Abstract
Molecules that control the lineage commitment of hematopoietic stem cells (HSCs) may allow the expansion of enriched progenitor populations for both research and therapeutic uses. In an effort to better understand and control the differentiation of HSCs to megakaryocytes, we carried out an image-based screen of a library of 50,000 heterocycles using primary human CD34+ cells. A class of naphthyridinone derivatives was identified that induces the differentiation of common myeloid progenitors (CMP) to megakaryocytes. Kinase profiling and subsequent functional assays revealed that these compounds act through inhibition of platelet-derived growth factor receptor (PDGFR) signaling in CMPs. Such molecules may ultimately have clinical utility in the treatment of thrombocytopenia.
Hematopoietic stem cells (HSCs) are among the best-characterized adult stem cells and have been used clinically for over 30 years in bone-marrow transplantation (1). Nonetheless, therapeutic applications of HSCs remain limited by a lack of robust, well-defined conditions for their self-renewal and directed differentiation. Recently, we and others identified small molecules and proteins for the ex vivo expansion of both human adult and umbilical-cord blood–derived HSCs (2, 3). For example, a purine derivative was identified that expands CD34+ cells by antagonizing the aryl hydrocarbon receptor; the expanded cells engraft efficiently in an nonobese diabetic (NOD) SCID gamma mouse model (4). Similarly, small molecules that control the differentiation of CD34+ cells to megakaryocyte or red-blood-cell lineages might be used in the treatment of genetic blood diseases and cancer, or enable the production of blood products ex vivo. In particular, we have developed a high-throughput image-based screen to identify small molecules that control the differentiation of human CD34+ cells to megakaryocytes. Such molecules should prove useful tools for elucidating the underlying cellular processes that regulate megakaryopoiesis. In addition, transplantation of ex vivo expanded megakaryocytic progenitor cells might provide a strategy for shortening the duration of thrombocytopenia associated with high-dose chemotherapy and hematopoietic stem-cell transplantation (5). Indeed the number of megakaryocytic progenitors present in transplants has been correlated with platelet recovery (6), and several clinical trials using expanded peripheral blood stem cells and bone marrow have demonstrated success in alleviating thrombocytopenia after high-dose chemotherapy (7, 8).
Mixtures of hematopoietic growth factors have been developed for the ex vivo expansion of megakaryocytes. Although thrombopoietin (TPO) is clearly the major physiological regulator of this process (9), several other cytokines, including interleukins-3, -1, -11, and -6, granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor, have been shown to be important for normal platelet formation (10). In contrast to TPO, which acts throughout megakaryopoiesis, these cytokines affect megakaryopoiesis during the early stages of megakaryocyte development. However, even the best-reported conditions for the ex vivo production of hematopoietic lineages yield a low percentage of megakaryocyte precursors (< 5–15%) (11). Here we report the identification of a class of small molecules that directs CD34+ cell differentiation down the megakaryocyte lineage by high-throughput cell-based screening of primary human CD34+ cells. The physiological target of these molecules appears to be platelet-derived growth factor receptor (PDGFR), and modulation of this signaling pathway may be useful to facilitate ex vivo megakaryocyte expansion for therapeutic applications.
Results and Discussion
High-Throughput Chemical Screen.
