Abstract
The benefits of protected areas (PAs) for biodiversity have been questioned in the context of climate change because PAs are static, whereas the distributions of species are dynamic. Current PAs may, however, continue to be important if they provide suitable locations for species to colonize at their leading-edge range boundaries, thereby enabling spread into new regions. Here, we present an empirical assessment of the role of PAs as targets for colonization during recent range expansions. Records from intensive surveys revealed that seven bird and butterfly species have colonized PAs 4.2 (median) times more frequently than expected from the availability of PAs in the landscapes colonized. Records of an additional 256 invertebrate species with less-intensive surveys supported these findings and showed that 98% of species are disproportionately associated with PAs in newly colonized parts of their ranges. Although colonizing species favor PAs in general, species vary greatly in their reliance on PAs, reflecting differences in the dependence of individual species on particular habitats and other conditions that are available only in PAs. These findings highlight the importance of current PAs for facilitating range expansions and show that a small subset of the landscape receives a high proportion of colonizations by range-expanding species.
Keywords: conservation, climate change adaptation, nature reserves
More than 10% of the Earth’s land surface has already been designated as protected area (PA) (1, 2), and there are calls to expand protection to 17% of the land (3, 4). However, the importance of a PA approach to conservation is open to question in the context of anthropogenic climate change and other environmental drivers that are causing species to shift their distributions. Terrestrial species’ distributions are shifting to higher latitudes and elevations (5–7), many species are at increased risk of extinction (8, 9), and the composition of biological communities is changing (10, 11). These observations, combined with predicted future changes to the composition of biological communities inside PAs (12–16), call into question (i) the long-term protection provided to species by PAs, because species may shift out of the sites where they were previously considered to be protected, and (ii) the legislative basis for protection in situations where legal PA designation stems from the occurrences of particular species or biological communities (17, 18) that may not remain within the PAs in the future. PAs have, on occasion, been downgraded or dedesignated in the face of competing demands (19), and there are suggestions that a PA approach could be outmoded (20) or that underperforming PAs should be replaced (21).
However, the overall risk to a species from climate change (and other large-scale drivers of distribution change) depends on the balance between losses of populations within the former range, on the one hand, and gains associated with the colonization of new regions where the climate or other conditions improve (8, 9). PAs could, therefore, continue to play a critical role for conservation if they protect habitats that are colonized by species shifting into new regions. Many studies have assessed the potential performance of PAs under climate change (12–16), but empirical evidence is lacking in terms of the capacity of PAs to accommodate changes to the distributions of breeding populations of species. The analyses presented here consider the value of PAs in the context of facilitating range expansions at species’ leading-edge boundaries, and they provide an empirical assessment of whether species disproportionately colonize PAs as they expand their distributions into new regions.
Our study considers distribution changes in a variety of species and taxonomic groups, capitalizing on the detailed distribution records that exist for wildlife in Britain since the 1960s, especially in southern Britain (where most of the range-expanding species occur). An estimated 84% of species, drawn from many different taxonomic groups, have shifted their ranges north in Britain (6, 22), a phenomenon directly linked to regional warming (7). However, species vary in the rates at which their ranges have shifted, because individual species are affected by different combinations of climatic and additional nonclimatic factors (7). Nonetheless, climate change is the reason why the predominant direction of range expansion is north: the species that we consider here were historically restricted to the warmer southern parts of Britain and belong to those taxonomic groups previously shown to be shifting north. The PAs that we consider are Sites of Special Scientific Interest (Fig. 1A), the primary terrestrial wildlife conservation designation in Great Britain (GB), and they correspond to International Union for the Conservation of Nature level IV of protection. We present two sets of analyses. The first set is for seven focal species for which there are high-quality data on the exact locations of colonizing populations based on full survey data across the species’ GB ranges. The second set is for 256 invertebrate species from groups for which distribution data are less complete but records are sufficiently robust that we can assess whether the patterns revealed by the seven focal species are observed more widely.
