Abstract
HIV modulates plasmacytoid dendritic cell (pDC) activation via Toll-like receptor 7, inducing type I IFN and inflammatory cytokines. Simultaneously, pDCs up-regulate the expression of indoleamine 2,3 dioxygenase (IDO), which is essential for the induction of regulatory T cells (Tregs), which function to down-modulate immune activation. Here we demonstrate the crucial importance of the noncanonical NF-κB pathway in the establishment of this immunoregulatory phenotype in pDCs. In response to HIV, the noncanonical NF-κB pathway directly induces IDO and involves the recruitment of TNF receptor-associated factor-3 to the Toll-like receptor/MyD88 complex, NF-κB–inducing kinase-dependent IκB kinase-α activation, and p52/RelB nuclear translocation. We also show that pDC-induced Tregs can inhibit conventional DC (cDC) maturation partially through cytotoxic T-lymphocyte antigen (CTLA)-4 engagement. Furthermore, CTLA-4 induces IDO in cDCs in a NF-κB–inducing kinase-dependent way. These CTLA-4–conditioned cDCs can in turn induce Treg differentiation in an IDO-dependent manner. Thus, the noncanonical NF-κB pathway is integral in controlling immunoregulatory phenotypes of both pDCs and cDCs.
The mechanisms of generation and the role of CD4+ regulatory T cells (Tregs) in HIV remain under investigation, but may be key to understanding and controlling the damaging immune activation observed in chronic infection (1). Treg frequency has been shown to inversely correlate with immune activation (2, 3). Furthermore, deregulation of Treg function and Treg/Th17 cell ratios may impair beneficial mucosal Th17 responses during chronic infection (4). These data suggest that Tregs can play an important role in HIV infection by modulating immune activation during acute and chronic infection. The precise mechanisms of Treg generation in HIV infection are still largely unknown, however. In this context, plasmacytoid dendritic cells (pDCs), the major type I IFN producers on viral infection, seem to play an important role in Treg generation. pDCs can promote the differentiation of Tregs through several mechanisms, including the expression of inducible T-cell costimulatory ligand or the tryptophan-metabolizing enzyme indoleamine 2,3 dioxygenase (IDO) (5–8). We recently demonstrated that HIV-1–activated pDCs induce Treg differentiation from naïve CD4+ T cells through a CD4-, endocytosis- and Toll-like receptor (TLR)-7–dependent pathway (7). Importantly, using the competitive inhibitor 1-methyl-tryptophan and siRNA knockdown, we determined that expression of the enzyme IDO by pDC is absolutely required for Treg generation.
An immunomodulatory role for IDO and Tregs has been suggested in animal models of simian immunodeficiency virus infection (9, 10), where nonpathogenic infection was correlated with IDO expression in blood and lymphoid tissue. Taken together, these data implicate the IDO pathway as a critical element in the induction of Tregs in HIV infection and the control of associated deleterious inflammatory consequences. Thus, elucidating the molecular mechanisms of IDO induction and its consequences on the differentiation of Tregs in the setting of HIV infection is important.
Results
Induction of IDO in pDC Is Independent of the Canonical NF-κB Pathway.
IDO induction on HIV stimulation in pDCs is dependent on the TLR-7–MyD88 pathway, as demonstrated previously by an siRNA knockdown of TLR-7 and MyD88 (7). These experiments were performed with HIV laboratory strains, however. We stimulated primary pDCs with several primary HIV isolates, all of which induced IDO in the pDCs (Fig. 1A), confirming the physiological relevance of this pathway. To identify the signaling pathways that induce IDO in pDCs, we focused on the activation of TLR by HIV. pDCs express both TLR-7 and TLR-9, but only TLR-7 is triggered by HIV (11). One consequence of TLR-7 triggering is recruitment of the MyD88/IRAK4/TNF receptor-associated factor (TRAF)-6 complex, which in turn leads to activation of the IκB kinase (IKK)-α/IKK-β/IKK-γ (NEMO) complex for activation of the canonical NF-κB pathway through ubiquitylation of IκBs and translocation of p50/RelA into the nucleus (12). In cooperation with the MAP kinase pathway and AP-1 translocation, the NEMO complex coordinates the transcription of inflammatory cytokines, such as TNF-α, and through recruitment of interferon regulatory factor 7 induces type I IFN transcription in a TRAF3-dependent manner (12–14).
