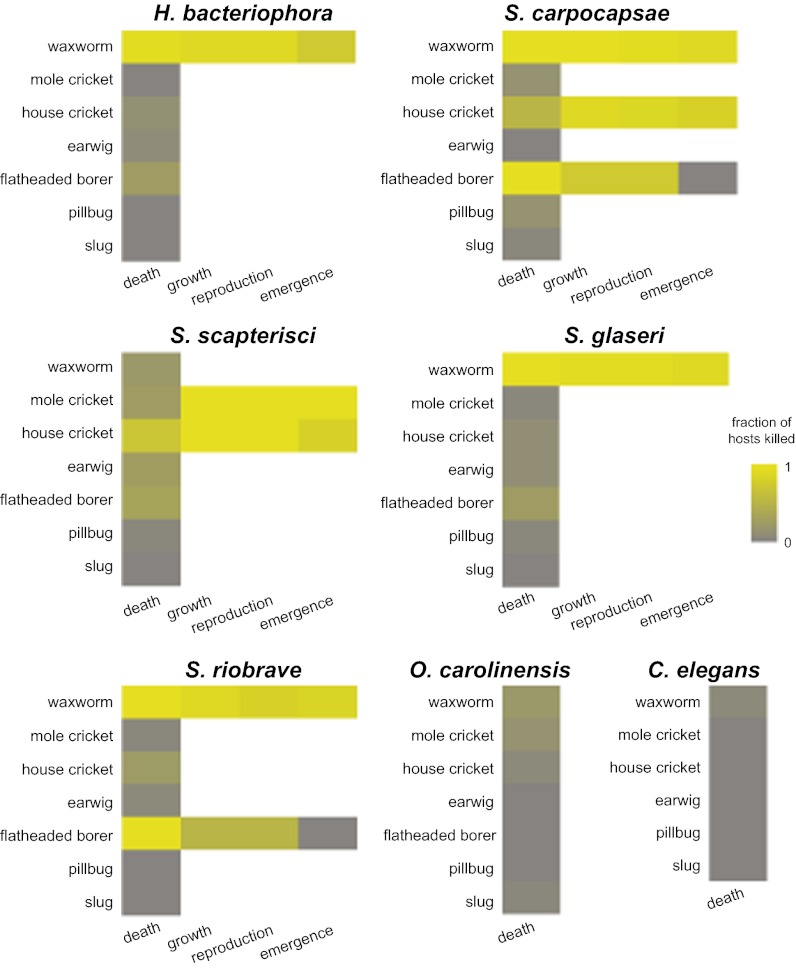
We then tested the virulence, i.e., the disease-producing power (59), of the six different EPNs toward the seven potential hosts. EPN virulence usually is tested by exposing potential hosts to a defined number of IJs (typically between 1 and 1,000 per potential host) (54, 60, 61). Previous work suggests that using high doses of IJs in mortality experiments allows poor host suitability to be overcome by the high number of parasites (35). Therefore, in our virulence assays, individual host animals were exposed to 100 IJs, and host survival was scored after 48 h. When the EPNs successfully killed the host, we subsequently scored EPN growth, reproduction, and emergence from host cadavers. We found that EPN virulence varied greatly among species (Fig. 2 and Dataset S6). For example, S. carpocapsae was virulent toward three of the seven species tested, whereas O. carolinensis was not virulent toward any of these species at the concentration of IJs tested. Overall, we found that waxworms are very efficient hosts for most EPNs: All species except S. scapterisci and O. carolinensis were highly successful in parasitizing waxworms. This result could reflect the proclivity of these species to infect lepidopteran hosts or the isolated environment of larval waxworms; as pests of beehives, they are unlikely to have evolved behavioral and immune defenses against soil-dwelling EPNs. It could also reflect unintentional laboratory selection toward virulence in waxworms, because most of these species have been maintained in waxworms after being collected from the wild. As expected, we found that S. scapterisci was most virulent toward crickets. In our assay, S. scapterisci was not as efficient at killing its natural host, the mole cricket, as it was at killing the house cricket: Only 25% of mole crickets were killed, compared with 71% of house crickets. However, the mole crickets that were killed successfully were the most effective hosts: 100% of the mole cricket cadavers supported S. scapterisci growth, reproduction, and emergence (Fig. 2 and Dataset S6). We note that S. scapterisci has been shown to be extremely effective at killing both house crickets and mole crickets at higher IJ densities than we tested here (39). Flatheaded borers proved to be dead-end hosts for both S. carpocapsae and S. riobrave: Although the EPNs could infect borers and in some cases could grow and reproduce inside borer cadavers, emergence of IJs from borer cadavers was never observed (Fig. 2 and Dataset S6). None of the EPNs was able to kill earwigs, pillbugs, or slugs successfully in our assay (Fig. 2 and Dataset S6). Thus, at this inoculum (100 IJs per host), EPNs differ in their range of hosts.
Fig. 2.
EPNs differ in their virulence toward potential hosts. Graphs show the virulence of each nematode toward the panel of potential hosts. Values for “death” represent the fraction of hosts that died within 48 h following exposure to nematodes. Values for “growth,” “reproduction,” and “emergence” represent the fraction of dead hosts that supported nematode growth, reproduction, and emergence, respectively. The frequency of death following exposure to nematodes was scored for all potential hosts; growth, reproduction, and emergence were scored only when host killing was observed at statistically significant levels. Each virulence assay consisted of a single potential host and 100 IJs. n = 20–50 assays for all invertebrates except flatheaded borers; n = 8–12 assays for flatheaded borers because of the limited availability of these insects. For each EPN–host combination, statistical significance was determined relative to an uninfected control using a χ2 test. Mean values for death, growth, reproduction, and emergence are given in Dataset S6.
CO2 Is a Host-Seeking Cue for Both Generalist and Specialist EPNs.