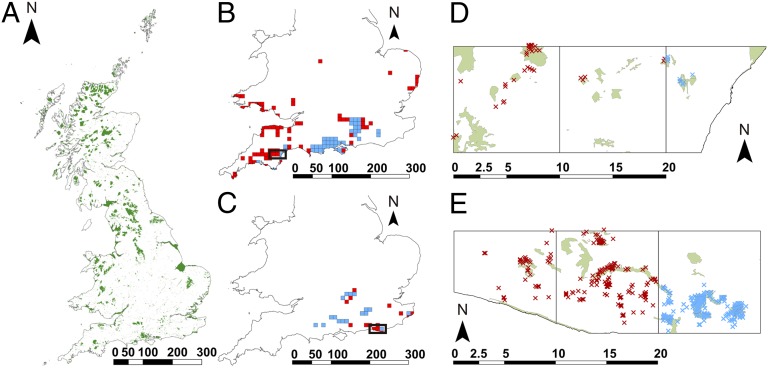
Fig. 1.
(A) Locations of PAs (Sites of Special Scientific Interest) within Great Britain. (B) Dartford warbler S. undata distribution change, showing 10 × 10-km grid squares considered to be core (occupied before and since the end of 1991; blue squares) and colonized (occupied since 1991 only; red squares). (C) Silver-spotted skipper H. comma distribution change, showing 10 × 10-km grid squares considered to be core (occupied during 1970–1982 and 1995–2010; blue squares) and colonized (not occupied 1970–1982 but occupied 1995–2010; red squares). (D) Expanded view of the 10 × 10-km grid squares highlighted in B showing the location of PAs in green, the locations of S. undata records in core areas (blue), and the locations of records in colonized areas (red). (E) Expanded view of the 10 × 10-km grid squares highlighted in C showing the location of PAs in green, the locations of H. comma records in core areas (blue), and the locations of records in colonized areas (red). Scale bars are in kilometers.
Results
Seven Focal Species with Intensive Survey Data.
The first analysis considers PA use by seven localized but, nonetheless, expanding species (five birds and two butterflies) that have been subject to exhaustive repeat surveys, during which potential breeding sites have been surveyed irrespective of the PA status of the land (23–30) (Table 1 and SI Materials and Methods). These species are the only expanding species in the United Kingdom (UK) for which such high-quality data are available. The bird species were monitored as part of a rolling program of coverage of a wider suite of rare UK breeding species, but the program represents all of the species in the program with ranges that have expanded during its lifetime. The butterflies are the two species subject to repeat patch-based monitoring that have also expanded over the period considered. The species were not selected for their associations with PAs, which can be seen from the fact that the different species vary from heavily to hardly associated with PAs (Table 1).
Table 1.
Use of PAs by species within newly colonized parts of their geographic distributions compared with the availability of PAs within the 10 × 10-km landscapes colonized
| Species | Habitat | Region | Period (first to last survey dates) | Number of 10-km grid squares colonized | Proportion of recorded colonizations in PAs | Proportion of PA land available* | PA use ratio† | Wilcoxon v value‡ | P |
| Butterflies | |||||||||
| Hesperia comma | Calcareous grassland | England | 1982–2002 | 24§ | 0.64 | 0.04 | 14.4 | 297 | <0.0001 |
| Polyommatus bellargus | Calcareous grassland | Dorset, England | 1978–1999 | 15§ | 0.45 | 0.11 | 4.2 | 114 | 0.0012 |
| Birds | |||||||||
| Botaurus stellaris | Reedbeds | United Kingdom | 1990–2008¶ | 41‖ | 0.41 | 0.13 | 3.2 | 577 | 0.029 |
| Burhinus oedicnemus | Arable, grassland, heathland | England | 1985–2010¶ | 24‖ | 0.07 | 0.03 | 2.0 | 61 | NS |
| Caprimulgus europaeus | Woodland, heathland | United Kingdom | 1981–2004¶ | 125‖ | 0.08 | 0.08 | 1.1 | 1,993 | NS |
| Lullula arborea | Heathland, woodland | United Kingdom | 1986–2006¶ | 79‖ | 0.42 | 0.06 | 7.1 | 2,485 | <0.0001 |
| Sylvia undata | Heathland | United Kingdom | 1974–2006¶ | 82‖ | 0.74 | 0.14 | 5.4 | 3,000 | <0.0001 |
NS, not significant.
*Proportion of the land classed as PA within 10 × 10-km grid squares with colonization.
†PA use ratio calculation is detailed in SI Materials and Methods; values > 1 indicate disproportionate use of PA land.
‡The v values are sums of ranks assigned to the differences with positive signs.
§Number of 10 × 10-km squares containing colonizations of individual sites (species recorded as absent from individual sites during the first survey year shown).