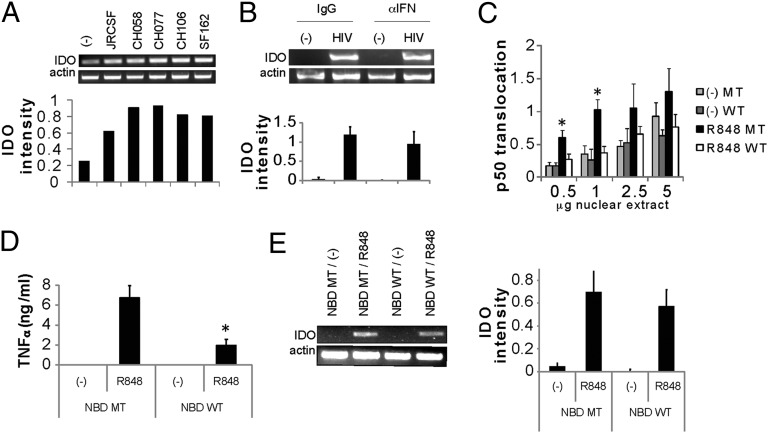
Fig. 1.
HIV induces IDO in pDCs. (A) Primary pDCs were incubated for 24 h with an HIV laboratory strain (SF162) or infectious molecular clones (JR-CSF, CH058, CH077, or CH106). Expression of IDO and actin were monitored by RT-PCR, and normalized IDO expression was quantified by densitometry. One representative experiment of three is displayed. (B) Primary pDCs were preincubated with blocking monoclonal antibodies for IFN-α, IFN-β, and IFN-α/β-R2 (α−IFN) or isotype controls (IgG) for 1 h, followed by the addition of HIVMN (300 ng/mL p24Eq) for 18 h. RNA was extracted, and IDO or β-actin levels were measured by RT-PCR. Normalized IDO expression in three experiments was quantified, and is represented as mean ± SD. (C) NBD inhibits p50 translocation induced by TLR-7. GEN pDCs were preincubated with NBD [either WT or mutant (MT)] for 1 h, after which R848 was added for 30 min and nuclear translocation of p50 was measured. Pooled data from three experiments are shown (*P < 0.05, Student t test). (D) GEN pDCs were preincubated with NBD (50 μM) for 1 h, followed by the addition of R848. TNF-α was measured after an 18-h incubation by cytokine bead array (BD Biosciences). Results are shown as mean ± SD of an experiment conducted in triplicate (*P < 0.05, Student t test). Data in C and D are representative of at least three experiments. (E) Expression of IDO in GEN pDCs was measured by RT-PCR after incubation with the NBD peptide and stimulation by R848. Normalized IDO expression in three experiments was quantified and is represented as mean ± SD.
In human pDCs, blockade of type I IFN did not reduce HIV-induced IDO expression, indicating that it is not required for IDO regulation (Fig. 1B) (15). We then examined the roles of other downstream TLR pathways in IDO induction. To determine whether the canonical NF-κB pathway is required for IDO induction, we made use of a NEMO-binding domain inhibitory peptide (NBD) to inhibit the interaction of NEMO with IKK-β (16). Activation of the GEN pDC line through TLR-7 (with its agonist resiquimod/R848) induced activation of the canonical NF-κB pathway, measured by p50 nuclear translocation (Fig. 1C). Preincubation with the NBD peptide (WT) inhibited p50 translocation compared with the control peptide (mutant), and also inhibited TNF-α production (Fig. 1 C and D). However, NBD peptide blockade did not inhibit IDO induction (Fig. 1E), indicating that the canonical NF-κB pathway is not involved in IDO transcription.
Noncanonical NF-κB Pathway Is Required for TLR-Induced IDO Expression.
