Abstract
Neurons in the CNS of higher vertebrates lose their ability to regenerate their axons at a stage of development that coincides with peak circulating thyroid hormone (T3) levels. Here, we examined whether this peak in T3 is involved in the loss of axonal regenerative capacity in Purkinje cells (PCs). This event occurs at the end of the first postnatal week in mice. Using organotypic culture, we found that the loss of axon regenerative capacity was triggered prematurely by early exposure of mouse PCs to T3, whereas it was delayed in the absence of T3. Analysis of mutant mice showed that this effect was mainly mediated by the T3 receptor α1. Using gain- and loss-of-function approaches, we also showed that Krüppel-like factor 9 was a key mediator of this effect of T3. These results indicate that the sudden physiological increase in T3 during development is involved in the onset of the loss of axon regenerative capacity in PCs. This loss of regenerative capacity might be part of the general program triggered by T3 throughout the body, which adapts the animal to its postnatal environment.
Keywords: axon regeneration, cerebellum, neural development, lentiviral vectors
In higher vertebrates, there is a period of development during which CNS neurons can regenerate their axons after injury (1). The molecular mechanisms underlying the subsequent loss of this ability are not fully understood, but the onset of an inhibitory environment in the developing CNS is known to prevent axon regeneration (1–3), together with intrinsic neuronal maturation (4–6). In mice, this event takes place during the first postnatal week for neurons of the entorhinal cortex and motor cortex, as well as retinal ganglion cells and Purkinje cells (PCs) (7–11). Interestingly, this loss of regenerative capacity coincides with a strong increase in circulating thyroid hormone (T3) levels. T3 levels in serum are low during mouse prenatal development but increase sharply (more than 1,000-fold) during the first week after birth before decreasing to adult levels during the second week (12–14). This hormonal burst leads to profound changes in gene expression that are required to adapt the physiology of various organs to postnatal life (15).
We suspected that the loss of axonal regenerative ability might be one of these critical physiological changes. We therefore investigated the involvement of high T3 levels in the onset of the loss of regenerative capacity of postnatal PCs. Because Krüppel-like factor 9 (Klf9) is a transcriptional target of T3 in some neuronal cell types (16, 17) and is involved in the developmental loss of regenerative ability of retinal ganglion cells (5), we also examined whether T3 acts on PCs by regulating Klf9 expression.
Results
Early Exposure to T3 Accelerates the Loss of Ability to Regenerate PC Axons.
To investigate the effect of early T3 exposure on axon regeneration ability, we used a coculture assay to quantify the regenerative capacity of PCs (18, 19). PCs from newborn mice [postnatal day (P) 0] were grown in organotypic culture for 7 d in vitro (div) and were then axotomized and placed in front of the ventral half of a cerebellar slice taken from age-matched (P0 + 7 div) calbindin-deficient mice (Calb1−/−) for another 7 div to allow for regeneration (Fig. 1A). Hence, all Calb1-immunoreactive axons in the ventral half of the slice corresponded to regenerative axons. Two parameters were measured to determine regeneration capacity: the area covered by Calb1+ regenerating axons and the mean length of the three longest regenerating axons in the Calb1−/− slice (Fig. S1). Hereafter, we refer to the area of regeneration as an indirect index for the number of PCs able to regenerate, whereas the mean length of the three longest axons serves as an indirect index for the growth rate of regenerating axons.
Fig. 1.
T3 accelerates the developmental loss of PC axon regenerative capacity. (A) Slice culture regeneration assay. Cerebellar slices from newborn mice (P0) were cultured, thus preserving PCs and their targets (deep cerebellar nuclei neurons) in the same slice. Axotomy (axt) was performed in vitro. After 7 div, the ventral halves containing the deep cerebellar nuclei neurons were amputated and replaced by the ventral half of a cerebellar slice taken from age-matched (P0 + 7 div) Calb1−/− mice. Hence, all Calb1-immunoreactive axons in the ventral half of the slice were regenerative axons. These slice cocultures were kept for another 7 div to allow regeneration to proceed. Photomicrographs of cocultured slices immunostained with Calb1 antibodies are shown: untreated coculture (B), T3-treated cocultures (C and E), and Gö6976-treated cocultures (D and E). The dotted lines indicate the sites of axotomy, and arrowheads show regenerating axons. Note that the axons are thicker in T3-treated cultures than in untreated cultures. (Scale bar: 475 μm.) (F and G) Quantitative analysis of regeneration in the presence and absence of T3 and Gö6976. Axotomy was always performed after 7 div. (H and I) Quantitative analysis of regeneration to determine the time dependency of the T3 effect. Axotomy was performed at various time points (0, 3, 5, 7, and 14 div). All cultures were grown in the presence of Gö6976, with (black bars) or without (white bars) T3. The area covered by Calb1+ regenerating axons (F and H) and mean length of the three longest regenerating axons in the Calb1−/− slice (G and I) are shown. Values are means ± SEM [***P < 0.001, nonparametric Kruskal–Wallis one-way ANOVA with the Mann–Whitney post hoc test (F and G) and two-way ANOVA (T3 effect and age) with post hoc protected least significant difference (PLSD) of the Fisher exact test (H and I)]. In F and G, n = 27, 27, 34, and 46 for Ctrl, T3, Gö6976, and Gö6976 + T3, respectively. In H and I, n = 24, 32, 24, 22, and 23 in the absence of T3 and n = 23, 36, 20, 21, and 22 in the presence of T3 for axotomy at 0, 3, 5, 7, and 14 div, respectively.