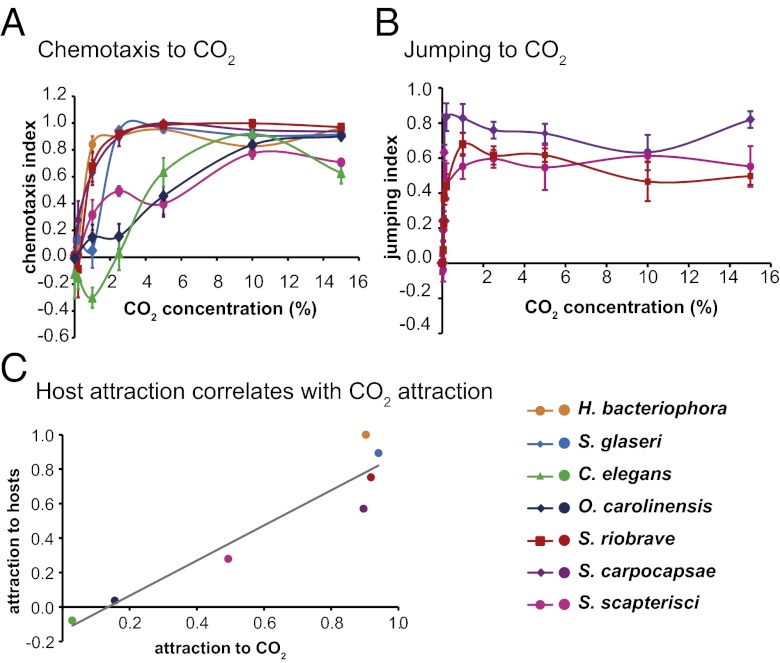
We then examined the host-derived odorants that stimulate host-seeking behavior. We first examined responses to CO2, which is emitted by all animals as a byproduct of respiration and is a host cue for a wide range of parasites, including many types of parasitic nematodes (2, 8, 62). To examine the chemotactic response to CO2, we used a CO2 chemotaxis assay in which worms were allowed to distribute on a plate in a CO2 concentration gradient (13). We found that all the tested EPNs are attracted to CO2 (Fig. 3A and Dataset S7), and all three of the jumping species jumped in response to CO2 (Fig. 3B and Dataset S7). However, the attractiveness of CO2 varied among EPNs, with S. scapterisci and O. carolinensis showing less attraction to low concentrations of CO2 than the other species (Fig. 3A and Dataset S7). Responses to low CO2 concentrations were highly correlated with overall host attraction, suggesting that differences in overall host attraction may be attributable to differences in CO2 sensitivity among EPNs (Fig. 3C). Thus, CO2 is an important host-seeking cue for both specialist and generalist EPNs.
Fig. 3.
CO2 stimulates host-seeking behavior of EPNs. (A) Chemotaxis of EPN IJs and C. elegans dauers to CO2. n = 5–23 trials. Data for H. bacteriophora and S. carpocapsae are from Hallem et al. (13). (B) Jumping of EPNs to CO2. n = 2–7 trials. (C) Host attraction correlates with CO2 attraction. The x-axis indicates the chemotaxis index in response to 2.5% CO2; the y-axis indicates the normalized sum of the chemotaxis indices toward all hosts. The best-fit linear trendline is shown. R2 = 0.90. Mean, n, and SEM values for each assay are given in Dataset S7.
Requirement for CO2 Varies for Different EPN–Host Combinations.
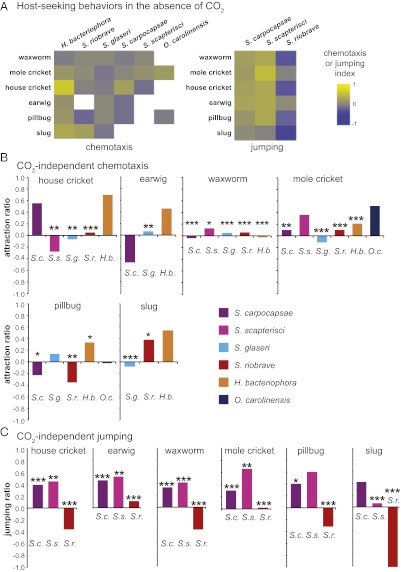
To test whether CO2 is required for host attraction, we assayed the response to live hosts in the presence of soda lime, which removes CO2 (13). We found that for all EPN–host combinations, chemotaxis was reduced in the absence of CO2 (Fig. 4A and Datasets S1 and S8). However, the extent of the reduction varied greatly for different EPNs and different hosts. For example, none of the EPNs were attracted to waxworms in the absence of CO2, whereas mole crickets, house crickets, and earwigs were still attractive to some EPNs but not to others (Fig. 4B and Dataset S8). Removal of CO2 did not render any hosts significantly repulsive (chemotaxis index ≤ −0.2) (Fig. 4A). Host-evoked jumping also was reduced in the absence of CO2, and, as with chemotaxis, the requirement for CO2 differed for different EPN–host combinations (Fig. 4 A and C and Datasets S1 and S9). Thus, although CO2 is sufficient for eliciting host-seeking behavior from all EPNs, it is both necessary and sufficient for some EPN-host combinations but not for others. To test further the role of CO2 versus host-specific odors in host seeking, we performed a chemotaxis competition experiment with S. carpocapsae in which CO2 was introduced into one side of the chemotaxis plate and odor from a single mole cricket was introduced into the other side (Fig. S3). We found that S. carpocapsae prefers live mole crickets to 1% CO2 (Fig. S3), even though 1% CO2 is highly attractive to S. carpocapsae and attraction of S. carpocapsae to mole crickets is reduced greatly in the absence of CO2 (Fig. 4A). However, higher concentrations of CO2 are more attractive than mole crickets (Fig. S3). These results demonstrate that EPNs use both CO2 and host-specific odorants for host location.
Fig. 4.
Host-seeking behavior is reduced in the absence of CO2. (A) Chemotaxis to live hosts is reduced significantly when CO2 is removed from the host airstream using soda lime (P < 0.0001 for all species except O. carolinensis; P < 0.05 for O. carolinensis; two-factor ANOVA with replication). Chemotaxis with CO2 removed was tested only for EPN–host combinations in which host attraction was observed initially. Jumping to live hosts also is reduced when CO2 is removed from the host airstream using soda lime (P < 0.001, two-factor ANOVA with replication). n = 6–22 trials for chemotaxis and two to seven trials for jumping for each EPN–host combination. (B) Levels of CO2-independent attraction to potential hosts. Attraction ratios indicate the chemotaxis index for host attraction with CO2 removed divided by the chemotaxis index for host attraction with CO2. (C) Levels of CO2-independent jumping to potential hosts. Jumping ratios indicate the jumping index for host-evoked jumping with CO2 removed divided by the jumping index for host-evoked jumping with CO2. In B and C, asterisks indicate cases where the response to host with CO2 removed was significantly different from the response to host with CO2 present. ***P < 0.001; **P < 0.01; *P < 0.05, two-factor ANOVA with replication with a Bonferroni posttest. Mean, n, and SEM values for each assay in A are given in Dataset S1; P values for each posttest are given in Datasets S8 and S9.