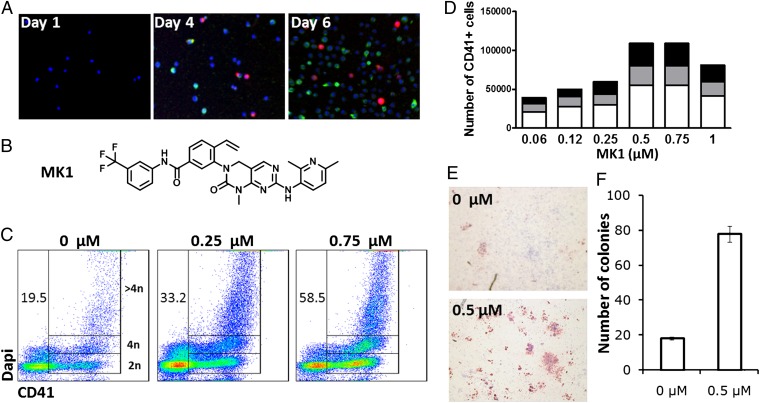
One limitation in identifying signals that promote megakaryopoiesis is a lack of suitable cell-line models. To overcome this we developed a low-volume high-throughput screen using advanced automation and imaging technologies. These features allow screens to be conducted on purified human CD34+ cells isolated from mobilized peripheral blood (mPB) from normal donors. To identify compounds that increase megakaryopoiesis, human CD34+ cells were cultured under conditions optimized for HSC expansion [serum-free media supplemented with TPO, stem cell factor, Flt3 ligand, and interleukin-6 (IL-6)] (12), and assayed for compounds that increase the number of CD41+ cells [CD41 is a selective megakaryocyte marker (13)]. When cultured under these conditions, CD34+ cells spontaneously differentiate into all of the different blood lineages, and depending on the donor, generate 3–15% megakaryocytes (based on CD41 expression) at 6 d. Adding IL-9, IL-11, or erythropoietin (EPO), three cytokines previously reported to increase megakaryocyte expansion (11, 14–17), to these four growth factors did not result in a higher number of megakaryocytes relative to input CD34+ cells (Fig. S1A). To carry out the screen, mPB CD34+ cells were plated into 1,536-well plates at a density of 250 cells per well in HSC expansion media, and on the same day, compounds from a library of 50,000 discrete heterocycles (18) were added (1 μM final concentration in 0.1% DMSO). After 6 d of incubation, the cells were immunostained for CD41 and CD71 expression (Fig. 1A). From the primary screen, a naphthyridinone (MK1, Fig. 1B) was identified that increases the percentage of CD41+ cells in a dose–response fashion (Fig. 1 C and D). At the optimal dose (EC90 = 750 nM), MK1 increased the absolute number of CD41+ cells threefold.
Fig. 1.
Screen of primary human mPB CD34+ cells identifies MK1 as an inducer of megakaryopoiesis. (A) Expression of CD41 (red) and CD71 (green) in mPB CD34+ cells cultured for 6 d in HSC expansion media. (B) Structure of MK1. (C) DNA content of mPB CD34+ cells cultured for 10 d in the presence of MK1 at the indicated concentrations. CD41+ cells containing 2N, 4N, and >4N are gated. Numbers represent the percentage of CD41+ cells. (D) Number of 2N CD41+ cells (white), 4N CD41+ cells (gray), and >4N CD41+ cells (black) from 5,000 mPB CD34+ cells cultured for 10 d in the presence of MK1 at the indicated concentrations. (E) CFU-Mk (red) generated from mPB CD34+ cells cultured in the presence or absence of MK1 (0.5 μM) for 8 d (F) Total CFU-Mk present in 5,000 mPB CD34+ cells expanded for 8 d in the presence or absence of MK1 (0.5 μM). Mean and SD are plotted from three individual plates within the same experiment (P < 0.01). Data are representative of a least three independent experiments.
Characterization of Biological Activity.
Ploidy analysis was carried out to ensure that the CD41+ cells were megakaryocytes. Megakaryocytes are 10–15 times larger than a typical red blood cell, averaging 50–100 μm in diameter (19). During its maturation, the megakaryocyte grows in size and replicates its DNA without cytokinesis (19); as a result, the nucleus of the megakaryocyte can become very large and lobulated, and in some cases, may contain up to 64N DNA, or 32 copies of the normal complement of DNA in a human cell. After 10 d in cell culture, mPB CD34+ cells treated with 0.75 μM MK1 showed a greater than twofold increase in the populations of CD41+ cells in the G1 (2N), G2 (4N), and greater than G2 (>4N) phase, consistent with formation of the megakaryocyte lineage (Fig. 1 C and D). The megakaryocyte precursors generated from MK1 treatment were also tested for their ability to generate megakaryocyte colonies (CFU-MK) in semisolid media optimized for megakaryocyte colony growth. After 8 d, 5,000 mPB CD34+ cells expanded with vehicle (DMSO, 0.1%) or MK1 (0.5 μM) were assayed for CFU-Mk content. MK1-treated cells generated a three- to fourfold increase in megakaryocyte colonies compared with vehicle alone, which indicates that compound treatment generates more megakaryocyte precursors that are able to differentiate and form colonies (Fig. 1 E and F).