When 30 nM T3 was added to the culture medium throughout the cell culture period, the regenerative capacity of PC axons was strongly inhibited (Fig. 1 B and C). The differences from untreated cultures were statistically significant both for the number of regenerating PCs and for the axon growth rate (Fig. 1 F and G). However, PC survival was very low in this experiment (Fig. 1 B and C and Fig. S2). PC death induced by axotomy can be prevented by inhibiting PKC with Gö6976 (18). When similar experiments were carried out in the presence of 1 μM Gö6976 (Fig. 1 D and E and Fig. S2), the two parameters reflecting regenerative capacity were enhanced in both the presence and absence of T3 (Fig. 1 D–G). In the presence of T3, no considerable regeneration was observed in slices with very high PC survival (Fig. 1E), whereas slices with lower PC survival showed marked regeneration in he absence of T3 (Fig. 1D). Thus, independent of the survival effect of Gö6976, T3 significantly reduced the capacity of axons to regenerate (Fig. 1 F and G). Remarkably, mean axonal length in slices grown in the presence of T3 was only 28% of that observed in the absence of T3 (Fig. 1G). Thus, T3 not only reduced the number of PCs capable of promoting axon regeneration but limited the axon growth rate of those that were able to regenerate.
We then investigated the time dependency of this effect by performing axotomy in the presence of Gö6976 at various time points (0, 3, 5, 7, and 14 div) and analyzing PC regeneration 7 d later. In the absence of T3, the capacity of PCs to regenerate axons decreased with time, as shown by the gradual decrease in the area of regeneration from 0 to 14 d (Fig. 1H) and the decrease in the average length of the three longest regenerative axons between 5 and 7 d (Fig. 1I). Addition of T3 throughout the cell culture period reduced the number of PCs able to regenerate (area) and the axon growth rate (longest axons) when axotomy was performed after 3 div (Fig. 1 H and I). Thus, early exposure to T3 accelerates the loss of PC regenerative capacity in our in vitro conditions.
Absence of T3 in Vivo Delays Developmental Loss of PC Axon Regenerative Ability in Organotypic Culture.
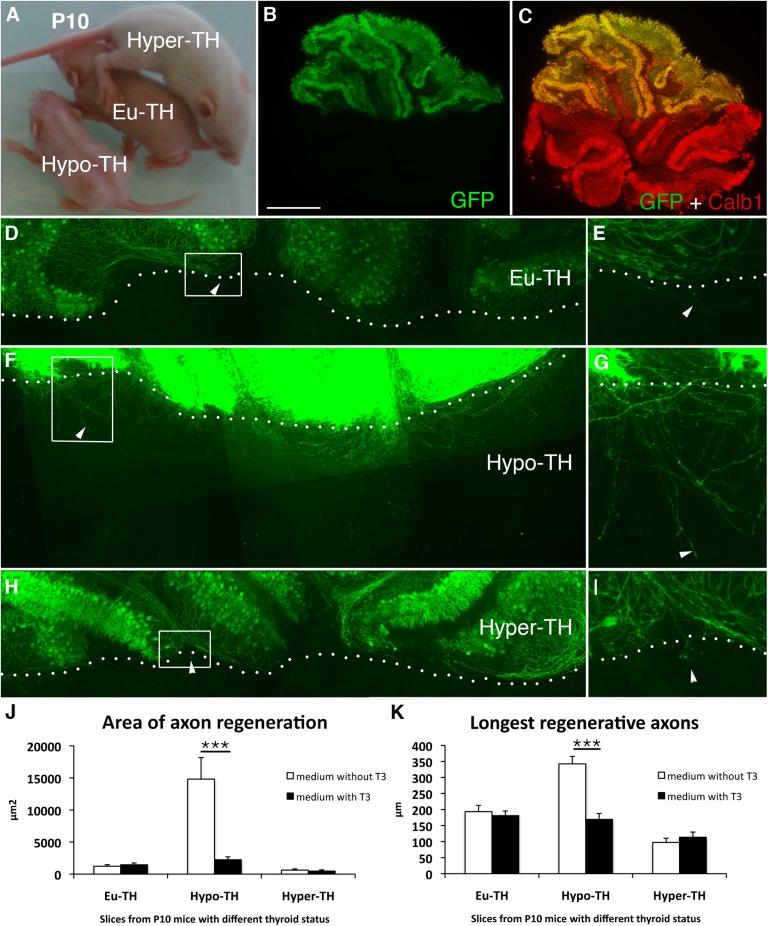