Diverse Array of Host-Derived Odorants Stimulates Host-Seeking Behaviors.
We next identified host-derived odorants that elicit host-seeking behavior. We previously used thermal desorption-gas chromatography-mass spectrometry (TD-GC-MS) to identify odorants emitted by waxworms and house crickets (13). We now have extended this analysis to all seven potential invertebrate hosts using TD-GC-MS and solid-phase microextraction-gas chromatography-mass spectrometry (SPME-GC-MS) (63). Overall, we identified 21 odorants emitted consistently and at relatively high abundance by the potential hosts (Fig. 5 and Fig. S4). (One of these odorants, p-dichlorobenzene, is a common pesticide that is unlikely to be insect derived.) The number of odorants we identified from each invertebrate ranged from nine for house crickets to two for waxworms to zero for slugs (Fig. 5). The fact that we identified more odorants from crickets than from waxworms is consistent with our finding that crickets evoke higher levels of CO2-independent attraction than waxworms (Fig. 4B) and suggests that the relative contributions to host seeking by CO2 versus host-specific odorants may depend, in part, on the number of odorants the host emits.
Fig. 5.
Host-derived odorants identified by TD-GC-MS and SPME-GC-MS. Each listed odorant was identified in at least two different experimental replicates at a relative abundance of ≥20,000 and with library matches of at least 95% confidence. Odorants identified from earwigs, flatheaded borers, and pillbugs and 2-propanone identified from house crickets were identified by SPME-GC-MS; all other odorants were identified by TD-GC-MS.
We then examined the behavioral responses to these odorants and found that many odorants strongly stimulated host-seeking behaviors (Fig. 6 and Dataset S10). Overall, we observed strong responses to at least one odorant identified from each of the tested invertebrates (with the exception of slugs, for which we did not successfully identify any odorants), suggesting that a wide variety of chemically diverse olfactory cues contribute to host-seeking behavior. The odorants that stimulated the strongest host-seeking responses differed for the different species; for example, 2-propanone, 4-methylphenol, and tetradecane were strongly attractive for S. carpocapsae but were repulsive or neutral for the other species (Fig. 6 and Dataset S10). In addition, all EPNs displayed unique chemotaxis and jumping odor–response profiles to host-derived odorants, with the exception of S. riobrave and O. carolinensis, whose chemotaxis odor–response profiles did not differ significantly (Fig. 6 and Datasets S11 and S12). Thus, most EPNs display species-specific responses to host-derived odorants.
Fig. 6.
EPN host-seeking behavior is stimulated by a wide variety of host-derived odorants. (A) Chemotaxis of EPNs to host-derived odorants. The order of both the nematodes and odorants in the heatmap was determined by hierarchical cluster analysis (Ward’s method). EPNs respond differently to different host-derived odorants (P < 0.001, two-factor ANOVA with replication). EPNs also displayed unique odor–response profiles (P < 0.05, two-factor ANOVA with replication, with a Bonferroni posttest), with the exception of S. riobrave and O. carolinensis, which did not differ from each other significantly. n = 4–10 trials for each EPN–odorant combination. Data for H. bacteriophora and S. carpocapsae responses to acetic acid, 2-butanone, dimethyl sulfone, ethanol, hexanal, 3-hydroxy-2-butanone, methyl acetate, α-pinene, propanol, propionic acid, γ-terpinene, and trimethylamine are from Hallem et al. (13). Mean, n, and SEM values for each assay are given in Dataset S10; P values for each posttest are given in Dataset S11. (B) Jumping of EPNs to host-derived odorants. The order of nematodes in the heatmap was determined by hierarchical cluster analysis (Ward’s method); the order of the odorants is as in A. EPNs respond differently to different host-derived odorants (P < 0.0001, two-factor ANOVA with replication), and the three species display unique jumping odor–response profiles (P < 0.001). n = 2–11 trials for each EPN–odorant combination. Mean, n, and SEM values for each assay are given in Dataset S10; P values for each posttest are listed in Dataset S12.
In the case of the cricket specialist S. scapterisci, we found that all the odorants that elicited a strong response (as defined by a chemotaxis or jumping index of ≥0.5) were cricket derived, and 7 of the 11 cricket-derived odorants elicited a positive chemotactic or jumping response (as defined by a chemotaxis or jumping index of ≥0.2). Thus, the odor–response profile of S. scapterisci appears to reflect its specialized host range.
Dose–response analysis indicated that, for chemotaxis behavior, most odorants were consistent attractants or repellants across concentrations (Fig. S5A and Dataset S13). The one exception was acetic acid, which was repulsive to S. carpocapsae at high concentrations but attractive at lower concentrations (Fig. S5A and Dataset S13). Jumping behavior was more dynamic across concentrations. One odorant, trimethylamine, inhibited S. scapterisci jumping at high concentrations but stimulated it at low concentrations; other odorants such as p-benzoquinone stimulated S. carpocapsae and S. scapterisci jumping at high concentrations but inhibited it at low concentrations (Fig. S5B and Dataset S14). These results suggest that EPNs may use olfactory cues to encode information about host proximity as well as host identity.