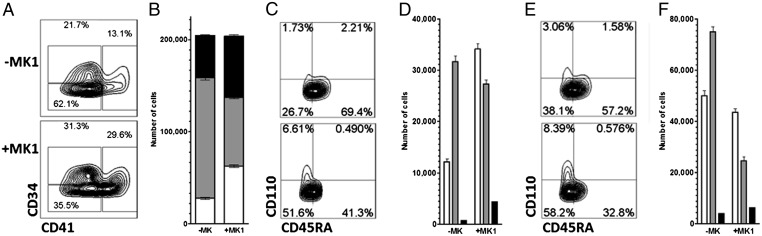
Differentiation of CD34+ cells to megakaryocytes involves progression through several different progenitor populations (20). The current model of hematopoiesis involves differentiation of the multipotent HSC to the common myeloid progenitor (CMP), which then differentiates into either granulocyte/monocyte progenitors (GMP) or megakaryocyte/erythrocyte progenitors (MEP). Each of these populations can be identified by flow cytometry using cell-surface markers (21) (Fig. S1B). To determine the effect of MK1 on HSC differentiation, cells were cultured for 10 d with MK1 and analyzed by flow cytometry (Fig. 2). This analysis revealed that MK1 promotes an increase in MEPs at the expense of GMPs (Fig. 2 C and D). Loss of GMPs is accompanied by a decrease in granulocytes and monocytes (Fig. 2 E and F). Cultures were performed in the presence of erythropoietin [EPO, a cytokine that is required for proliferation of erythrocyte precursors (22)] to determine if MK1 also impacts erythropoiesis (Fig. S2). As expected, EPO generated a robust expansion of erythroid progenitors. Including MK had no impact on erythropoiesis, but did result in the decrease of GMPs and an increase in MEPs and megakaryocytes as observed in the absence of EPO. These data suggest MK1 is directing CMPs down the MEP pathway.
Fig. 2.
MK1 promotes megakaryocyte differentiation at the expense of the granulocyte and monocyte lineages. (A) Phenotype of the progeny of 5,000 mPB CD34+ cells cultured for 10 d in the presence or absence of MK1 (0.75 μM). Megakaryocytes (CD41), stem and progenitor cells (CD41−CD34+), and single lineage progenitors/mature cells (CD41−CD34−) are gated. (B) Absolute number of megakaryocytes (white, P < 0.01), stem and progenitor cells (black), and single lineage progenitors/mature cells (gray) in A. (C) Phenotype of the stem and progenitor cells (CD41−CD34+) gated in A: CMPs (CD41−CD34+CD110−CD45RA−), GMPs (CD41−CD34+CD110−CD45RA+), and MEPs (CD41−CD34+CD110+CD45RA−) are gated. (D) Absolute numbers of CMPs (white), GMPs (gray), and MEPs (black) in C (P < 0.01). (E) Phenotype of single lineage progenitors/mature cells (CD41−CD34−) gated in A: Granulocyte or monocyte progenitors (CD41−CD34−CD110−CD45RA+), erythroid progenitors (CD41−CD34−CD110+CD45RA−), and mature cells (CD41−CD34−CD110−CD45RA−) are gated. (F) Absolute number of mature cells (white), granulocyte or monocyte progenitors (gray), and erythroid progenitors (black) in E (P < 0.02). Data are representative of at least three independent experiments.
To determine if MK1 acts directly on CMPs to increase megakaryopoiesis, CMPs, MEPs, and GMPs were purified by FACS from mPB CD34+ cells following culture in expansion media for 5 d (Fig. S1B), and the progenitor populations were cultured for an additional 5 d in the absence or presence of MK1. Treatment of CMPs with MK1 resulted in an increase in megakaryocyte differentiation similar to that seen in cultures initiated with CD34+ cells (Fig. S3 A and D). Flow-cytometry analysis revealed the cells were able to differentiate into both GMPs and MEPs (Fig. S3 B and C), confirming that the isolated cells were CMPs. These effects were also seen in the presence of EPO (Fig. S3), which further indicates that MK1 does not adversely affect erythrocyte differentiation (see Fig. S3D). MK1 (0.75 μM) had no effect on the proliferation or differentiation of purified GMPs, and very few (< 1%) CD41+ or GlyA+ cells were present (Fig. S4). Finally, treatment of MEPs with MK1 in the absence of EPO led to a modest increase in megakaryocytes and a reduction in GlyA+ erythrocyte progenitors (Fig. S5). Supplementing MEP cultures with EPO + MK1 resulted in a greater than twofold increase in CD41+ cells and the number of erythroid progenitors was unchanged (Fig. S5).