Recently, the noncanonical NF-κB pathway was implicated in CD40 and glucocorticoid-induced TNF receptor ligand-induced IDO expression in conventional dendritic cells (cDCs) and pDCs, respectively (17, 18). Activation of some cell surface receptors, including TNF family members and various TLRs (17, 19, 20), has been linked to the activation of an alternative NF-κB pathway in addition to the canonical NF-κB pathway. The noncanonical NF-κB pathway involves activation of NF-κB–inducing kinase (NIK) and phosphorylation of IKK-α dimers, leading to phosphorylation of p100, cleavage pf p100 into p52, and translocation of p52/RelB complexes to the nucleus (19, 21). Indeed, HIV activation in primary pDCs and TLR-7 engagement induced cleavage of p100 into p52 (Fig. 2A). Constitutive activation of and cleavage of p100 was observed in some instances, and HIV stimulation induced further activation (Fig. 2B). On average, HIV stimulation induced up-regulation of p52 by 3.33 ± 0.94-fold (P = 0.0257, Student t test; n = 5). The synthetic TLR-7 agonist R848 also induced p100 processing into p52 (Fig. 2C), as well as translocation of p52 to the nucleus, in GEN pDCs (Fig. 2D), indicating that the noncanonical NF-κB pathway is generally activated on TLR-7 triggering in pDCs. Elevation of p100 protein levels upon activation by R848 suggests coupled cotranslational processing of p100, as described previously (22).
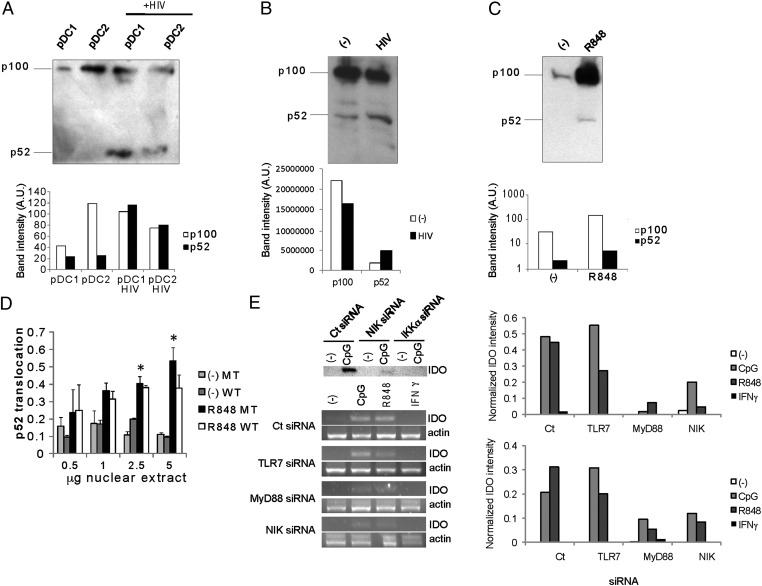
Fig. 2.
The noncanonical NF-κB pathway is required for IDO expression on TLR-7 triggering. (A) Primary pDCs were activated by HIV for 24 h, and then cleavage of p100 into p52 was measured by Western blot analysis after 18 h. (B) Constitutive cleavage of p100 in some primary pDCs (observed in two donors). (C) R848 induces cleavage of p100 into p52 after 18 h. Quantification of band intensity (arbitrary units) is presented for A, B, and C. (D) GEN pDCs were preincubated with the NBD peptide, either WT or mutant (MT), and then activated by R848 for 3 h, after which p52 was measured in nuclear extracts. R848 induced nuclear translocation of p52. Pooled data from three experiments are shown. (*P < 0.05, Student t test). (E) (Upper) GEN pDCs were transfected with NIK or IKK-α or control (Ct) siRNA, and stimulated by CpG oligonucleotide. IDO expression was measured by Western blot analysis. (Lower) GEN pDCs were transfected with TLR-7, MyD88, NIK, or control (Ct) siRNA, and then stimulated by CpG, R848 or 10 IU/mL of IFN-γ. IDO and actin expression was measured by RT-PCR after 18 h, and was inhibited on TLR, MyD88, and NIK knockdown. Relative expression of IDO by RT-PCR, normalized to actin, is presented on the right for two experiments.
Importantly, siRNA knockdown of NIK and IKK-α (Fig. S1), two crucial components for activating the noncanonical pathway, inhibited expression of IDO (Fig. 2E). Thus, in five experiments, NIK knockdown induced a 3.0 ± 1.4-fold inhibition of IDO expression (P < 0.05, Student t test). Activation of the noncanonical NF-κB pathway and processing of p100 are independent of IKK-β and NEMO, but strictly dependent on IKK-α (23), explaining why blockade of NEMO did not affect IDO expression. On the other hand, IKK-α is not strictly required for activation of the canonical NF-κB pathway (24). Knockdown of TLR-7, TLR-9, MyD88, and NIK all inhibited IDO expression by TLR-7 and TLR-9 agonists (Fig. 2E). In five independent experiments, knockdown of TLR-7 induced 60% ± 16% less IDO expression (P < 0.05, Student t test). Low levels of IFN-γ did not induce IDO expression under these conditions, as reported previously (25). These data indicate that TLR-7–mediated activation of the noncanonical NF-κB pathway is important for IDO expression in human pDCs.