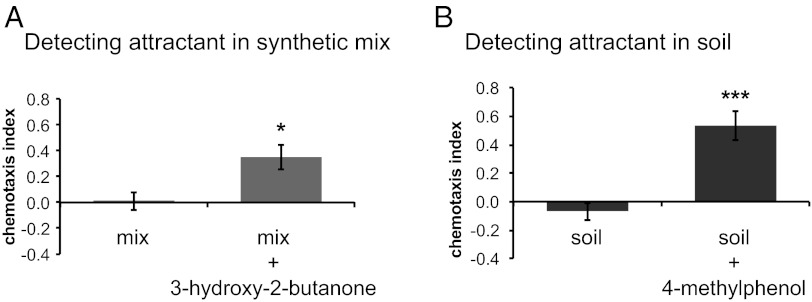
To explore further the role of host-specific odors in EPN host-seeking behavior, we examined the responses to attractive host-derived odorants in the presence of either a neutral mixture of host-derived odorants (i.e., odorants that we identified from hosts but that did not elicit a response when tested individually) (Fig. 6) or soil odor. We found that host-derived odorants that attracted EPNs when tested individually still were attractive in the presence of both the neutral odorant mixture and the soil odor (Fig. 7). Thus, EPNs can detect and respond to host-derived odorants even in the presence of other unrelated olfactory cues. These results suggest that EPNs are likely to use olfactory cues for host seeking even in complex soil environments.
Fig. 7.
EPNs detect and respond to host-derived odorants in the presence of complex odor mixtures. (A) Response of S. scapterisci IJs to a 10−1 dilution of the cricket-derived odorant 3-hydroxy-2-butanone in the presence of a synthetic mix containing 10−1 dilutions of hexanal, γ-terpinene, and p-dichlorobenzene. The left bar represents the response to the synthetic mix vs. a paraffin oil control. The right bar represents response to the synthetic mix vs. the synthetic mix with 3-hydroxy-2-butanone added. n = 6–9 trials for each condition. The response to the synthetic mix with 3-hydroxy-2-butanone added was significantly different from the response to the synthetic mix alone (P < 0.05, unpaired t test). (B) Response of S. carpocapsae IJs to 4-methylphenol in the presence of soil odor. The left bar represents the response to soil odor vs. an air control. The right bar represents response to 4-methylphenol plus soil odor vs. soil odor alone. n = 6 trials for each condition. The response to 4-methylphenol plus soil odor was significantly different from the response to soil odor alone (P < 0.001, unpaired t test). In addition, the response to 4-methylphenol in the presence of soil odor was not significantly different from the response to 4-methylphenol in the absence of soil odor (unpaired t test). Mean, n, and SEM values for each assay are given in Dataset S10.
Discussion
Heterorhabditis, Steinernema, and Oscheius are phylogenetically distant genera of EPNs that convergently have evolved similar entomopathogenic lifestyles. The entomopathogenic lifestyle is highly specialized: EPNs locate and infect insect larval hosts, deposit their bacterial symbiont into the host, rapidly kill the host, and then resume normal development (11). The convergence of three separate genera in the EPN guild therefore is a striking example of adaptive plasticity among nematodes. Our results demonstrate that even closely related EPNs display different odor–response profiles, raising the possibility that olfaction contributes to this adaptive plasticity.
Overall, we found that chemotaxis behaviors exhibit more species specificity than jumping behaviors. For example, the relative attractiveness of different potential hosts in a chemotaxis assay varied for different EPN species (Fig. 1B). By contrast, all the jumping species tested displayed the same relative host preferences; i.e., hosts that evoked higher levels of jumping for one species also evoked higher levels of jumping for the other species, and the reverse was also true (Fig. 1D). We also observed that odorants did not always stimulate equivalent responses for jumping and chemotaxis, indicating that these behaviors are controlled by different chemosensory cues and therefore may serve different functions in the host-seeking process. The evolution of jumping behavior likely played a major role in niche partitioning among EPNs, because jumping ambushers are found primarily in epigeal (soil–air interface) habitats, whereas cruisers often are found deeper in the soil column (64). However, our results suggest that odor-driven chemotaxis behavior may have played a more important role than odor-driven jumping behavior in further partitioning of the epigeal niche among jumping species. This suggestion is consistent with the possibility that jumping is a less specific short-range host-seeking strategy that facilitates rapid attachment to nearby hosts at the expense of specificity, whereas chemotaxis before jumping and tactile or other cues subsequent to jumping are used for host discrimination. However, it is possible that jumping also can be used as a long-range strategy for rapid movement toward potential hosts.
S. scapterisci is the only tested species known to have a narrow host range and for which a natural host, the mole cricket, has been convincingly demonstrated (38–40). We found that the olfactory responses of S. scapterisci reflect its host range: S. scapterisci IJs showed the highest virulence to orthopteran hosts and appeared to respond primarily to crickets and cricket-derived odorants (Figs. 1, 2, and 6). In addition, we found that S. scapterisci showed a reduced response to low concentrations (≤1%) of CO2 compared with most EPNs in a chemotaxis assay but not in a jumping assay (Fig. 3), and the response of S. scapterisci to mole crickets in a chemotaxis assay was not significantly different when CO2 was removed from the host airstream (Fig. 4A and Dataset S8). Thus, S. scapterisci may rely more than generalist EPN species on host-specific cues and less on CO2 for long-range host seeking. In addition, we found that S. scapterisci was attracted to the cricket-derived odorant 3-hydroxy-2-butanone even in the presence of a mixture of other odorants (Fig. 7A), suggesting that S. scapterisci is capable of responding to cricket-derived odorants even in complex odor environments. Taken together, our results suggest an important role for olfaction in the evolution of host specificity for S. scapterisci.
The lack of overlap in the odorants identified from the two cricket species (Fig. 5) suggests either that S. scapterisci uses different olfactory cues to locate the different species or that S. scapterisci relies on low-abundance odorants common to multiple cricket species that were not included in this study. However, we note that the odorant dimethyl sulfone, which we identified as a house cricket-derived odorant, also was identified from mole crickets but did not meet our stringent criteria for inclusion in our analysis (Fig. S4). Dimethyl sulfone elicited behavioral responses from S. scapterisci even at low concentrations (Fig. S5A), suggesting it may be an important orthopteran host-seeking cue.
O. carolinensis showed the lowest levels of host attraction in our assays, and, like S. scapterisci, the attraction of O carolinensis to CO2 declined at concentrations around 1% (Figs. 1B and 3A). O. carolinensis is one of two recently described EPNs in the genus Oscheius; these species are thought to have evolved an entomopathogenic lifestyle more recently than Heterorhabditis and Steinernema species (11, 21, 65). Thus, the olfactory system of O. carolinensis may be less highly specialized for insect parasitism than those of the more anciently evolved EPNs. It also is possible that none of the seven hosts tested are natural or preferred hosts for O. carolinensis. In support of this possibility, the closely related species Oscheius necromenus is associated with millipedes, which are noninsect arthropods in the class Diplopoda (66, 67).