We then investigated whether the absence of T3 in vivo delayed the loss of regenerative capacity beyond P10, the age at which it is normally completed (9, 18). Cerebellar explants were prepared from three groups of P10 littermates (euthyroid, hypothyroid, and hyperthyroid pups; Fig. 2A). The blood level of T3 was measured in each group of animals to validate their thyroid status (Table S1). In coculture assays (Fig. 2 B and C) performed in the absence of T3, the area covered by regenerating GFP+ PC axons was 12-fold higher in hypothyroid than in euthyroid cerebella (Fig. 2 D–G and J) and the regenerated axons were 77% longer (Fig. 2 D–G and K). Hyperthyroidism had no significant additional influence (Fig. 2 H–K). Addition of T3 to the culture medium strongly reduced axon regeneration by PCs from hypothyroid animals, although it did not affect PCs from euthyroid or hyperthyroid animals (Fig. 2 J and K). Thus, the absence of T3 in vivo prolongs the period during which PCs are able to regenerate in organotypic culture assays, whereas an excess of T3 does not seem to have any influence on this ability.
Fig. 2.
T3 depletion prolongs the period of developmental plasticity of PC axons. (A) To study PC regenerative capacity in P10 animals in T3-depleted conditions, we generated euthyroid (Eu-TH), hypothyroid (Hypo-TH), and hyperthyroid (Hyper-TH) pups of the L7-GFP-BAC line. Thyroid status can be detected visually: Euthyroid pups are bigger than hypothyroid pups but smaller than hyperthyroid pups. (B and C) Only half of the litter expressed GFP in all PCs when Swiss females were crossed with transgenic L7-GFP-BAC heterozygous males. The dorsal half of cerebellar slices from L7-GFP-BAC mice were cultured and apposed to the ventral half from their GFP-negative littermates. Thus, all the GFP-immunoreactive axons present in the ventral half were regenerative PC axons. The ventral half was visualized by immunostaining with anti-Calb1 antibodies (C). Photomicrographs of euthyroid (D and E), hypothyroid (F and G), and hyperthyroid (H and I) cocultures. E, G, and I are magnified views of D, F, and H, respectively. The arrowheads indicate regenerating axons, and the dotted lines indicate the sites of axotomy. Quantitative analysis of the area of axon regeneration (J) and the longest regenerative axons (K) is shown. The cocultures were grown in the presence (black bars) or absence (white bars) of T3. Values are means ± SEM (***P < 0.001, Kruskal–Wallis test with Mann–Whitney post hoc test). (Scale bar: B and C, 450 μm; D, F, and H, 240 μm; E, G, and I, 80 μm.) n = 17, 18, and 17 for Eu-TH, Hypo-TH, and Hyper-TH, and n = 15, 18, and 17 in the presence of T3 for Eu-TH, Hypo-TH, and Hyper-TH, respectively.
T3 Receptor α1 Is Involved in the T3-Induced Loss of PC Axon Regenerative Capacity in Organotypic Cultures.