Activation of NIK Correlates with TRAF3 Recruitment to the TLR–MyD88 Complex.
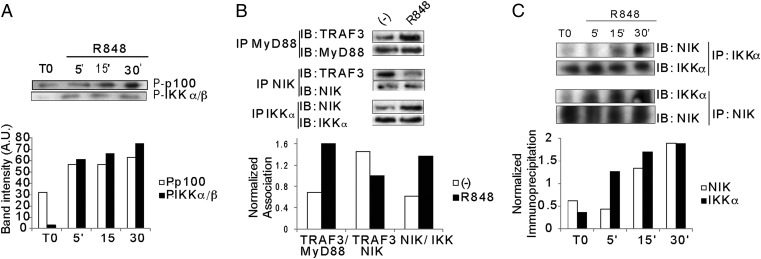
The upstream mechanisms by which the noncanonical pathway is activated remain to be fully characterized (19). NIK is crucial for the phosphorylation of IKK-α and the formation of a ternary complex with IKK-α and p100. In B cells, NIK expression is normally maintained at low levels through sequestration by TRAF3 and constitutive ubiquitylation by cellular inhibitor of apoptosis (cIAP). On ligation of B-cell activating factor receptor by B-cell activating factor, TRAF3 is recruited to the receptor and ubiquitylated, releasing NIK, which can then associate with IKK-α and p100. Given that endosomal TLR-7 triggering in pDCs is linked to recruitment of TRAF3 for interferon regulatory factor 7 phosphorylation (14) and possibly MAPK activation (26), we hypothesized that TRAF3 recruitment might free NIK for activation of the noncanonical NF-κB pathway. Triggering of TLR-7 on pDCs induced early phosphorylation of p100 (Fig. 3A); the average up-regulation at 30 min was 1.9 ± 0.3 fold (P = 0.045; n = 3). Immunoprecipitation of MyD88 showed an association with TRAF3 on TLR triggering (Fig. 3B). Furthermore, TRAF3 dissociated from NIK, as demonstrated by coimmunoprecipitation of NIK and TRAF3 (fold reduction of TRAF3–NIK association, 1.56 ± 0.23, P = 0.027, n = 3) (Fig. 3B). At the same time, immunoprecipitation of NIK and IKK-α demonstrated that NIK associates with IKK-α on TLR triggering (Fig. 3 B and C) (fold NIK binding to IKK-α at 30 min vs. time 0, 2.35 ± 0.79; P = 0.0054; n = 4). These data show that TRAF3 is recruited to the TLR/MyD88 complex, inducing dissociation of NIK from TRAF3 and association of NIK with IKK-α for p100 phosphorylation.
Fig. 3.
Mechanisms of NIK activation. (A) Kinetics of p100 and IKK-α/β phosphorylation on R848 stimulation of GEN pDCs. (B) NIK associates with IKK-α on R848 stimulation. IKK-α (Upper) and NIK (Lower) were immunoprecipitated, and association with NIK (Upper) and IKK-α (Lower) was probed by immunoblot analysis. (C) Recruitment of TRAF3 to the MyD88 complex and release of NIK. GEN pDCs were stimulated with R848 for 20 min, and MyD88 was immunoprecipitated to probe the association with TRAF3. NIK was immunoprecipitated at 30 min to probe for association/dissociation with TRAF3.
Noncanonical NF-κB Binding Sites Directly Bind RelB and Drive IDO Expression.