¶For bird species, colonizations relate to new site records after 1991 (details of each species in SI Materials and Methods).
‖Number of 10 × 10-km squares colonized (recorded as absent from the 10 × 10-km squares before 1992).
We examined whether colonization events since the 1970s and 1980s were disproportionally associated with PAs (Table 1). New colonizations were defined as locations (1-ha grid resolution) within areas (10 × 10-km national ordnance survey grid squares) where a species was recorded as absent during the first survey period but present during subsequent surveys. From 7% to 74% (median = 42%, mean = 40%) of colonizations occurred within PAs, depending on the species (Table 1). The association of colonization records with PAs was compared with the area of PAs in the landscape as a whole, which was defined as the area of PA-designated land within the same 10 × 10-km squares that had been colonized. The study species were a median of 4.2 times more likely to colonize PAs than expected given the availability of PA land within the landscapes colonized (Table 1). Five of seven species were significantly more associated with PAs than by chance (Table 1), and all seven species showed PA use ratios > 1 (one-tailed sign test for seven of seven species, P = 0.008).
Recent distribution changes are shown for two exemplar species in Fig. 1: the Dartford warbler Sylvia undata (Fig. 1B) (30) and the silver-spotted skipper butterfly Hesperia comma (Fig. 1C) (23, 28). These two species illustrate three common patterns. First, patterns of expansion are patchy at this coarse scale, which reflects the availability of potential habitats provided by dwarf shrub heathlands on acidic soils in the case of S. undata and dry grasslands on chalk hills in the case of H. comma. Second, species show east and west patterns of expansion (Fig. 1B) and range infilling (Fig. 1C) as much as north extension, reflecting habitat availability and longitudinal climatic gradients (warmer summers in the east and milder winters in the west). Third, at finer resolution, colonization records are disproportionately associated with PAs, but not every PA is colonized (Fig. 1 D and E). For H. comma, the four largest PAs that remain uncolonized (shown in Fig. 1E) do not support suitable habitats for this species (they contain floodplain meadows and coastal vegetation but not the dry calcareous grasslands used by H. comma).
Invertebrate Species Expansions from Volunteer-Collected Data.
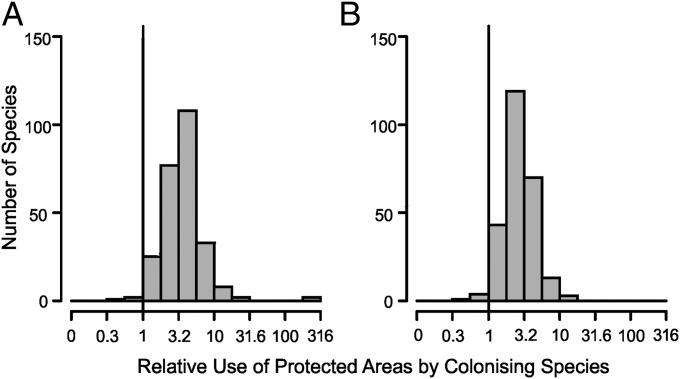
Our second analysis was for 256 invertebrate species from groups where distribution data were sufficient to split volunteer-collected records into two time periods (Materials and Methods), enabling us to identify colonizations. We were able to include 14 species of aquatic bugs, 32 butterfly species, 20 dragonfly and damselfly species, 22 species of grasshoppers and allies, 57 ground beetle species, 11 longhorn beetle species, 16 species of soldier beetles and allies, and 84 spider species. The 121,517 unique (after removing duplicates) 1-ha resolution colonization records for these species are not exhaustive, but they permit us to evaluate whether the conclusions for the seven focal study species hold across larger numbers of species; 251 of 256 species (98%) were recorded more frequently from PAs than expected given the occurrence of PAs within the landscapes colonized by each species (Wilcoxon signed rank test, v = 32,643, P < 0.0001) (Fig. 2A). This conclusion held regardless of taxonomic group (Wilcoxon signed rank tests, P ≤ 0.001 for each group separately; one-tailed sign test for eight of eight groups, P = 0.004) (Fig. 3A). Two ways to estimate the background distribution of biological recording by volunteers, and hence establish that the observed patterns for invertebrate species emerge from biased colonization of PAs and not biased recording, are analyses of records of the 25% most widespread resident (comparator) species (which occur widely both inside and outside PAs) (SI Materials and Methods) and analysis of multispecies recording events (SI Materials and Methods). These analyses show that records of colonizing species were even more strongly associated with PAs than the widespread comparator species that were already resident in the regions being colonized, and the conclusions also hold for an analysis of sites for which there is certain knowledge that they were visited by biological recorders (SI Materials and Methods).