Taken together, our results show that MK1 promotes megakaryopoiesis from both CMP and MEPs. We observed that cultures initiated with CMPs showed a less-marked MK1-induced MEP increase compared with cultures initiated with HSCs. This may result from the difference in treatment duration (5 versus 10 d, respectively). It may also be the case that within a population of sorted CMPs, many cells are already primed toward either the MEP or the GMP lineage. In contrast, in cultures initiated with HSCs, every newly generated CMP is naïve, resulting in a larger pool of cells that are potentially MK1 responsive.
Mechanism of Action.
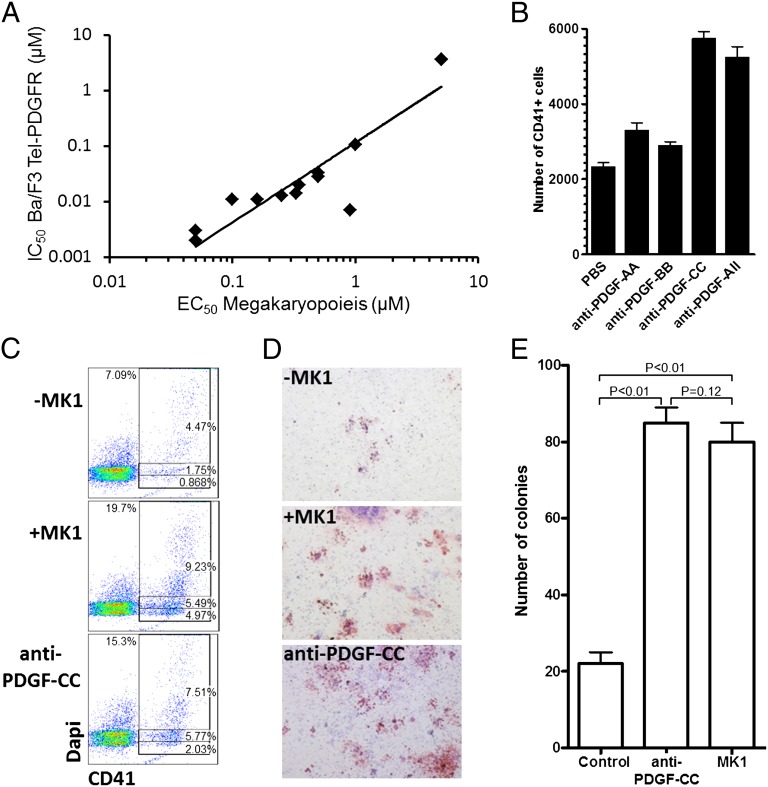
To identify the mechanism by which MK1 increases megakaryopoiesis, we carried out kinase-profiling experiments. The top 12 hit compounds confirmed from the primary screen (based on EC50 values) were analyzed using a panel of activated tyrosine-kinase–dependent cellular assays described by Melnick et al. (23). Briefly, the compounds were tested against a panel of Ba/F3 cell lines stably expressing different TEL–tyrosine kinase fusions, on whose activity they depend for survival. The compounds were found to inhibit growth of the cell lines expressing the PDGFR-TEL fusion. A correlation (R2 = 0.82) between activity in the mPB CD34+ cell assay and inhibition of PDGFR (Fig. 3A) was observed, which suggests that MK1 may affect megakaryocyte differentiation via inhibition of PDGFR signaling. The PDGF family of growth factors consists of five different disulfide-linked dimers composed of four different polypeptide chains encoded by four different genes (24, 25). These isoforms, PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD act through two receptor tyrosine kinases PDGFRs (α and β) that form three dimeric isoforms (αα, αβ, and ββ) (26).
Fig. 3.