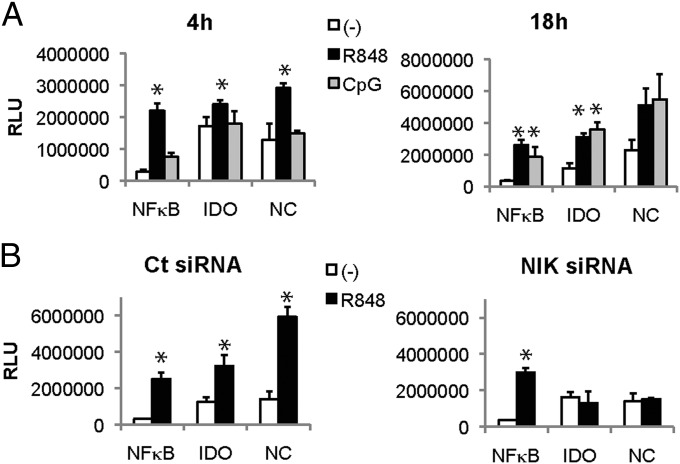
The IDO promoter contains three partial p52/RelB binding sites (AGGAGACACA, GGGAGACAGA, and AGGAGAAAGA), the consensus noncanonical binding sequence being PuGGAGAPyTTPu (27) around position −2000 (28). To determine whether these binding sites are sufficient to interact with p52/RelB and induce gene transcription on TLR triggering, we transfected pDCs with plasmids containing repeats of one IDO promoter sequence (AGGAGAAAGA), the consensus noncanonical NF-κB binding sequence (GGGAGATTTG), or the canonical NF-κB binding site (GGGGACTTTCC) (27) ahead of the firefly luciferase gene. R848 triggering induced luciferase expression by 4 h, driven by the canonical NF-κB promoter, the full noncanonical promoter, and, importantly, the IDO promoter (Fig. 4). CpG also induced IDO promoter-driven luciferase activity, albeit with slower kinetics (Fig. 4A). NIK knockdown by siRNA abolished expression of luciferase driven by the IDO or the noncanonical promoter sequences, but not of luciferase driven by the canonical NF-κB binding site (Fig. 4B), controlling for the specificity of the noncanonical-driven luciferase induction. This finding suggests that TLR-7 and TLR-9 triggering induces NIK activation and p52/RelB binding to the upstream IDO promoter sequences for gene expression.
Fig. 4.
The partial noncanonical NF-κB site in the INDO gene promoter drives luciferase reporter activity on TLR triggering. (A) GEN pDCs were transfected with reporter plasmids encoding luciferase driven by five repeats of the canonical NF-κB site (NFKB), five repeats of the partial noncanonical NF-κB sites in the INDO promoter (IDO), or the full noncanonical NF-κB site (NC). Luciferase activity was measured after 4 h and 18 h on stimulation with R848 and CpG. (B) Transfected GEN pDCs were stimulated by R848 after transfection with control (Ct) or NIK siRNA, and luciferase activity was measured at 4 h (*P < 0.05, Student t test).
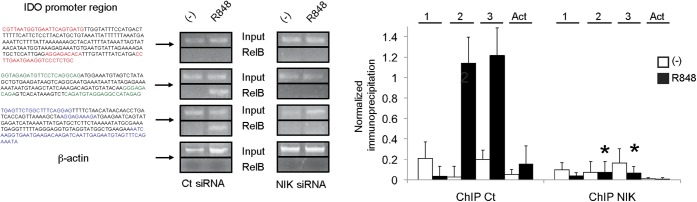
To demonstrate direct binding of p52/RelB to the IDO promoter, we performed ChIP analysis by RelB pull-down. RelB immunoprecipitation showed binding to two of three noncanonical binding sequences in the IDO promoter (GGGAGACAGA and AGGAGAAAGAT, the two proximal sequences) (Fig. 5). Again, NIK knockdown abrogated binding of RelB to the promoter sequences, indicating that NIK activation is required for RelB translocation and binding to the IDO promoter sequences. Taken together, these data demonstrate that TLR-7 triggering is linked to noncanonical NF-κB activation, leading to binding of p52/RelB to the IDO promoter and IDO transcription.
Fig. 5.
RelB binds to the INDO promoter on TLR-7 triggering. RelB was immunoprecipitated upon R848 stimulation of control (Ct) or NIK siRNA-transfected GEN pDCs. Binding of RelB to three different regions in the IDO promoter containing partial noncanonical NF-κB sites was measured by ChIP. Regions amplified are highlighted, bracketing the noncanonical sites. Quantification of immunoprecipitation was performed for three experiments, by normalizing the intensity of each immunoprecipitated band to its input. Comparison of immunoprecipitation of regions 1, 2, and 3 or actin (Act) is represented on the right in the Ct siRNA-transfected and NIK siRNA-transfected cells (*P < 0.05, Student t test). RelB binds to the two most proximal regions in a NIK-dependent fashion.
pDC-Induced Tregs Induce an Immunoregulatory Phenotype in cDCs Through Cytotoxic T-Lymphocyte Antigen-4 Modulation of the Noncanonical NF-κB Pathway and IDO Expression.