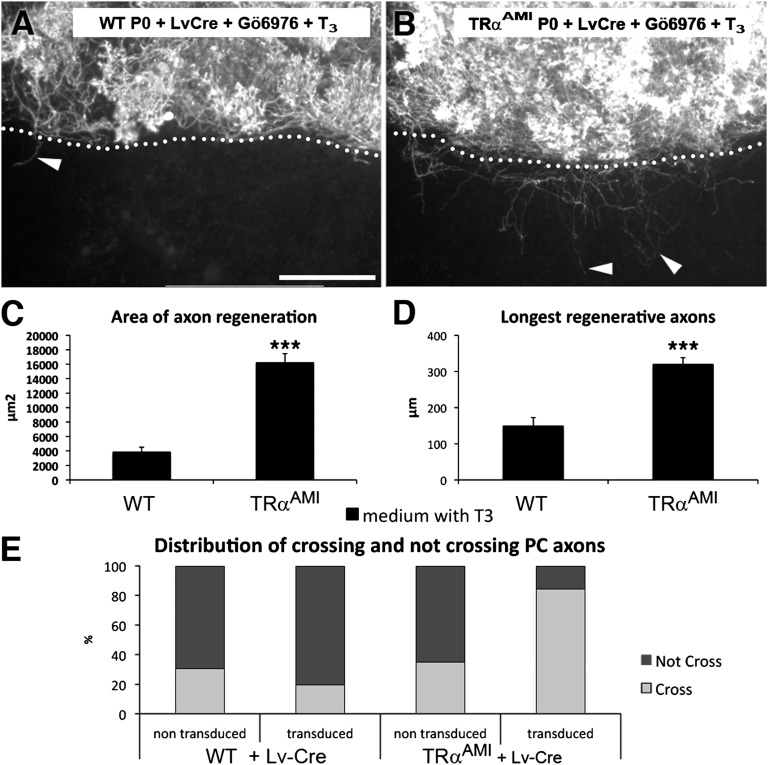
T3 acts directly on gene transcription by binding to the T3 receptor (TR) α1 or TRβ1/2 [thyroid hormone receptor alpha1 (THRA1) and thyroid hormone receptor beta1/2 (THRB1/2) according to the MGI nomenclature] nuclear receptors, encoded by the genes TRα and TRβ [Thra and Thrb (20)]. Both receptors are expressed by PCs (21–23). We used a mouse model (TRαAMI line) in which CRE/loxP recombination is used to trigger the expression of a dominant-negative mutant receptor, TRα1L400R (24). Cerebellar slices from newborn TRαAMI mice were transduced with a lentiviral vector (Lv) expressing Cre recombinase driven by the CMV promoter (Fig. S3 A and B) and were grown in the presence of T3. Gö6976 was added at the time of axotomy for 48 h to increase survival (Fig. S3C). It is important to note that the regenerative axons elongated in a WT TRα1 environment (Calb1−/− slices). T3 exposure failed to inhibit the regenerative ability of PCs expressing mutated TRα1L400R (Fig. 3 A and B), whose axons covered an area fourfold larger than those of control PCs, and whose axons were more than twice as long (Fig. 3 C and D).
Fig. 3.
TRα1 mediates the T3-induced loss of PC regenerative capacity. Photomicrographs of newborn mouse (P0) cocultured slices transduced with Lv encoding the Cre recombinase (LvCre) and immunostained with anti-Calb1 antibodies: slices from WT (A) and TRαAMI (B) mouse lines. The dotted lines indicate the site of axotomy, and the arrowheads point to regenerating axons. (Scale bar: 240 μm.) Quantitative analysis of PC axon regeneration: area of axon regeneration (C) and longest regenerative axons (D). All cultures were grown in the presence of Gö6976 at the time of axotomy to increase PC survival. Values from WT and TRαAMI animals are plotted as the mean ± SEM (***P < 0.001, Mann–Whitney test). n = 14 WT and 66 TRαAMI. (E) Distribution of crossing and noncrossing PC axons (Fig. S4 C and I). The histogram illustrates percentages of PCs transduced or nontransduced with Lv-Cre on WT or TRαAMI slices that either cross or do not cross the site of axotomy. The frequency distribution of sections in the four groups was compared between the various experimental conditions using the Fisher exact test (***P < 0.001). WT: n = 32 nontransduced, n = 15 transduced; TRαAMI: n = 66 nontransduced, n = 77 transduced.
To determine whether this effect was cell-autonomous, we compared the behavior of Cre-transduced PCs vs. nontransduced PCs on slices from WT and TRαAMI animals (Fig. S4). The majority of PCs expressing TRα1L400R were able to regenerate, whereas the majority of those expressing TRα1 did not regenerate (Fig. 3E). Although our results do not exclude the involvement of TRβ1/2, they clearly show that T3 acts on TRα1 and inhibits PC axonal regeneration in a cell-autonomous manner in organotypic culture.
Krüppel-Like Factor 9 Mediates the Inhibitory Effect of T3 on PC Axon Regeneration.