MK1 is a PDGFR antagonist. (A) Correlation (R2 = 0.82) between EC50 for megakaryopoiesis and IC50 for PDGFR inhibition. (B) Absolute number of CD41+ cells derived from 1,000 mPB CD34+ cells cultured for 10 d in the presence of vehicle (PBS), neutralizing antibodies against PDGF-AA, PDGF-BB, and PDGF-CC (all at 200 μg/mL) or a combination of all three antibodies (each at 200 μg/mL). All combinations have P < 0.01 when related to vehicle. (C) DNA content of mPB CD34+ cells cultured for 10 d in the presence or absence of MK1 (0.75 μM) or neutralizing antibodies against PDGF-CC (200 μg/mL). Total CD41+ cells and CD41+ cells containing 2N, 4N, and >4N are gated. (D) CFU-Mk (red) generated from mPB CD34+ cells cultured in the presence or absence of MK1 (0.75 μM) or neutralizing antibodies against PDGF-CC (200 μg/mL) for 8 d. (E) Total CFU-Mk present in 5,000 mPB CD34+ cells expanded for 8 d in the presence or absence of MK1 (0.5 μM) or neutralizing antibodies against PDGF-CC (200 μg/mL). Mean and SD are plotted from three individual plates within the same experiment. Data are representative of at least three independent experiments.
Based on the results of the kinase-profiling experiment, we used quantitative PCR to investigate expression of the PDGFRs and different PDGF isoforms in cultivated CD34+ cells and their progeny. This analysis showed that PDGF-CC is the predominant form of PDGF expressed during the first 7 d of CD34+ cell culture (the time frame in which we begin to observe the effect) (Fig. S6A). PDGF-AA and -BB are not expressed until day 7 of culture. PDGFR-α and -β are not expressed at high levels on freshly isolated CD34+ cells, but are present on isolated CMPs and on CD41+ cells, consistent with the ability of MK1 to affect megakaryocyte differentiation at the CMP stage (Fig. S6B).
Based on these gene expression observations, we investigated the hypothesis that inhibition of PDGFR signaling was responsible for the effect of MK1 on megakaryopoiesis, by using neutralizing antibodies to the different PDGFs. The addition of neutralizing antibodies against PDGF-CC (200 μg/mL) to the culture also promoted megakaryopoiesis in a manner similar to MK1 (Fig. 3B); whereas, neutralizing antibodies against PDGF-AA and -BB had a lower effect. Neutralization of PDGF-CC increased both the percentage of CD41+ cells and the absolute number of CD41+ cells two- to threefold (Fig. 3 B and C and Fig. S7). Including the anti–PDGF-CC antibody also resulted in increased number of high ploidy (>4N) cells and of CFU-Mk (Fig. 3 C–E). Like MK1, anti–PDGF-CC increased the number of MEPs and led to a reduction in GMP number (Fig. S7). Treatment of isolated CMPs with anti–PDGF-CC increased megakaryopoiesis (Fig. S8). In addition, anti–PDGF-CC increased the number of megakaryocytes (∼25%) from purified MEPs similarly, and had no effect on GMPs (Fig. S8). Finally, cotreatment with MK1 and anti–PDGF-CC neutralizing antibody did not show any additive effect on megakaryopoiesis (Fig. S9A). These experiments strongly support the hypothesis that PDGFR is the biological target of MK1 that is responsible for increased megakaryopoiesis.
Finally, to confirm the hypothesis that MK1 is a PDGFR inhibitor, we measured the ability of MK1 to inhibit PDGF-CC–mediated PDGFR phosphorylation in U138-malignant glioma (MG) cells, a glioblastoma cell line expressing high levels of PDGFR. Pretreatment with MK1 significantly and dose-responsively lowered the levels of phosphorylated PDGFR measured in these cells after a 10-min PDGF-CC stimulation (Fig. S9B). MK1 also inhibited the PDGF-CC–induced phosphorylation of PLCγ1, a major downstream target of PDGFR (Fig. S9C).
Taken together, these data suggest that PDGF-CC is expressed throughout CD34+ cell differentiation and acts on CMPs to inhibit megakaryopoiesis. Inclusion of the PDGFR antagonist MK1 counteracts these effects thereby promoting megakaryocyte differentiation. The observation that PDGF-CC is expressed by CD34+ cells may explain why previous efforts supplementing cultures with exogenous PDGF failed to identify a role for PDGF in megakaryopoiesis (27). The observation that the action of MK1 happens at the expense of GM differentiation, but not at the expense of erythrocyte differentiation, further supports the notion that MK1 acts at the CMP stage. Despite unchanged total cell numbers, a larger proportion of MK1 and anti–PDGF-CC–treated cells remained CD34+ (Fig. 2 and Fig. S7), suggesting that antagonizing PDGF-CC activity may slow down the differentiation process. The vast majority of the remaining CD34+ cells are CMPs, indicating that cells may encounter a bottleneck at that stage. This is consistent with our observation that PDGF-CC antagonism blocks differentiation to the GMP lineage, thereby reducing the possible outlets available to CMPs, and suggests that MK1 skews but does not accelerate CMP differentiation, causing an accumulation of cells in the CMP stage. In contrast, the MEP stage seems to be much more transient, at least in culture, as their levels appear repeatedly much lower than their CMP progenitors or their CD41+ progeny.