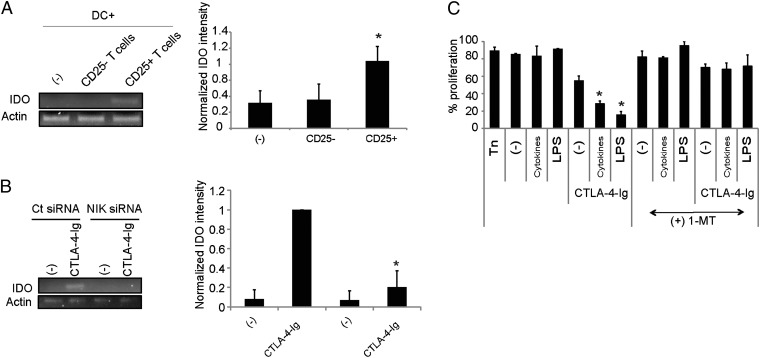
In earlier work, we reported that IDO expression by pDCs on HIV or TLR agonist activation regulates the differentiation of Foxp3+CD127lowCD25high Tregs from naïve CD4+ T cells (inducible Tregs) that can inhibit autologous naïve CD4+ T-cell proliferation and the maturation of and inflammatory cytokine secretion by cDCs (7). Knockdown of IDO abrogates the differentiation of these Tregs. We investigated the mechanisms by which Tregs suppress cDC phenotype and function. pDC-induced Foxp3+ Tregs express cytotoxic T-lymphocyte antigen (CTLA)-4 (Fig. S2), and CTLA-4 was found to be partially implicated in their inhibition of cDC activation. Whereas the Tregs containing the CD25+ fraction (7) of pDC-activated CD4+ T cells blocked TNF-α secretion by LPS-stimulated cDCs, CTLA-4–blocking antibodies partially restored TNF-α secretion (Fig. S3). Furthermore, the Treg-containing CD25+ fraction of pDC-activated CD4+ T cells induced IDO in monocyte-derived dendritic cells (moDCs) (Fig. 6A). As described previously, CTLA-4-Ig induced IDO expression in cDCs (29–31) (Fig. 6B), supporting a direct role of CTLA-4 on Tregs in this effect. Furthermore, NIK knockdown in cDCs abrogated the induction of IDO induced by CTLA-4-Ig (Fig. 6B). Thus, these data confirm the involvement of the noncanonical NF-κB pathway in IDO up-regulation by CTLA-4 in cDCs. CTLA-4-Ig–treated cDCs were functionally impaired, displaying impaired inflammatory cytokine secretion (Fig. S4). Moreover, when cocultured with naïve CD4+ T cells, they induced the differentiation of CD25+ T cells capable of inhibiting the proliferation of autologous naïve CD4+ T cells in a secondary suppressor assay (Fig. 6C and Fig S5). CTLA-4-Ig synergized with LPS to facilitate DC-mediated Treg induction (Fig. 6C). Addition of the IDO inhibitor 1-methyl tryptophan blocked the generation of Tregs by CTLA-4-Ig–treated cDCs, demonstrating that Treg generation is IDO-dependent (Fig. 6C), as described previously (32). Thus, CTLA-4-Ig–treated DCs up-regulate IDO and can induce a population of CD25+ Tregs that have a suppressor function.
Fig. 6.
CTLA-4–dependent induction of IDO in moDCs. (A) R848-activated primary pDCs were used to stimulated allogeneic naïve CD4+ T cells, and CD25+ and CD25− cells were purified after 7 d. Each T-cell fraction was added to pDC-autologous moDCs and stimulated by CD3 antibodies. IDO expression was measured by RT-PCR in moDCs. Normalized IDO expression from three experiments is displayed (*P < 0.05, Student t test). (B) CTLA-4-Ig was added to moDCs, transfected by control (Ct) or NIK siRNA, and IDO expression was measured by RT-PCR. Normalized IDO expression from three experiments is displayed (*P < 0.05, Student t test). (C) moDCs were incubated with CTLA-4-Ig and a cytokine mixture comprising TNFα, IL-1β, and prostaglandin E2 (C) or LPS. The cells were then added to allogeneic naïve CD4+ T cells and cultured for 7 d. The IDO inhibitor 1-methyl-tryptophan was added or not during the culture. CD25+ T cells were purified and added to carboxyfluorescein succinimidyl ester (CFSE)-labeled, T-cell autologous naïve CD4+ T cells stimulated by CD3/CD28. Proliferation of the naïve T cells was measured as CFSElow cells (*P < 0.05, Student t test). Results shown are representative of at least three experiments.