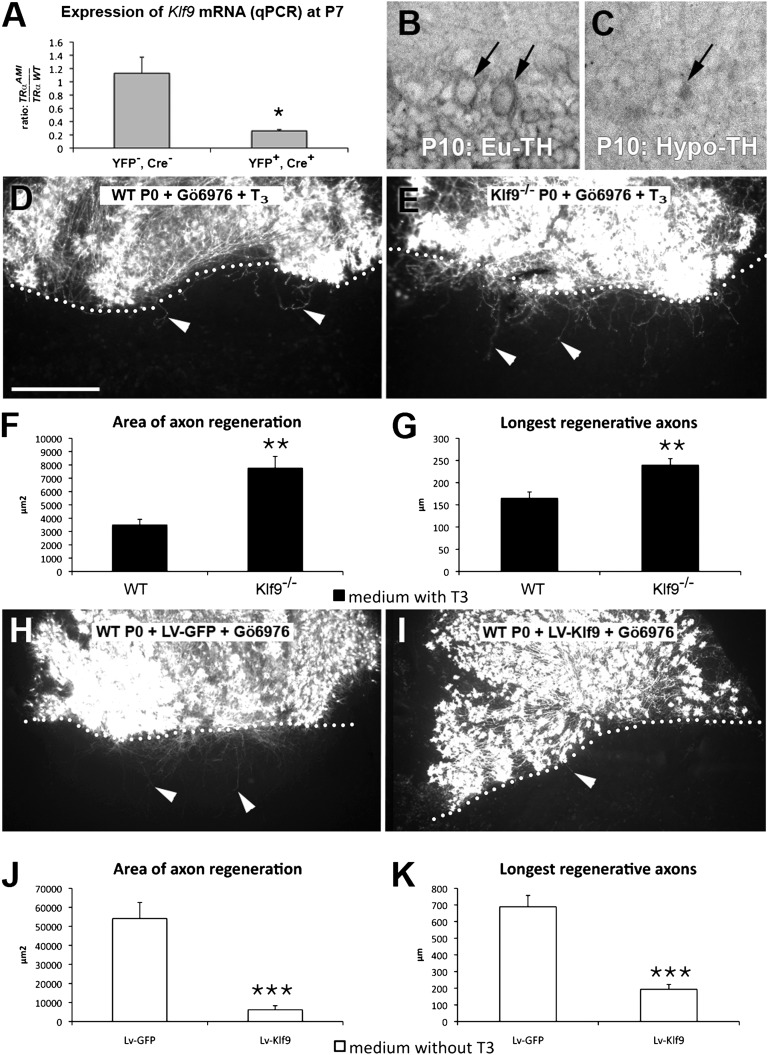
Klf9 is a T3 target gene in several neuronal cell types (16, 17). It encodes a transcription factor identified as an inhibitor of axonal regeneration in murine retinal ganglion cells (5). To determine whether Klf9 is under the control of TRα1 in PCs, we crossed the TRαAMI mouse line with the ROSA26-lox-STOP-lox-EYFP floxed and Ptf1a Cre deleter line (Fig. S5A). After dissociating the cerebella of P7 animals, YFP-expressing cells (PCs and interneurons) were sorted out by FACS to measure the expression of Klf9 mRNA by quantitative RT-PCR. Among the cells expressing KLF9, only PCs express TRα1L400R and YFP (Fig. S5B). They showed a considerable reduction of Klf9 mRNA by 75% (Fig. 4A).
Fig. 4.
KLF9 reduces the capacity of PCs to regenerate their axons. (A) Graphic representation of Klf9 mRNA expression in the cerebellum at P7. Shown here is the ratio of Klf9 expression in TRαAMI/+ and TRα WT (TRα WT) mice, both in a ROSA26-lox-STOP-lox-EYFP (R26YFP)/+; Ptf1aCre/+ background. The left bar represents YFP− cells (granule cells and other cell types), and the right bar represents YFP+ cells (PCs and interneurons; a description of the experiment is provided in Fig. S5). When TRα1L400R is expressed in PCs, the Klf9 mRNA level is reduced by fourfold compared with WT (*P < 0.05, Mann–Whitney test). Photomicrographs of in situ hybridization of euthyroid (Eu-TH; B) and hypothyroid (Hypo-TH; C) sections from P10 mice with a Klf9 probe; the arrows point to the PC layers. Photomicrographs of newborn WT (D) and Klf9−/− mouse (E) dorsal slices apposed to Calb1−/− ventral slices, cultured in the presence of T3, and immunostained with anti-Calb1 antibodies are shown. Quantitative analysis of the area of axon regeneration (F) and the longest regenerative axons (G) is shown. Photomicrographs of dorsal cerebellar slices transduced with GFP-expressing Lv (LV-GFP; H) or KLF9-expressing Lv (LV-KLF9; I), apposed to Calb1−/− ventral slices cultured in the absence of T3, and immunostained with anti-Calb1 antibodies are shown. Quantitative analysis of the area of axon regeneration (J) and the longest regenerative axons (K) is shown. The dotted lines indicate the site of axotomy, and the arrowheads point to regenerating axons. Black bars represent cultures grown in the presence of T3, and white bars represent those grown in the absence of T3. All cultures were grown in the presence of Gö6976 at the time of axotomy to increase PC survival. (Scale bars: B and C, 60 μm; D, E, H, and I, 240 μm.) Values are means ± SEM (**P < 0.01, ***P < 0.001 Mann–Whitney test). n = 32 WT, 41 KO, 14 Lv-GFP, and 13 Lv-KLF9.