Fig. 2.
The association of colonizing invertebrate species with PAs. (A) Colonization of PAs relative to PA availability (i.e., fraction of records from PAs ÷ fraction of land covered by PAs); a value greater than one (vertical black line) indicates that a colonizing species was recorded in PAs more frequently than expected from the availability of PA land in colonized 10 × 10-km grid squares (10 indicates 10 times more frequently; n = 256 species). (B) The same as in A after the exclusion of urban, suburban, and arable land (n = 255 species).
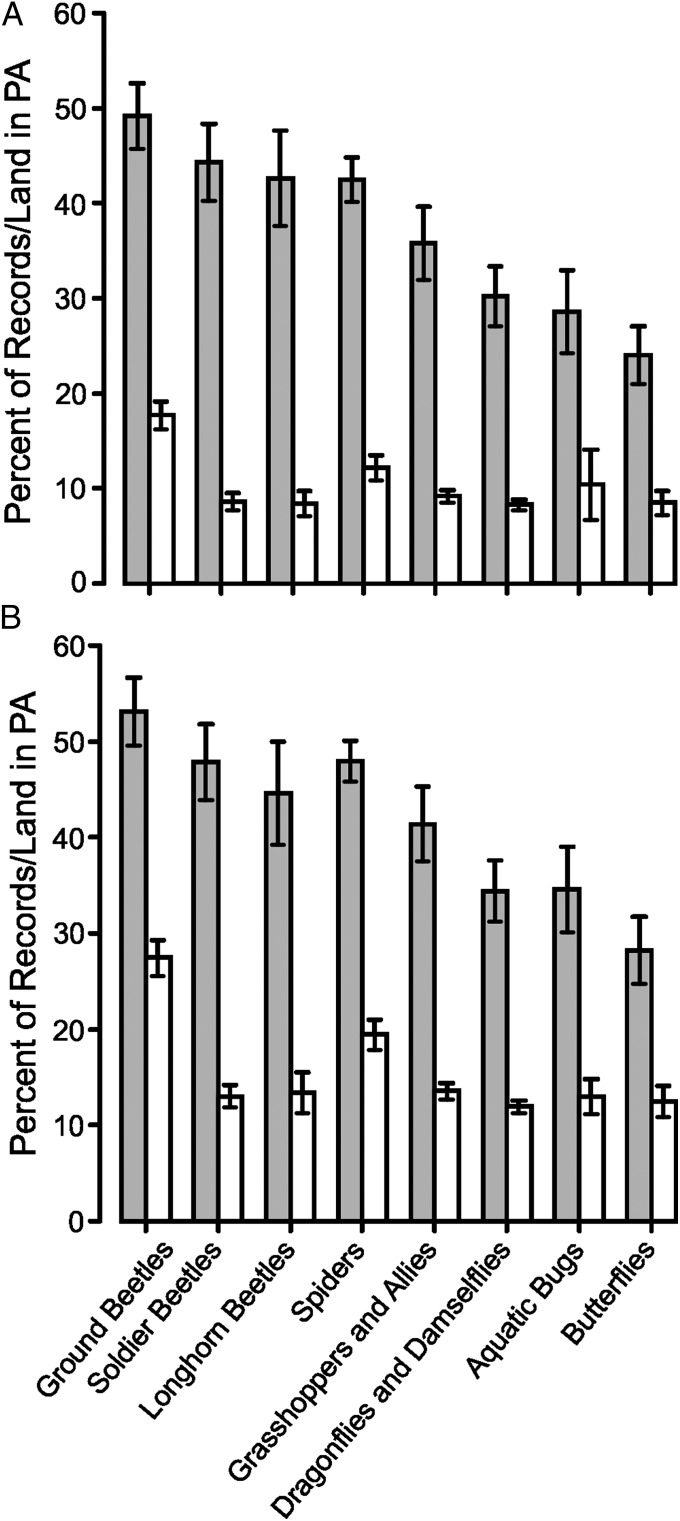
Fig. 3.