Our virulence assays revealed that all EPNs, even those with very broad host ranges such as S. carpocapsae, are able to infect some insects better than others (Fig. 2). Thus, virulence varies greatly for different EPN–host combinations. However, we note that the number of IJs to which hosts are exposed is positively correlated with both the number of nematodes entering the host and the number of resultant infections (68). Many EPNs are capable, at high doses, of infecting a wide variety of insect larvae and even some noninsect invertebrates (61, 69–71). Thus, it is likely that at least some of the potential hosts we tested that appeared resistant to EPN infection can serve as hosts if exposed to a high enough concentration of IJs. We also note that host efficiency is determined not only by the rate of host killing but also by the level of reproduction supported by the host (35), and reproduction levels were not tested here.
A comparison of host virulence with host-evoked chemotaxis and jumping behaviors revealed that some EPNs are attracted to invertebrate species that are not effective hosts (Figs. 1 and 2). This finding is consistent with the observation that EPNs can engage in phoresy—a relationship in which nematodes use an organism for transportation to new environmental niches—with both nonhost insects and noninsect invertebrates such as isopods and earthworms (72–74). Attraction to nonhosts in the absence of hosts may offer a survival advantage to EPNs by facilitating dispersal to more favorable environmental niches. It also is possible that in some cases olfactory preferences can lead EPNs to pursue nonhosts or dead-end hosts. Host selection is a complex process that can be broken down into multiple steps, including host location, host attachment, host recognition, and host penetration (19, 57). Host attraction is only one component of this process, and other behaviors such as those that mediate host recognition and penetration may prevent the fatal decision to infect an inappropriate host. We note that the gastropod-parasitic nematode Phasmarhabditis hermaphrodita, which is in the Rhabditid family and is closely related to C. elegans, H. bacteriophora, and O. carolinensis, also displays host-seeking behavior toward various species of gastropods (75–77).
In addition to examining responses to live hosts, we examined responses to CO2 and other host-derived odorants. We found that all EPNs tested are attracted to CO2 and that CO2 sensitivity is positively correlated with overall host attraction (Fig. 3). Thus, CO2 is a critical host-seeking cue for EPNs regardless of host-seeking strategy or host range. However, the importance of CO2 as a host-seeking cue varies for different hosts. For example, CO2 appears to be more important for attraction to waxworms than to crickets: Waxworms were no longer attractive to any of the EPNs in the absence of CO2, but crickets were still attractive to some but not all EPNs (Fig. 4). In addition, S. carpocapsae preferred mole cricket odor to 1% CO2 in a competition chemotaxis assay, demonstrating that at least some live hosts are more attractive than low concentrations of CO2 alone (Fig. S3). The importance of CO2 also varies for different EPNs. For example, in the absence of CO2, S. riobrave responded to slugs only, and, in fact, host-evoked chemotaxis and jumping were suppressed in many cases in the absence of CO2 (Fig. 4). Consistent with the reliance of S. riobrave on CO2, we did not identify any host-derived odorants that were strong attractants for S. riobrave, and we identified only one host-derived odorant that strongly stimulated jumping (Fig. 6). These results suggest that EPNs differ in the extent to which their olfactory systems have evolved to mediate specific host–parasite interactions: Some EPNs rely primarily on CO2 for host location, but others use CO2 in combination with host-specific odorants. We also found that at least some EPNs are attracted to host-specific odorants even in the presence of complex mixtures (Fig. 7), further confirming an important role for host-specific odorants in host location.
EPNs inhabit all continents except Antarctica and have been isolated from diverse soil ecosystems ranging from forests in Germany to coastlands in Kenya to the arctic regions of Russia (78–80). Because of their strikingly diverse biogeography, EPNs are promising biocontrol agents for nearly all climates and locales and have been used successfully throughout the world to control a wide variety of insect pests (81). However, the commercial success of EPNs as biocontrol agents often is unpredictable. For example, S. scapterisci has proven to be as effective as chemical pesticides for the control of mole crickets and now is used widely on golf courses, pastures, and other grassy terrains subject to mole cricket infestation (40, 81). In contrast, EPNs have been much less successful against Colorado potato beetles, chafers, and armyworms (81). A better understanding of how EPNs locate hosts and discriminate among potential hosts may be useful for enhancing the efficacy of EPNs as biocontrol agents.
The ability to find and infect hosts using host-emitted chemosensory cues is essential for many endoparasites, such as parasitic nematodes and schistosomes, as well as for many ectoparasites such as blood-feeding insects, ticks, and lice (82–86). We show that EPNs respond differently to the odors of different potential hosts, and we identify a number of host-derived odorants that stimulate strong attractive and repulsive behavioral responses. Our results provide a foundation for future investigations into the mechanisms of these responses.
Materials and Methods
All nematode strains were cultured as previously described (13). Mole crickets, earwigs, flatheaded borers, pillbugs, and slugs were collected from their natural habitats in the greater Los Angeles area (Fig. S2); waxworms and house crickets were purchased commercially from American Cricket Ranch or Petco. Chemotaxis and jumping assays were performed as previously described (13). For virulence assays, individual hosts were placed in Petri dishes lined with filter paper containing 100 IJs. Survival was scored after 48 h, growth and reproduction in host cadavers was scored after 5 d, and emergence from host cadavers was scored after 10 d for all hosts except house crickets, for which survival was scored after 5 d. TD-GC-MS was performed as previously described (13), and the procedure for SPME-GC-MS was modified from Villaverde et al. (63). A detailed description of all materials and methods used in this study is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Todd Ciche, Heidi Goodrich-Blair, S. Patricia Stock, Byron Adams, and the Caenorhabditis Genetics Center for nematode and bacterial stocks; Nathan Dalleska and the California Institute of Technology Environmental Analysis Center for help with TD-GC-MS, SPME-GC-MS, and subsequent analysis; Richard Menking for help in collecting insects around the California Institute of Technology campus; John Rodriguez and the Rio Hondo golf course grounds crew for access to the grounds and help in setting up mole cricket traps; Chris Cronin for help with the design and maintenance of mole cricket sound traps and mole cricket collection; Joseph Tang, John DeModena, Brian Anderson, Michael Castillo, Esther Thompson, and Beth Dillman for help with mole cricket collection; Joseph Vanderwaart and the University of California, Los Angeles Statistical Consulting Center for help with statistical analysis; and James Baldwin and Jagan Srinivasan for critical reading of the manuscript. We also thank the reviewers of this manuscript for their insightful comments. This work was supported by National Institutes of Health US Public Health Service Training Grant T32GM07616 (to A.R.D.), the Howard Hughes Medical Institute (of which P.W.S. is an investigator), and a Summer Undergraduate Research Fellowship (to B.K.). E.A.H. is an Alfred P. Sloan Research Fellow and a Rita Allen Foundation Scholar.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 13896 (volume 109, number 35).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211436109/-/DCSupplemental.