To address the possibility that KLF9 might mediate the inhibitory effect of T3 on PC axon regeneration, we then compared Klf9 expression between hypothyroid and euthyroid animals by in situ hybridization at P10. Klf9 mRNA was detected in both PCs and inner granule cells (Fig. 4B), and its level was markedly decreased by T3 depletion in both neuron populations (Fig. 4C). Similar results were obtained when the expression of β-gal was quantified in the Klf9 KO mice. In this mouse line, Klf9 was replaced by the reporter gene lacZ, and it is therefore driven by the Klf9 promoter (Fig. S6).
To determine whether T3 acts on PCs through KLF9 expression, we compared the effect of T3 on regeneration of PCs from Klf9−/− and WT mice on Calb1−/− slices in the presence of Gö6976 (Fig. 4 D–G). Klf9−/− PCs displayed a smaller T3-induced decrease in regenerative capacity than WT PCs: The area and length of regenerative PC axons elongating in a WT environment for KLF9 were, respectively, twofold and 1.45-fold higher (Fig. 4 F and G). These moderate effects contrasted with the dramatic effect of T3 on WT PCs (Fig. S7).
We finally investigated whether KLF9 overexpression itself inhibited PC axon regeneration in a WT KLF9 environment in vitro. When cerebellar slices of newborn mice were transduced with an Lv encoding Klf9 and grown in the absence of T3, the capacity of PCs to regenerate axons was markedly lower than that of PCs expressing GFP (Fig. 4 H–K, in the presence of Gö6976). To determine whether this effect was cell-autonomous, we compared PCs transduced with either Lv-KLF9 or control Lv-GFP, as well as nontransduced PCs of the same slices. Almost 90% of the Lv-KLF9–transduced PCs were unable to regenerate even in the absence of T3, whereas in all three control PC populations, the majority of PCs regenerated (Fig. S8).
Together, our findings demonstrate that the T3-induced developmental loss of PC axon regenerative capacity is largely mediated by KLF9.
Discussion
This study shows that T3 is a key factor to determine the time at which PCs lose their ability to regenerate their axons in organotypic cultures. This function of T3 is mainly mediated by TRα1 and involves its downstream target Klf9. This effect of T3 on developing mammalian CNS neurons is a unique finding, because T3 had previously been shown to promote axon regeneration in sectioned adult mammal nerves in the peripheral nervous system (25).
Role of T3, TRα1, and KLF9 in the Developmental Loss of PC Axon Regenerative Ability.
We have previously shown that newborn mouse PCs lose the ability to regenerate their axons by the end of the first week in organotypic culture (18, 19). In these experiments, T3 was present in the culture medium through the addition of 25% (vol/vol) horse serum. Here, in the absence of T3 (using serum-free medium), PCs lose their ability to regenerate later. Thus, T3 determines the onset of the loss of PC regenerative capacity, an event that coincides with peak circulating levels of T3.
The respective functions of the T3 nuclear receptors TRα1 and TRβ1 in PCs are controversial. In vivo PC dendritogenesis is reduced in mice expressing dominant-negative TRα1L400R or TRα1R384C (26, 27), as well as in ex vivo in TRα KO mice (28). However, the TRβ1 dominant-negative mutation also affects PC differentiation (29). Here, we found that TRα1L400R expression by PCs was sufficient to protect them from the inhibitory effect of T3 on axon regeneration. This effect was cell-autonomous because the behavior of PCs was dependent on which form of TRα1 was expressed; TRα1L400R-expresssing PCs were able to regenerate, whereas WT TRα1-expressing PCs were not. Thus, our work demonstrates the involvement of TRα1 but does not rule out an eventual implication of TRβ1.
Several lines of evidence suggest that Klf9 is a direct transcriptional target of T3 (17). Here, we show that this is also likely to be the case in PCs. The Klf9 gene encodes a transcription factor that, together with Klf4, regulates the developmental loss of the regenerative capacity of retinal ganglion cell axons (5). We used gain- and loss-of-function approaches to establish that KLF9 largely mediates the T3-induced loss of PC regenerative ability during development. Altogether, our results show the involvement of the T3/TRα1/KLF9 pathway in the loss of PC axon regenerative ability during development.
T3 Orchestrates a General Change in Vertebrate Brain Properties, Reducing Plasticity.