Mean (± SE) percentages of records per species from PAs for the eight taxonomic groups considered (gray bars), compared with the background availability of PA land (open bars). (A) The 100 × 100-m resolution records of colonizing species are more likely to be in PAs than at random given the availability of PA land in the colonized 10 × 10-km squares (Wilcoxon signed rank tests, P < 0.001 for each taxon). (B) The same as in A after the exclusion of urban, suburban, and arable land (Wilcoxon signed rank tests, P < 0.001 for each taxon).
A characteristic of both PAs and colonizing species is that they tend to be scarce in the most heavily modified environments. We examined if this characteristic could coincidentally be driving the association of these invertebrate species with PAs by repeating the analysis but excluding heavily modified urban, suburban, and arable areas, which are rarely used by most species. Of the 255 species that still had sufficient data for analysis, 247 (97%) species were still recorded more frequently from PAs than expected given the availability of PAs and other nonintensively used land within the landscapes colonized by each species (Wilcoxon signed rank test, v = 32,101, P < 0.0001) (Fig. 2B). This association held for all eight taxonomic groups (Wilcoxon signed rank tests, P < 0.001 for each group separately; one-tailed sign test for eight of eight groups, P = 0.004) (Fig. 3B). Thus, our general conclusions concerning the value of PAs from these less-exhaustive surveys are not due to the presence of heavily human-dominated areas within colonized landscapes.
Variation Among Species.
Both the focal and invertebrate species showed considerable variation in their associations with PAs in the areas that they colonize. The PA use ratios of the seven focal species varied from 1.1 to 14.4, ranging from near-equivalent colonization of sites inside and outside PAs to very high dependence on PAs by some habitat specialists. The two focal study species that were not significantly more associated with PAs than expected by chance were those species with the lowest habitat specificities (nightjar Caprimulgus europaeus in open woodland and heathland and stone curlew Burhinus oedicnemus on arable farmland and open heaths and grasslands) (Table 1). Across all seven focal species, approximately one-half of the new locations that were colonized fell outside PAs, indicating that, although PAs are important for colonizations, they are not the only places in these British landscapes that provide suitable breeding conditions for the focal species.
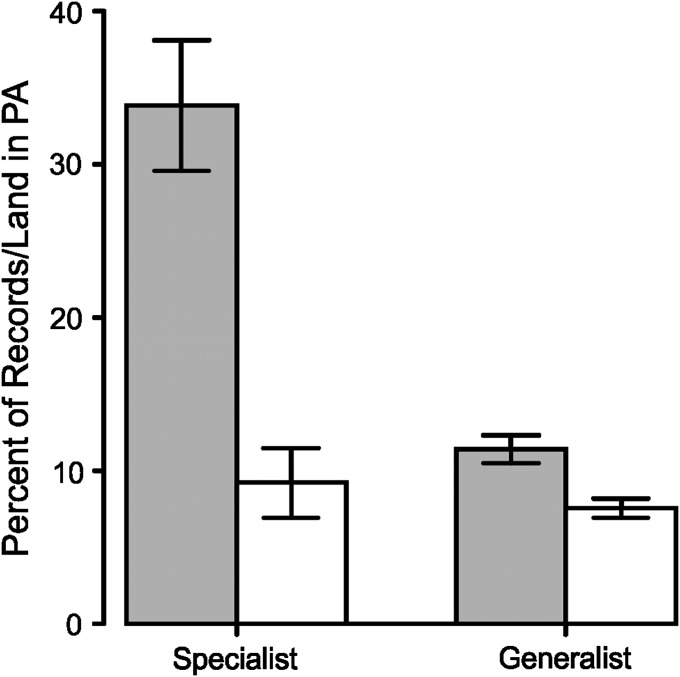
The same pattern of variation in PA use can also be seen among the much larger number of invertebrate species (ranging from a few species more likely to be recorded outside PAs to some species having great dependence on PAs, with PA use ratios of greater than 10) (Fig. 2). Substantial variation in the associations of species with PAs exists within all of the major taxonomic groups considered, which may be associated with levels of habitat specificity. For example, butterflies have been classified into habitat specialists and generalists (wider countryside species) (31). Colonizing habitat specialist butterflies have a median PA use ratio of 3.85 in the regions that they have colonized, whereas the corresponding ratio for generalists is only 1.33 (Fig. 4). Hence, we conclude overall that some species are heavily dependent on PA conditions for colonization, particularly if they are habitat specialists, whereas others also find breeding areas in the wider (non-PA) countryside.
Fig. 4.