Feedback inhibition of megakaryocyte differentiation by PDGF has interesting parallels to the regulation of megakaryocyte and platelet production by TPO. In this system, the amount of free TPO is regulated by TPO receptors on platelets. As platelet numbers increase, the amount of bioavailable TPO is reduced, leading to decreased megakaryopoiesis. PDGF is also highly expressed by platelets and it is tempting to speculate that PDGF may also inhibit megakaryopoiesis in vivo especially when platelet levels are high. Because the effect of MK1 is observed in the presence of saturating TPO, PDGFR inhibition may provide a unique approach to increase platelets in thrombocytopenic patients alone or in combination with TPO.
Materials and Methods
CD34+ Cell Culture.
All experiments were performed in HSC expansion media [StemSpan SFEM (StemCell Technologies) supplemented with 1× antibiotics and the following recombinant human cytokines; thrombopoietin, IL6, Flt3 ligand, and stem cell factor (100 ng/mL; R & D Systems)] unless otherwise indicated. Recombinant human EPO, IL-9, and IL-11 were purchased from R & D Systems. Human CD34+ cells were purified from fresh human leukophoresed-mobilized peripheral blood (AllCells) or from umbilical-cord blood (Bioreclamation), as noted, using direct CD34 progenitor cell isolation kits (Miltenyi Biotec) following manufacturer's protocols. CD34+ cells were resuspended in HSC expansion medium (5 × 104 cells/mL) before being aliquoted in 384-well plates (Greiner Bio-One). Compounds were added immediately after plating. Cells were transferred to larger well plates, and fresh medium (containing cytokines and compound) was added as needed to keep the cell density between 3 × 105 and 1 × 106 cells/mL Cells were cultured at 37 °C in 5% CO2.
High-Throughput Confocal Imaging.
Stained 384-well plates were imaged by the Opera confocal-imaging reader and analyzed with the Opera software (Evotec Technologies). An air ×10 lens was used to capture six images at both wavelengths (460 and 535 nm), representing different locations in a single well. For image analysis, Hoechst-stained nuclei and FITC/PE-positive cell bodies were detected by an algorithm that selects for positive cell bodies and nuclei within a range of fluorescent emission values and sizes, as determined by fitting parameters to negative controls (DMSO, 0.1%) where 15% of the cells are CD41 positive.
Flow Cytometry.
Multicolor analysis for cell phenotyping was performed on a LSR II flow cytometer (Becton Dickinson). Cell sorting was performed on an FACSDiVa (Becton Dickinson). Cells were stained in staining media [HBSS supplemented with FBS [2% (vol/vol)] and EDTA (2 mM)] at 4 °C for 1 h with APC anti-human CD110 (BD Bioscience), PerCP anti-CD34 (BD Bioscience), FITC anti-CD45RA (e-Bioscience), PECy7 anti-CD41 (BD Bioscience), and PE anti-GlyA (BD Bioscience), then washed with staining media and analyzed.
Determination of Hematopoietic Cell Subsets.
The percentages of indicated cell subsets were determined from aliquots of the cell culture at each time point. Flow-cytometry results of these subsets are given as percentage of the total population. The total cell number in each treatment group was determined by trypan blue exclusion. Absolute numbers of each population in the culture were calculated from the total number of cells multiplied by the percentage of each population.
Colony-Forming Assays.
CFU-MKs were generated by seeding cells into the MegaCult-detect megakaryocyte progenitor kit (StemCell Technologies) following manufacturer’s instructions. Colonies were scored on day 12 with an inverted microscope at 40× magnification. Colony content of the expansion culture was calculated as follows: the average number of scored colonies from three dishes × total mononuclear cell number/input cell number. Total mononuclear cells were determined by multiplying the number of cells per milliliter by the culture volume.