References
- 1.Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: Structure and function in animal parasitic nematodes. Vet Parasitol. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 2.Haas W. Parasitic worms: Strategies of host finding, recognition and invasion. Zoology (Jena) 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 3.Haas W, et al. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol Res. 2005;95:30–39. doi: 10.1007/s00436-004-1257-7. [DOI] [PubMed] [Google Scholar]
- 4.Torr P, Heritage S, Wilson MJ. Vibrations as a novel signal for host location by parasitic nematodes. Int J Parasitol. 2004;34:997–999. doi: 10.1016/j.ijpara.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Granzer M, Haas W. Host-finding and host recognition of infective Ancylostoma caninum larvae. Int J Parasitol. 1991;21:429–440. doi: 10.1016/0020-7519(91)90100-l. [DOI] [PubMed] [Google Scholar]
- 7.Safer D, Brenes M, Dunipace S, Schad G. Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc Natl Acad Sci USA. 2007;104:1627–1630. doi: 10.1073/pnas.0610193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciacca J, et al. Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol Int. 2002;51:53–62. doi: 10.1016/s1383-5769(01)00105-2. [DOI] [PubMed] [Google Scholar]
- 9.Root RB. Niche exploitation pattern of blue-gray gnatcatcher. Ecol Monogr. 1967;37(4):317–350. [Google Scholar]
- 10.Dillman AR, Sternberg PW. Entomopathogenic nematodes. Curr Biol. 2012;22:R430–R431. doi: 10.1016/j.cub.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillman AR, et al. An entomopathogenic nematode by any other name. PLoS Pathog. 2012;8:e1002527. doi: 10.1371/journal.ppat.1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciche T. 2007. The biology and genome of Heterorhabditis bacteriophora. Wormbook, www.WormBook.org.
- 13.Hallem EA, et al. A sensory code for host seeking in parasitic nematodes. Curr Biol. 2011;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaugler R, LeBeck L, Nakagaki B, Boush GM. Orientation of the entomogenous nematode Neoaplectana carpocapsae to carbon dioxide. Environ Entomol. 1980;9(5):649–652. [Google Scholar]
- 15.O’Halloran DM, Burnell AM. An investigation of chemotaxis in the insect parasitic nematode Heterorhabditis bacteriophora. Parasitology. 2003;127:375–385. doi: 10.1017/s0031182003003688. [DOI] [PubMed] [Google Scholar]
- 16.Prot JC. Migration of plant-parasitic nematodes towards plant roots. Revue Nematol. 1980;3(2):305–318. [Google Scholar]
- 17.Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. Entomopathogenic nematodes: Systematics, phylogeny and bacterial symbiosis. In: Nguyen KB, Hunt DJ, editors. Nematology Monographs and Perspectives. Vol 5. Leiden-Boston: Brill; 2007. pp. 693–733. [Google Scholar]
- 18.Viney ME, Thompson FJ, Crook M. TGF-β and the evolution of nematode parasitism. Int J Parasitol. 2005;35:1473–1475. doi: 10.1016/j.ijpara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Lewis EE, Campbell J, Griffin C, Kaya H, Peters A. Behavioral ecology of entomopathogenic nematodes. Biol Control. 2006;38:66–79. [Google Scholar]
- 20.Dowds BCA, Peters A. Virulence mechanisms. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI Publishing; 2002. pp. 79–98. [Google Scholar]
- 21.Ye W, Torres-Barragan T, Cardoza YJ. Oscheius carolinensis n. Sp. (Nematoda: Rhabditidae), a potential entomopathogenic nematode from vermicompost. Nematol. 2010;12(1):121–135. [Google Scholar]
- 22.Ciche TA, Ensign JC. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol. 2003;69:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martens EC, Heungens K, Goodrich-Blair H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J Bacteriol. 2003;185:3147–3154. doi: 10.1128/JB.185.10.3147-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams BJ, Nguyen KB. 2002. Taxonomy and systematics. Entomopathogenic Nematology, ed Gaugler R (CABI Publishing, New York), pp 1–33.