Mean (± SE) percentages of records per butterfly species (gray bars) from PAs for colonizing habitat specialists (n = 18 species) and generalists (wider countryside species; n = 14), compared with the background availability of PA land in areas of colonization (open bars). Specialists are more strongly associated than generalists with PAs (Mann–Whitney U test comparison of specialist vs. generalist PA use ratios; W = 26, n1 = 18, n2 = 14, P < 0.0001).
Discussion
The results identify an ecological phenomenon, where a small percentage of the land surface receives a disproportionately large number of colonizations: on average, 40% of colonizations by the focal species fell into the 8.4% of the landscapes that were designated as PAs. It has long been known that most species occupy a small percentage of any given landscape, but this finding is most commonly interpreted as being a consequence of past population losses, whereas we show that it can also arise through localized colonization patterns.
Although the PAs considered here were not designated with colonizing species in mind, they perform the function of facilitating range expansion for a large number of species responding to climate change and other drivers of distribution change. The explanation as to why PAs are colonized disproportionately probably involves a combination of factors, reflecting the diversity of ecological requirements seen across the many species considered. The seven focal species show different levels of dependence on climate change and other factors (e.g., habitat quality, disturbance) (SI Materials and Methods) as they expand into new regions, and this diversity of drivers and responses is likely to extend to the larger number of invertebrate species considered. Nonetheless, the disproportionately poleward direction of range expansion across the many species considered is attributable to recent climate change (7, 22).
The value of PAs for colonization may be associated with their condition before designation as well as their subsequent protection and management. The best preexisting habitats are most likely to be designated as PAs, and these sites might have been colonized anyway, irrespective of legal designation. However, subsequent active reserve management may then improve habitat quality for potential colonists, and PAs also gain legal protection from major land use changes along with a general reduction in the threats prevalent in the wider countryside (e.g., chemical inputs, persecution, and disturbance). These postdesignation effects increase the distinction in the quality of habitats inside PAs compared with those habitats outside PAs. The relative importance of different PA attributes is likely to vary among species and sites, and additional research is needed to evaluate which attributes are most important in particular situations. The species included in the invertebrate analysis are not the primary focus for current site protection or conservation management in PAs (especially before their arrival), so the PAs principally provided suitable habitats for these expanding species to colonize, rather than species-specific conservation management. The five focal species that colonized PAs significantly more frequently than expected (Table 1) likewise benefit principally from the habitats provided within PAs, just like habitat specialist butterflies (Fig. 4), with the benefits deriving from both the predesignation condition of sites and the subsequent postdesignation improvement of habitat quality (23–25, 28–30) (SI Materials and Methods).
PAs disproportionately provide suitable conditions for the establishment of new local populations, which in turn provide emigrant individuals that are available to fuel the next stage of expansion. Conservation strategies that aim to facilitate the range expansions of species at their leading-edge range margins as they respond to climate change and other environmental drivers should, thus, retain high-quality habitats within existing PAs to provide targets for colonization—at least within landscapes that are heavily modified by human activities, such as those areas in lowland Britain. Additional research is needed to identify whether this conclusion is replicated in regions where there is less distinction between the habitats that are present inside and outside of PAs and whether this conclusion is replicated in other taxonomic groups.
Even in heavily modified landscapes, PAs are not the only locations colonized by species, and additional non-PA habitats are likely to be critical to the successful expansion of many species in fragmented landscapes. This need is reflected in the high level of variation among species in their reliance on PAs; slightly more than one-half of the locations colonized by the focal species were nondesignated sites (Table 1). Some of these non-PA locations represent potential sites that could be incorporated within the PA network in the future (i.e., habitats that are as unusual as those habitats already designated), but in other cases it would be more appropriate to safeguard the areas through conservation easements or other means (e.g., B. oedicnemus requires protection from farm machinery on arable farmland).
In conclusion, many species are only expected to be able to survive climate change if they are able to colonize new regions, replacing the populations that are lost at the trailing edges of their distributions. Our study shows that species disproportionately colonize PAs as they expand into new regions, and hence that current PAs remain valuable for conservation.
Materials and Methods
Species Selection.