Ploidy.
Cells were harvested and washed with staining media. They were then labeled with FITC anti-human CD41. After washing, the cells were resuspended in paraformaldehyde (2%) and incubated at room temperature for 10 min before the addition of DAPI (1 μg/mL) for another 10 min. The cells were washed and analyzed by flow cytometry.
Kinase Profiling.
The activated tyrosine-kinase–dependent Ba/F3 cellular assays were described by Melnick et al. (23).
PDGFR Phosphorylation Assays.
U138-MG cells were cultivated in DMEM + 10% FBS. Before the experiment, cells were serum starved overnight then treated with MK1 or vehicle at the indicated concentrations, and incubated for a minimum of 30 min at 37 °C. PDGF-CC was then added to the cultures to a final concentration of 100ng/mL After 10 min of incubation at 37 °C, cells were washed with cold PBS, then lysed. The lysis buffer used depended on the downstream application: ELISA or Western blot (see below).
ELISA.
Cell lysis and all subsequent steps were performed with the following kits, according to the manufacturer’s instructions: Pathscan Phospho-PDGF receptor α/β (panTyr) sandwich ELISA kit (7235; Cell Signaling) and Pathscan total PDGF receptor α sandwich ELISA kit.
Western Blots.
Cells were lysed with 100 μL of RIPA buffer supplemented with a protease inhibitor mixture (Sigma). Following incubation on ice for 20 min, the lysed cells were passed through a 261/2 syringe needle and centrifuged (15,000 g, 20 min at 4 °C). Cell lysates were denatured by boiling in SDS sample buffer (Invitrogen) containing 5% β-mercaptoethanol. Proteins were electrophoresed, transferred onto a PVDF membrane and probed with anti-PLCγ1 (2822; Cell Signaling) or anti-phospho-PLCγ1 (2821; Cell Signaling) antibodies at suggested concentrations in tris-buffered saline containing 0.1% tween 20 and 5% (vol/vol) BSA. Blots were then incubated with HRP-conjugated secondary antibodies and detected with a chemiluminescent substrate (Thermo Scientific).
qPCR Analysis.
RNA was extracted using RNAeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Purified RNA was subjected to cDNA synthesis using Quantitect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. The cDNA served as a template for the amplification of PDGF-a, PDGF-b, PDGF-c, PDGF-d, PDGFR-α, PDGFR-β, and the housekeeping gene TATA binding protein (TBP) by real-time PCR, using a 384-well plate in a total volume of 12.5 μL, which contained 1 μL of cDNA, 0.5 μL of primer at 5 μM, 4.75 μl H20, and 6.25 μL of SYBR Green Master Mix (Applied Biosystems). Reactions were amplified on an ABI-7900HT using standard parameters. Transcripts were quantitated by comparative CT method and normalized to TBP. A melting curve analysis was performed to verify the specificity of the amplified PCR products. The following primers were used: TBP: forward CCACTCACAGACTCTCACAAC, reverse CTGCGGTACAATCCCAGAACT; PDGF-a: forward CCAGCGACTCCTGGAGATAGA, reverse CTTCTCGGGCACATGCTTAGT, PDGF-b: forward TCTCTGCTGCTACCTGCGT, reverse GTGGGAGCGGGTCATGTTC, PDGF-c: forward ATTCACAGCCCAAGGTTTCCT, reverse GGGTCTTCAAGCCCAAATCTTT, PDGF-d: forward CCGGCTCATCTTTGTCTACAC, reverse CAAGTCTGTGAGGTGATTGCTC, PDGFRα: forward GCAAAGGCATCACAATGCTGG, reverse GCACATTCGTAATCTCCACTGTC, PDGFRβ: forward AGACACGGGAGAATACTTTTGC, reverse AGTTCCTCGGCATCATTAGGG.
Supplementary Material
Acknowledgments
We thank C. Trussell for cell sorting and technical assistance with flow cytometry, Ben Wen, and Valentina Molteni (all from the Genomics Institute of the Novartis Research Foundation) for helpful discussions. This work was supported by the Skaggs Institute for Chemical Biology (P.G.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212545109/-/DCSupplemental.