The role of T3 in PC dendritic development and synaptogenesis has been clearly demonstrated in vivo (30, 31) and in vitro (21, 32). T3 also acts on other cell types (30, 31). In particular, it promotes the differentiation of oligodendrocytes (33), which express molecules that inhibit axon growth (2, 3). Furthermore, the loss of axonal regenerative capacity coincides with myelination (1, 34). Thus, T3 could indirectly abrogate PC regenerative capacity by promoting oligodendrocyte differentiation and myelin formation. However, we have previously shown that PCs lose their ability to regenerate their axons in the presence of T3, even in the absence of oligodendrocytes (19). In our coculture essay, the environment in which axotomized axon elongate is independent of other conditions (i.e., with slices from Calb1−/− being WT for TRα1 or KLF9 expression), we show that the action of T3 via TRα1 and KLF9 on PC regenerative capacity is cell-autonomous. The transition from the ability to the inability to regenerate axons is likely due to profound modifications of both neuron differentiation and the environment. Interestingly, our results also reveal that this transition is permanent, because once the P10 animals have been exposed to T3, the regenerative ability cannot be reversed by omitting T3 in the slice culture medium. Thus, T3 controls at least two important processes that reduce brain plasticity. We found recently that the promotion of myelin formation by T3 is not a cell-autonomous process but is secondary to some events taking place in neurons (35). T3 would thus orchestrate a complex network of cellular interactions, which all contribute to determine the correct timing for PCs to lose their regenerative capacity, suggesting that the loss of axonal regenerative ability could be part of a general process of brain maturation. It remains to be shown whether this maturation also involves other neuronal cell types.
The role of T3 in terminating axon regeneration during development seems to be conserved across many vertebrate species. Xenopus laevis, for example, displays robust regeneration of neural structures as larvae but partially or totally loses this capability after T3-induced metamorphosis (36, 37). In chicks, circulating T3 levels increase before hatching (38), and this coincides with the loss of axon regenerative capacity (39). Birds are classified as precocial or altricial according to their degree of maturation and physiological capabilities at hatching. In precocial birds, thyroid function is already well developed during the later part of incubation and hatchings have relatively mature sensory and locomotor capabilities, whereas thyroid maturation occurs mainly after hatching in altricial birds, as is also the case with their sensory and motor functions (38). Interestingly, in sheep, which are mature at birth, the increase in circulating T3 levels occurs before birth (40) and axon regenerative ability is lost in the late embryonic period (41). This implies that humans likely lose their axon regenerative ability before birth, because the T3 peak occurs prenatally (40).
Thus, our results indicate that the physiological T3 burst in mammals is involved in the developmental loss of PC axon regenerative capacity. This effect may be part of a more general process of T3-dependent maturation that readies the animal for its postnatal environment.
Materials and Methods
A complete description of the material and methods used in this study is provided in SI Materials and Methods.
Animals.
All animal procedures were approved by the Ile de France Ethics Committee (p3/2009/020).
Slice Culture.
Cerebellar organotypic cultures were prepared from newborn (P0) and P10 Swiss mice as previously described (19, 32). Each experiment was performed three times with at least four animals each time. For each experiment, the number of slices is given in the figure legend, together with the statistic tests.
Supplementary Material
Acknowledgments
We thank Teddy Fauquier for providing various mouse lines and Isabelle Caillé, Ekrem Dere, Fatiha Nothias, and Alain Trembleau for helpful discussions. This work was supported financially by the Centre National de la Recherche Scientifique, Université Pierre et Marie Curie (Grant ANR-07-NEURO-043-01) and International Foundation for Research in Paraplegia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119853109/-/DCSupplemental.
References
- 1.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14(1):118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu Rev Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. [DOI] [PubMed] [Google Scholar]
- 4.Cai D, et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Field PM, Raisman G. Failure of axon regeneration in postnatal rat entorhinohippocampal slice coculture is due to maturation of the axon, not that of the pathway or target. Eur J Neurosci. 1995;7:1164–1171. doi: 10.1111/j.1460-9568.1995.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen DF, Jhaveri S, Schneider GE. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc Natl Acad Sci USA. 1995;92:7287–7291. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusart I, Airaksinen MS, Sotelo C. Purkinje cell survival and axonal regeneration are age dependent: an in vitro study. J Neurosci. 1997;17:3710–3726. doi: 10.1523/JNEUROSCI.17-10-03710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianola S, Rossi F. Evolution of the Purkinje cell response to injury and regenerative potential during postnatal development of the rat cerebellum. J Comp Neurol. 2001;430(1):101–117. doi: 10.1002/1096-9861(20010129)430:1<101::aid-cne1017>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Oishi Y, Baratta J, Robertson RT, Steward O. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: Effects of age and neurotrophic factors. J Neurotrauma. 2004;21:339–356. doi: 10.1089/089771504322972121. [DOI] [PubMed] [Google Scholar]