We included all seven expanding species for which high-quality data were available from previous detailed surveys. These species were surveyed at high resolution without reference to the PA status of survey locations (details of the surveys, ecological requirements, and climate sensitivities are given in SI Materials and Methods). For the 256 species of invertebrates analyzed, we used species selection and date criteria identified in the work by Hickling et al. (6), which was updated to 2010; we included aquatic bugs (from first recording period in 1970–1980 to second recording period in 1990–2010), butterflies (1970–1982 to 1995–2010), dragonflies and damselflies (1960–1970 to 1985–2010), grasshoppers and allies (1960–1970 to 1985–2010), ground beetles (1965–1975 to 1990–2010), longhorn beetles (1960–1970 to 1985–2010), soldier beetles and allies (1965–1975 to 1990–2010), and spiders (1965–1975 to 1990–2010). These eight taxonomic groups represent separate invertebrate recording schemes in Britain.
National distribution data used in this paper were sourced from the National Biodiversity Network Gateway (http://data.nbn.org.uk/), Biological Records Centre (http://www.brc.ac.uk/), and Butterfly Conservation (http://www.butterfly-conservation.org/). Sites of Special Scientific Interest shape files were obtained from Natural England (http://www.naturalengland.org.uk/), Countryside Council for Wales (http://www.ccw.gov.uk/), and Scottish Natural Heritage (http://www.snh.gov.uk/). Land cover maps were obtained from the Natural Environment Research Council Centre for Ecology and Hydrology (http://www.ceh.ac.uk/LandCoverMap2000.html).
Resolution of Data and Associations with PAs.
We considered 100 × 100-m (1 ha) grid resolution records from the second period to be colonizations if they fell within landscapes (defined as 10 × 10-km grid squares) where that species had not been observed in the first recording period but where other species within the same taxonomic group had been recorded (i.e., recorders had visited the 10-km square in the first period). Within the 10 × 10-km grid squares colonized, we calculated (i) PA use for each species as the fraction of 100 × 100-m resolution records that fell within PAs (using ArcMap 9.3.1; cells of 1 ha that partially overlapped PA land were assigned to PA land in proportion to the fractional overlap) and (ii) background PA availability as the fraction of PA land in the same 10 × 10-km grid squares. We calculated a PA use ratio (i.e., PA use ÷ PA availability), with a value > 1 indicating disproportionate use of PA land. For statistical tests, PA use and PA availability were compared with Wilcoxon signed rank tests. Each species was regarded as a replicate in the multispecies analyses, and each 10 × 10-km grid square was regarded as a replicate for species with detailed surveys (Table 1). One-tailed probabilities are shown given the unidirectional hypothesis (all significant results remain significant with two-tailed tests). For the analysis that excluded the most heavily modified environments, we removed urban, suburban, and arable land cover (using Centre for Ecology and Hydrology Landcover 2000 data) and then repeated the analysis for the remaining land.
Supplementary Material
Acknowledgments
We thank the many, mainly volunteer, recorders responsible for data collection. We also thank referees for suggestions to improve the manuscript. We thank The Aquatic Heteroptera Recording Scheme, Biological Records Centre, British Arachnological Society (Spider Recording Scheme), British Dragonfly Society (Dragonfly Recording Scheme), British Trust for Ornithology, Butterfly Conservation, Cantharoidea and Buprestoidea Recording Scheme, Cerambycidae Recording Scheme, Countryside Council for Wales, Forestry Commission, Ground Beetle Recording Scheme, Joint Nature Conservation Committee, Natural England, Natural Environment Research Council, Orthoptera Recording Scheme, Royal Society for the Protection of Birds, and Scottish Natural Heritage for data and/or financial support for surveys. The project was funded by a Knowledge Exchange Grant from the Natural Environment Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All data are derived from existing databases, which are listed in the text.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210251109/-/DCSupplemental.
References
- 1.Chape S, Harrison J, Spalding M, Lysenko I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos Trans R Soc Lond B Biol Sci. 2005;360:443–455. doi: 10.1098/rstb.2004.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soutullo A. Extent of the global network of terrestrial protected areas. Conserv Biol. 2010;24:362–363. doi: 10.1111/j.1523-1739.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- 3.Convention on Biological Diversity 2010. Decision Adopted by the Conference of the Parties to the Convention of Biological Diversity at its Tenth meeting. Available at http://www.cbd.int/doc/decisions/COP-10/cop-10-dec-02-en.pdf. Accessed June, 2011.