- 25.Downes MJ, Griffin CT. Dispersal behavior and transmission strategies of the entomopathogenic nematodes Heterorhabditis and Steinernema. Biocontrol Sci Technol. 1996;6(3):347–356. [Google Scholar]
- 26.Lewis EE. Behavioral ecology. In: Gauger R, editor. Entomopathogenic Nematology. New York: CABI Publishing; 2002. pp. 205–223. [Google Scholar]
- 27.Campbell JF, Kaya HK. How and why a parasitic nematode jumps. Nature. 1999;397:485–486. [Google Scholar]
- 28.Maitland DP. Locomotion by jumping in the Mediterranean fruit-fly larva Ceratitis capitata. Nature. 1992;355:159–161. [Google Scholar]
- 29.Campbell JF, Kaya HK. Influence of insect-associated cues on the jumping behavior of entomopathogenic nematodes (Steinernema spp.) Behavior. 2000;137(5):591–609. [Google Scholar]
- 30.Campbell JF, Kaya HK. Mechanism, kinematic performance, and fitness consequences of jumping behavior in entomopathogenic nematodes (Steinernema spp.) Can J Zool. 1999;77(12):1947–1955. [Google Scholar]
- 31.Campbell JF, Gauger R. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) Behaviour. 1993;126(3):155–169. [Google Scholar]
- 32.Grewal PS, Lewis EE, Gauger R, Campbell JF. Host finding behavior as a predictor of foraging strategy in entomopathogenic nematodes. Parasitol. 1993;108(4):207–215. [Google Scholar]
- 33.Schmidt J, All JN. Attraction of Neoaplectana carpocapsae (Nematoda: Steinernematidae) to common excretory products of insects. Environ Entomol. 1979;8(1):55–61. [Google Scholar]
- 34.Rasmann S, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 35.Lewis EE, Ricci M, Gaugler R. Host recognition behavior predicts host suitability in the entomopathogenic nematode Steinernema carpocapsae (Rhabditida: Steinernema) Parasitol. 1996;113:573–579. doi: 10.1017/s0031182000067627. [DOI] [PubMed] [Google Scholar]
- 36.Poinar GO., Jr . Nematodes for Biological Control of Insects. Boca Raton, FL: CRC; 1979. [Google Scholar]
- 37.Hodson AK, Friedman ML, Wu LN, Lewis EE. European earwig (Forficula auricularia) as a novel host for the entomopathogenic nematode Steinernema carpocapsae. J Invertebr Pathol. 2011;107:60–64. doi: 10.1016/j.jip.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen KB, Smart GC. Steinernema scapterisci n. sp. (Rhabditida: Steinernematidae) J Nematol. 1990;22:187–199. [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen KB, Smart GC. Pathogenicity of Steinernema scapterisci to selected invertebrates. J Nematol. 1991;23:7–11. [PMC free article] [PubMed] [Google Scholar]
- 40.Frank JH. Steinernema scapterisci as a biological control agent of scapteriscus mole crickets. In: Hajek AE, Glare TR, O'Callaghan M, editors. Use of Microbes for Control and Eradication of Invasive Arthropods, Progress in Biological Control. The Netherlands: Springer; 2009. [Google Scholar]
- 41.Kaya HK. Soil ecology. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC; 1990. pp. 93–111. [Google Scholar]
- 42.Nguyen KB, Hunt DJ. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial symbioses. Nematology Monographs and Perspectives. Vol 5. Nguyen KB, Hunt DJ, editors. Leiden-Boston: Brill; 2007. pp. 283–294. [Google Scholar]
- 43.Cabanillas HE, Raulston JR. Impact of Steinernema riobravis (Rhabditida: Steinernematidae) on the control of Helicoverpa zea (Lepidoptera: Noctuidae) in corn. J Econ Entomol. 1995;88(1):58–64. [Google Scholar]
- 44.Shapiro DI, McCoy CW. Virulence of entomopathogenic nematodes to Diaprepes abbreviatus (Coleoptera: Curculionidae) in the laboratory. J Econ Entomol. 2000;93:1090–1095. doi: 10.1603/0022-0493-93.4.1090. [DOI] [PubMed] [Google Scholar]
- 45.Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ. Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. BioControl. 2011;59(2):123–129. [Google Scholar]
- 46.Poinar GOJ. Description and biology of a new insect parasitic Rhabditoid, Heterorhabditis bacteriophora n. Gen. N. Sp. (Rhabditida; Heterorhabditidae n. Fam.) Nematologica. 1975;21(4):463–470. [Google Scholar]
- 47.Weiser J. Neoaplectana carpocapsae n. Sp. (Angullulinata: Steinernematidae) novy, cizopasnic housenik obalece jableeneho, Carpocapsa pomonella l. Westnik Ceskoslovenske Zoologicke Spolecnosti. 1955;19:44–52. [Google Scholar]
- 48.Stock SP, Gardner SL, Wu FF, Kaya HK. Characterization of two Steinernema scapterisci populations (Nemata: Steinernematidae) using morphology and random amplified polymorphic DNA markers. J Helminth Soc Wash. 1995;62(2):242–249. [Google Scholar]
- 49.Glaser RW, Fox H. A nematode parasite of the japanese beetle (Popillia japonica newm.) Science. 1930;71:16–17. doi: 10.1126/science.71.1827.16-b. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Gaugler R, Cui L. Variations in immune response of Popillia japonica and Acheta domesticus to Heterorhabditis bacteriophora and Steinernema species. J Nematol. 1994;26:11–18. [PMC free article] [PubMed] [Google Scholar]
- 51.Lacey LA, Kaya HK, editors. Field Manual of Techniques in Invertebrate Pathology: Application and Evaluation of Pathogens for Control of Insects and Other Invertebrate Pests. 2007. (Springer, The Netherlands) [Google Scholar]
- 52.Nielsen DG. Studying biology and control of borers attacking woody plants. Bull ESA. 1981;27(4):251–260. [Google Scholar]
- 53.Poinar GO, Paff M. Laboratory infection of terrestrial isopods (Crustacea: Isopoda) with Neoaplectanid and Heterorhabditid nematodes (Rhabditida: Nematoda) J Invertebr Pathol. 1985;45(1):24–27. [Google Scholar]
- 54.Sicard M, Raimond M, Prats O, Lafitte A, Braquart-Varnier C. Pathogenic effect of entomopathogenic nematode-bacterium complexes on terrestrial isopods. J Invertebr Pathol. 2008;99:20–27. doi: 10.1016/j.jip.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Jaworska M. Laboratory infection of slugs (Gastropoda: Pulmonata) with entomopathogenic nematodes (Rhabditida: Nematoda) J Invertebr Pathol. 1993;61(2):223–224. [Google Scholar]
- 56.Poinar GO. Non-insect hosts for the entomogenous Rhabditoid nematodes Neoaplectana (Steinernematodae) and Heterorhabditis (Hetororhabditidae) Rev Nematol. 1989;12(4):423–428. [Google Scholar]