Taken together, our findings show that HIV-activated pDCs induce Tregs in a noncanonical NF-κB– and IDO-dependent pathway. These Tregs in turn induce IDO expression in bystander DCs via CTLA-4, further amplifying Treg differentiation in a process of “infectious tolerance.”
Discussion
Our data indicate that the noncanonical NF-κB pathway is required for stimulating the expression of IDO in pDCs in response to HIV and TLR triggering, and for facilitating the differentiation of Tregs from naïve CD4+ T cells. Once generated, these Tregs can induce IDO expression in cDC through activation of the noncanonical NF-κB pathway. This requires engagement of CTLA-4 on Tregs and its presumed ligands on cDCs (CD86 and CD80). Previous studies have demonstrated synergy between CTLA-4-Ig and CD40 triggering for IDO expression (31) in cDCs, and these interactions are known to act through noncanonical NF-κB signaling to modulate IDO transcription (17). A crucial role for the noncanonical pathway in IDO induction also has been uncovered on GITR triggering in pDCs, together with type I IFN (18). Our observation that CTLA-Ig can induce IDO in human cDCs adds to the described cell-extrinsic role of CTLA-4, mediating CD80 and CD86 depletion on the surface of antigen-presenting cells through transcell endocytosis (33).
The precise mechanisms linking TLR activation to noncanonical NF-κB activation are unclear (26). Our data indicate that TRAF3 is recruited to the MyD88 complex upon TLR engagement, which releases NIK from TRAF3-mediated destabilization. Indeed, TRAF3 is known to regulate the turnover of NIK, through interaction with TRAF2/cIAP and K48-linked ubiquitylation of NIK. Recruitment of TRAF3 to TLR-4 leads to TRAF3 K-48 and/or K63 ubiquitylation for MAPK or type I IFN signaling, respectively (34), which may allow the release of NIK from TRAF3/TRAF2/cIAP-mediated ubiquitylation and its accumulation for auto-activation and triggering of the noncanonical NF-κB pathway. TRAF3 is essential for type I IFN signaling (19), and its recruitment to MyD88 in the endosomes on TLR-7 triggering (13, 35) may allow the release of NIK and result in stabilization. Although the noncanonical pathway is typically a slow process, possibly owing to the kinetics of NIK synthesis and stabilization, cotranslational processing of p100, or compartmentalization of TLR signaling for IFN-α induction, rapid activation of the noncanonical NF-κB pathway also has been observed in pDCs activated through GITR (18) or on LPS stimulation of monocytic cells (20), and may comprise early initiation followed by an amplification phase. Early activation may result from constitutive noncanonical NF-κB activation, which is further enhanced on receptor triggering (Fig. 1). Basal RelB expression may finally determine the strength and kinetics of noncanonical NF-κB signaling (36). The results from the present study, although obtained mostly with the human GEN pDC line, also likely apply to primary pDCs, given that GEN cells share many of the functional attributes of primary pDCs, including TLR-induced IDO expression (37, 38). Recent studies in primary mouse pDCs also support the idea that IDO expression is controlled by the noncanonical NF-κB pathway upon triggering by TNF family receptors or TGF-β (37, 38).
The transcriptional control of IDO is complex and context-dependent. Although type I and type II IFNs alone can induce IDO in many cell types through STAT1, they are not necessarily strictly required for IDO expression in some instances, such as after systemic injection of TLR-9 agonists (39). In our studies and those of others (15), type I IFNs are not required for IDO induction in human pDCs. IDO can be induced independent of STAT1 and IFN regulatory factor 1 by inflammatory cytokines (e.g., TNF-α, IL-6) that activate p38 MAPK and NF-κB pathways (40, 41), by prostaglandin E2 acting through prostaglandin receptor EP2 (42) or by TGF-β (37, 38), underscoring the complexity of signaling required for IDO induction in different cell types and physiological situations. So-called “reverse signaling” through cell surface receptors [e.g., GITR (28)] activates the noncanonical NF-κB pathway, which in synergy with IFN-α induces IDO in murine pDCs, presumably through STAT1 phosphorylation (18). In human pDC, it appears that PI3K-p38 MAPK mediated phosphorylation of STAT1 can occur independently of type I interferons after TLR stimulation (43, 44). Therefore, based on our data and others’ (15) we speculate that direct TLR activation of STAT1 may bypass the requirement for IFN signaling, and p52/RelB may act in synergy with or facilitate STAT1 binding to IFN-stimulated response element or IFN-γ–activated sequence elements for IDO induction (Fig. S6). Other pattern-recognition receptors, such as Dectin-1, can activate the noncanonical NF-κB pathway; however, RelB activation is concomitantly antagonized by Dectin-1–induced Raf-1 activation to induce Th1- and Th17-polarizing cytokines (45), reflecting an integrated regulation of the pathway with differential impacts on T-cell differentiation.