- 12.Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregón MJ. Thyroid hormones in tissues from fetal and adult rats. Endocrinology. 1994;134:2410–2415. doi: 10.1210/endo.134.6.8194467. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier K, et al. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadj-Sahraoui N, Seugnet I, Ghorbel MT, Demeneix B. Hypothyroidism prolongs mitotic activity in the post-natal mouse brain. Neurosci Lett. 2000;280(2):79–82. doi: 10.1016/s0304-3940(00)00768-0. [DOI] [PubMed] [Google Scholar]
- 15.Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: Paradox or duality? Mol Cell Endocrinol. 2009;313(1-2):36–49. doi: 10.1016/j.mce.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Martel J, Cayrou C, Puymirat J. Identification of new thyroid hormone-regulated genes in rat brain neuronal cultures. Neuroreport. 2002;13:1849–1851. doi: 10.1097/00001756-200210280-00003. [DOI] [PubMed] [Google Scholar]
- 17.Denver RJ, Williamson KE. Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology. 2009;150:3935–3943. doi: 10.1210/en.2009-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoumari AM, Wehrlé R, De Zeeuw CI, Sotelo C, Dusart I. Inhibition of protein kinase C prevents Purkinje cell death but does not affect axonal regeneration. J Neurosci. 2002;22:3531–3542. doi: 10.1523/JNEUROSCI.22-09-03531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouslama-Oueghlani L, Wehrlé R, Sotelo C, Dusart I. The developmental loss of the ability of Purkinje cells to regenerate their axons occurs in the absence of myelin: An in vitro model to prevent myelination. J Neurosci. 2003;23:8318–8329. doi: 10.1523/JNEUROSCI.23-23-08318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 21.Heuer H, Mason CA. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor alpha1. J Neurosci. 2003;23:10604–10612. doi: 10.1523/JNEUROSCI.23-33-10604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallis K, et al. The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24:1904–1916. doi: 10.1210/me.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley DJ, Young WS, 3rd, Weinberger C. Differential expression of alpha and beta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci USA. 1989;86:7250–7254. doi: 10.1073/pnas.86.18.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quignodon L, Vincent S, Winter H, Samarut J, Flamant F. A point mutation in the activation function 2 domain of thyroid hormone receptor alpha1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol Endocrinol. 2007;21:2350–2360. doi: 10.1210/me.2007-0176. [DOI] [PubMed] [Google Scholar]
- 25.Panaite P-A, Barakat-Walter I. Thyroid hormone enhances transected axonal regeneration and muscle reinnervation following rat sciatic nerve injury. J Neurosci Res. 2010;88:1751–1763. doi: 10.1002/jnr.22344. [DOI] [PubMed] [Google Scholar]
- 26.Venero C, et al. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes Dev. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fauquier T, et al. Severe impairment of cerebellum development in mice expressing a dominant-negative mutation inactivating thyroid hormone receptor alpha1 isoform. Dev Biol. 2011;356:350–358. doi: 10.1016/j.ydbio.2011.05.657. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K, et al. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA. 2001;98:3998–4003. doi: 10.1073/pnas.051454698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portella AC, et al. Thyroid hormone receptor β mutation causes severe impairment of cerebellar development. Mol Cell Neurosci. 2010;44(1):68–77. doi: 10.1016/j.mcn.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev. 1997;18:462–475. doi: 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- 31.Koibuchi N. The role of thyroid hormone on cerebellar development. Cerebellum. 2008;7:530–533. doi: 10.1007/s12311-008-0069-1. [DOI] [PubMed] [Google Scholar]
- 32.Boukhtouche F, et al. Induction of early Purkinje cell dendritic differentiation by thyroid hormone requires RORα. Neural Dev. 2010;5:18. doi: 10.1186/1749-8104-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 34.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 35.Picou F, Fauquier T, Chatonnet F, Flamant F. A bimodal influence of thyroid hormone on cerebellum oligodendrocyte differentiation. Mol Endocrinol. 2012;26:608–618. doi: 10.1210/me.2011-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beattie MS, Bresnahan JC, Lopate G. Metamorphosis alters the response to spinal cord transection in Xenopus laevis frogs. J Neurobiol. 1990;21:1108–1122. doi: 10.1002/neu.480210714. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs KM, Chittur SV, Szaro BG. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. Eur J Neurosci. 2011;33:9–25. doi: 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- 38.McNabb FMA. Avian thyroid development and adaptive plasticity. Gen Comp Endocrinol. 2006;147(7):93–101. doi: 10.1016/j.ygcen.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Hasan SJ, Keirstead HS, Muir GD, Steeves JD. Axonal regeneration contributes to repair of injured brainstem-spinal neurons in embryonic chick. J Neurosci. 1993;13:492–507. doi: 10.1523/JNEUROSCI.13-02-00492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher DA, Polk DH, Wu SY. Fetal thyroid metabolism: A pluralistic system. Thyroid. 1994;4:367–371. doi: 10.1089/thy.1994.4.367. [DOI] [PubMed] [Google Scholar]
- 41.Meuli-Simmen C, et al. The fetal spinal cord does not regenerate after in utero transection in a large mammalian model. Neurosurgery. 1996;39:555–560, discussion 560–561. doi: 10.1097/00006123-199609000-00024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.