- 57.Kaya HK, Gaugler R. Entomopathogenic nematodes. Annu Rev Entomol. 1993;38:181–206. [Google Scholar]
- 58.McGrath PT, et al. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapiro-Ilan DI, Fuxa JR, Lacey LA, Onstad DW, Kaya HK. Definitions of pathogenicity and virulence in invertebrate pathology. J Invertebr Pathol. 2005;88:1–7. doi: 10.1016/j.jip.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey L, editor. Manual of Techniques in Insect Pathology. San Diego: Academic Limited; 1997. [Google Scholar]
- 61.de Doucet MM, Bertolotti MA, Giayetto AL, Miranda MB. Host range, specificity, and virulence of Steinernema feltiae, Steinernema rarum, and Heterorhabditis bacteriophora (Steinernematidae and Heterorhabditidae) from Argentina. J Invertebr Pathol. 1999;73:237–242. doi: 10.1006/jipa.1998.4831. [DOI] [PubMed] [Google Scholar]
- 62.Klowden MJ. Blood, sex, and the mosquito. Bioscience. 1995;45(5):326–331. [Google Scholar]
- 63.Villaverde ML, Juarez MP, Mijailovsky S. Detection of Tribolium castaneum (herbst) volatile defensive secrections by solid phase microextraction-capillary gas chromatrophy (SPME-CGC) J Stored Prod Res. 2007;43(4):540–545. [Google Scholar]
- 64.Lewis EE, Campbell JF. Entomopathogenic nematode host search strategies. In: Lewis EE, Campbell JF, Sudhdeo MVK, editors. The Behavioral Ecology of Parasites. New York: CABI Publishing; 2002. pp. 13–39. [Google Scholar]
- 65.Zhang C, et al. Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. J Invertebr Pathol. 2008;98:153–168. doi: 10.1016/j.jip.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Shulte F. The association between Rhabditis necromena Sudhaus & Schulte, 1989 (Nematoda: Rhabditidae) and native and introduced millipedes in South Australia. Nematologica. 1989;35(1):82–89. [Google Scholar]
- 67.Kiontke K, Sudhaus W. Ecology of Caenorhabditis species. WormBook. 2006. www.WormBook.org. [DOI] [PMC free article] [PubMed]
- 68.Shapiro-Ilan DI, Gaugler R, Tedders WL, Brown I, Lewis EE. Optimization of inoculation for in vivo production of entomopathogenic nematodes. J Nematol. 2002;34:343–350. [PMC free article] [PubMed] [Google Scholar]
- 69.Poinar GO, Jr, Thomas GM. Laboratory infection of spiders and harvestmen (Arachnida: Araneae and Opiliones) with Neoaplectana and Heterorhabditis nematodes. J Arachnol. 1985;13(3):297–302. [Google Scholar]
- 70.Samish M, Glazer I. Infectivity of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to female ticks of Boophilus annulatus (Arachnida: Ixodidae) J Med Entomol. 1992;29:614–618. doi: 10.1093/jmedent/29.4.614. [DOI] [PubMed] [Google Scholar]
- 71.de Oliveira Vasconcelos V, et al. Steinernema glaseri Santa Rosa strain (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora CCA Strain (Rhabditida: Heterorhabditidae) as biological control agents of Boophilus microplus (Acari: Ixodidae) Parasitol Res. 2004;94:201–206. doi: 10.1007/s00436-004-1178-5. [DOI] [PubMed] [Google Scholar]
- 72.Eng MS, Preisser EL, Strong DR. Phoresy of the entomopathogenic nematode Heterorhabditis marelatus by a non-host organism, the isopod Porcellio scaber. J Invertebr Pathol. 2005;88:173–176. doi: 10.1016/j.jip.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Kruitbos LM, Heritage S, Wilson MJ. Phoretic dispersal of entomopathogenic nematodes by Hylobius abietis. Nematol. 2009;11(3):419–427. [Google Scholar]
- 74.Campos-Herrera R, Trigo D, Gutiérrez C. Phoresy of the entomopathogenic nematode Steinernema feltiae by the earthworm Eisenia fetida. J Invertebr Pathol. 2006;92:50–54. doi: 10.1016/j.jip.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Rae RG, Robertson JF, Wilson MJ. The chemotactic response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditida) to cues of Deroceras reticulatum. Nematol. 2006;8(2):197–200. [Google Scholar]
- 76.Rae RG, Robertson JF, Wilson MJ. Chemoattraction and host preference of the gastropod parasitic nematode Phasmarhabditis hermaphrodita. J Parasitol. 2009;95:517–526. doi: 10.1645/GE-1637.1. [DOI] [PubMed] [Google Scholar]
- 77.Hapca S, Crawford J, Rae R, Wilson M, Young I. Movement of the parasitic nematode Phasmarhabditis hermaphrodita in the presence of mucus from the host slug Deroceras reticulatum. Biol Control. 2007;41(2):223–229. [Google Scholar]
- 78.Eilenberg J, Hokkanen HMT, editors. An Ecological and Societal Approach to Biocontrol. 2006. (Springer, The Netherlands) [Google Scholar]
- 79. Vega FE, Kaya H,K eds (2012) Insect Pathology, 2nd Ed (Academic Press, London)
- 80.Hominick WM. Biogeography. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI Publishing; 2002. pp. 115–143. [Google Scholar]
- 81.Shapiro-Ilan DI, Gouge DH, Koppenhofer AM. Factors affecting commercial success: Case studies in cotton, turf, and citrus. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI Publishing; 2002. [Google Scholar]
- 82.Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proc Natl Acad Sci USA. 2011;108:12987–12995. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sukhdeo SC, Sukhdeo MVK. Trematode behaviours and the perceptual worlds of parasites. Can J Zool. 2004;82(2):292–315. [Google Scholar]
- 84.McMahon C, Guerin PM. Attraction of the tropical bont tick, Amblyomma variegatum, to human breath and to the breath components acetone, NO and CO2. Naturwissenschaften. 2002;89:311–315. doi: 10.1007/s00114-002-0317-z. [DOI] [PubMed] [Google Scholar]
- 85.Osterkamp J, Wahl U, Schmalfuss G, Haas W. Host-odor recognition in two tick species is coded in a blend of vertebrate volatiles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185(1):59–67. doi: 10.1007/s003590050366. [DOI] [PubMed] [Google Scholar]
- 86.Mikheev VN, Pasternak AF, Valtonen ET. Tuning host specificity during the ontogeny of a fish ectoparasite: Behavioural responses to host-induced cues. Parasitol Res. 2004;92:220–224. doi: 10.1007/s00436-003-1044-x. [DOI] [PubMed] [Google Scholar]