Overall, our results point to a central role of noncanonical NF-κB signaling in the establishment of tolerance through the control of IDO expression and Treg generation by pDCs and cDCs during HIV infection. Type I IFN secreted by pDCs on HIV infection may amplify induction of IDO in an autocrine/paracrine fashion through either STAT1 signaling or the noncanonical NF-κB pathway (46), further expanding the spectrum of IDO-expressing cells and strengthening IDO associated immune modulation. These results provide molecular targets to modulate immune activation in HIV and other pathologies involving pDC activation (47). Chronic activation of TLR by HIV or bacterial products in pDCs and cDCs, accompanied by chronic type I IFN and inflammatory cytokine secretion, likely participates in disease progression. Manipulation of Treg expansion in HIV infection through the noncanonical pathway regulation of IDO in pDCs and cDCs may limit the damaging T-cell overactivation that characterizes progressive disease.
Materials and Methods
Primary pDCs were stimulated for 24 h by 30 ng/mL of p24 equivalent HIV laboratory strains (SF162) or infectious molecular clones (JR-CSF, CH058, CH077, and CH106), obtained through the Center for HIV-AIDS Immunology. pDCs and the GEN line were stimulated by 2 μg/mL of CpG oligonucleotide (GGGGACGACGTCGTGGGGGGG; Integrated DNA Technologies) or 0.5–1 μM resiquimod (R848; 3M). Detailed information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Center for HIV/AIDS Vaccine Immunology for their support. This work was supported by National Institutes of Health Grants 5 RC1AI087897, 5R01AI071078, 1R01AI081848, and 5 U19AI067854; and Collaboration for AIDS Vaccine Discovery Grant 38645 from the Bill and Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204032109/-/DCSupplemental.
References
- 1.Sempere JM, Soriano V, Benito JM. T regulatory cells and HIV infection. AIDS Rev. 2007;9:54–60. [PubMed] [Google Scholar]
- 2.Card CM, et al. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 3.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3-expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol. 2008;83:254–262. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: Implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito T, et al. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase–dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 14.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 15.Boasso A, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May MJ, et al. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 17.Tas SW, et al. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 18.Grohmann U, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 19.Häcker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber J, et al. Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci USA. 2006;103:5899–5904. doi: 10.1073/pnas.0510996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 22.Mordmüller B, Krappmann D, Esen M, Wegener E, Scheidereit C. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep. 2003;4:82–87. doi: 10.1038/sj.embor.embor710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dejardin E, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 24.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Puccetti P. On watching the watchers: IDO and type I/II IFN. Eur J Immunol. 2007;37:876–879. doi: 10.1002/eji.200737184. [DOI] [PubMed] [Google Scholar]
- 26.Häcker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 27.Bonizzi G, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puccetti P, Grohmann U. IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 29.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 30.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 31.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 32.Jürgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma–triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009;114:3235–3243. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng PH, et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. 2010;11:70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shih VF, Tsui R, Caldwell A, Hoffmann A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belladonna ML, et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 38.Pallotta MT, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 39.Wingender G, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 40.Fujigaki H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 41.Fujigaki S, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma–independent mechanism. Eur J Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Domizio J, et al. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114:1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takauji R, et al. CpG-DNA–induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002;72:1011–1019. [PubMed] [Google Scholar]
- 45.Gringhuis SI, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 46.Du Z, et al. Non-conventional signal transduction by type 1 interferons: The NF-kappaB pathway. J Cell Biochem. 2007;102:1087–1094. doi: 10.1002/jcb.21535. [DOI] [PubMed] [Google Scholar]
- 47.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.