References
- 1.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Lyssiotis CA, et al. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl. 2011;50:200–242. doi: 10.1002/anie.201004284. [DOI] [PubMed] [Google Scholar]
- 3.Peled T, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 4.Boitano AE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eapen M, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 6.Begemann PG, et al. Correlation of time to platelet engraftment with amount of transplanted CD34+CD41+ cells after allogeneic bone marrow transplantation. J Hematother Stem Cell Res. 2002;11:321–326. doi: 10.1089/152581602753658501. [DOI] [PubMed] [Google Scholar]
- 7.Paquette RL, et al. Culture conditions affect the ability of ex vivo expanded peripheral blood progenitor cells to accelerate hematopoietic recovery. Exp Hematol. 2002;30:374–380. doi: 10.1016/s0301-472x(02)00770-1. [DOI] [PubMed] [Google Scholar]
- 8.McNiece I. Delivering cellular therapies: Lessons learned from ex vivo culture and clinical applications of hematopoietic cells. Semin Cell Dev Biol. 2007;18:839–845. doi: 10.1016/j.semcdb.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Kirito K, Kaushansky K. Transcriptional regulation of megakaryopoiesis: Thrombopoietin signaling and nuclear factors. Curr Opin Hematol. 2006;13:151–156. doi: 10.1097/01.moh.0000219660.03657.4b. [DOI] [PubMed] [Google Scholar]
- 10.Szalai G, LaRue AC, Watson DK. Molecular mechanisms of megakaryopoiesis. Cell Mol Life Sci. 2006;63:2460–2476. doi: 10.1007/s00018-006-6190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bruyn C, Delforge A, Martiat P, Bron D. Ex vivo expansion of megakaryocyte progenitor cells: Cord blood versus mobilized peripheral blood. Stem Cells Dev. 2005;14:415–424. doi: 10.1089/scd.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 12.Murray LJ, et al. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp Hematol. 1999;27:1019–1028. doi: 10.1016/s0301-472x(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MV, Ralph SJ, Tan AS. Cell-lineage antigens of the stem cell-megakaryocyte-platelet lineage are associated with the platelet IIb-IIIa glycoprotein complex. Blood. 1985;66:76–85. [PubMed] [Google Scholar]
- 14.Fujiki H, et al. Role of human interleukin-9 as a megakaryocyte potentiator in culture. Exp Hematol. 2002;30:1373–1380. doi: 10.1016/s0301-472x(02)00966-9. [DOI] [PubMed] [Google Scholar]
- 15.Cortin V, et al. Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp Hematol. 2005;33:1182–1191. doi: 10.1016/j.exphem.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga T, et al. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 2006;24:2877–2887. doi: 10.1634/stemcells.2006-0309. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, et al. An effective and simple expansion system for megakaryocyte progenitor cells using a combination of heparin with thrombopoietin and interleukin-11. Exp Hematol. 2005;33:1537–1543. doi: 10.1016/j.exphem.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Ding S, Gray NS, Wu X, Ding Q, Schultz PG. A combinatorial scaffold approach toward kinase-directed heterocycle libraries. J Am Chem Soc. 2002;124:1594–1596. doi: 10.1021/ja0170302. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, Bluteau D, Debili N, Vainchenker W. From hematopoietic stem cells to platelets. J Thromb Haemost. 2007;5(Suppl 1):318–327. doi: 10.1111/j.1538-7836.2007.02472.x. [DOI] [PubMed] [Google Scholar]
- 20.Geddis AE. Megakaryopoiesis. Semin Hematol. 2010;47:212–219. doi: 10.1053/j.seminhematol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edvardsson L, Dykes J, Olofsson T. Isolation and characterization of human myeloid progenitor populations—TpoR as discriminator between common myeloid and megakaryocyte/erythroid progenitors. Exp Hematol. 2006;34:599–609. doi: 10.1016/j.exphem.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Elliott S. Erythropoiesis-stimulating agents. Cancer Treat Res. 2011;157:55–74. doi: 10.1007/978-1-4419-7073-2_4. [DOI] [PubMed] [Google Scholar]
- 23.Melnick JS, et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci USA. 2006;103:3153–3158. doi: 10.1073/pnas.0511292103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Eriksson U. Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 2003;14:91–98. doi: 10.1016/s1359-6101(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 25.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 26.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TW, et al. Large generation of megakaryocytes from serum-free expanded human CD34+ cells. Biochem Biophys Res Commun. 2009;378:112–117. doi: 10.1016/j.bbrc.2008.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.