- 4.Harrop SR. Living in harmony with nature? Outcomes of the 2010 Nagoya Conference of the Convention on Biodiversity. J Environ Law. 2011;23:117–128. [Google Scholar]
- 5.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 6.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distribution of a wide range of taxonomic groups are spreading polewards. Glob Change Biol. 2006;12:450–455. [Google Scholar]
- 7.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 8.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 9.Maclean IMD, Wilson RJ. Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci USA. 2011;108:12337–12342. doi: 10.1073/pnas.1017352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-Megías A, Menéndez R, Roy D, Brereton T, Thomas CD. Changes to the composition of British butterfly assemblages over two decades. Glob Change Biol. 2008;14:1464–1474. [Google Scholar]
- 11.Thibault KM, Brown JH. Impact of an extreme climatic event on community assembly. Proc Natl Acad Sci USA. 2008;105:3410–3415. doi: 10.1073/pnas.0712282105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns CE, Johnston KM, Schmitz OJ. Global climate change and mammalian species diversity in U.S. national parks. Proc Natl Acad Sci USA. 2003;100:11474–11477. doi: 10.1073/pnas.1635115100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dockerty T, Lovett A, Watkinson A. Climate change and nature reserves: Examining the potential impacts, with examples from Great Britain. Glob Environ Change. 2003;13:125–135. [Google Scholar]
- 14.Araújo MB, Cabeza M, Thuiller W, Hannah L, Williams PH. Would climate change drive species out of reserves? An assessment of existing reserve-selection methods. Glob Change Biol. 2004;10:1618–1626. [Google Scholar]
- 15.Hole DG, et al. Projected impacts of climate change on a continent-wide protected area network. Ecol Lett. 2009;12:420–431. doi: 10.1111/j.1461-0248.2009.01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Araújo MB, Alagador D, Cabeza M, Nogués-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecol Lett. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin GE, Rehfisch MM. Shifting nonbreeding distributions of migratory fauna in relation to climatic change. Glob Change Biol. 2005;11:31–38. [Google Scholar]
- 18.Cliquet A, Backes C, Harris J, Howsam P. Adaptation to climate change: Legal challenges for protected areas. Utrecht Law Rev. 2009;5:158–175. [Google Scholar]
- 19.Mascia M, Pailler S. Protected area downgrading, downsizing and degazettement (PADDD) and its conservation implications. Conserv Lett. 2011;4:9–20. [Google Scholar]
- 20.Lovvorn JR, Grebmeier JM, Cooper LW, Bump JK, Richman SE. Modeling marine protected areas for threatened eiders in a climatically changing Bering Sea. Ecol Appl. 2009;19:1596–1613. doi: 10.1890/08-1193.1. [DOI] [PubMed] [Google Scholar]
- 21.Fuller RA, et al. Replacing underperforming protected areas achieves better conservation outcomes. Nature. 2010;466:365–367. doi: 10.1038/nature09180. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CD. Climate, climate change and range boundaries. Divers Distrib. 2010;16:488–495. [Google Scholar]
- 23.Thomas CD, et al. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- 24.Thomas JA, et al. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc Biol Sci. 2001;268:1791–1796. doi: 10.1098/rspb.2001.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert G, Tyler GA, Smith KW. Local annual survival of booming male Great Bittern Botaurus stellaris in Britain, in the period 1990–1999. Ibis. 2002;144:51–61. [Google Scholar]
- 26.Conway G, et al. Status and distribution of European nightjars Caprimulgus europaeus in the UK in 2004. Bird Study. 2007;54:98–111. [Google Scholar]
- 27.Conway G, et al. The status of breeding woodlarks Lullula arborea in Britain in 2006. Bird Study. 2009;56:310–325. [Google Scholar]
- 28.Wilson RJ, Davies ZG, Thomas CD. Modelling the effect of habitat fragmentation on range expansion in a butterfly. Proc Biol Sci. 2009;276:1421–1427. doi: 10.1098/rspb.2008.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wotton S, McIntyre R, Schmitt S, Gregory R, Brown A. Bittern Botaurus stellaris Monitoring in the UK: Summary of the 2011 Season. Sandy: RSPB; 2012. [Google Scholar]
- 30.Bradbury RB, Pearce-Higgins JW, Wotton SR, Conway GJ, Grice PV. The influence of climate and topography in patterns of territory establishment in a range-expanding bird. Ibis. 2011;153:336–344. [Google Scholar]
- 31.Asher J, et al. The Millennium Atlas of Butterflies in Britain and Ireland. Oxford: Oxford Univ Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.