Abstract
The evolutionary emergence of the egalitarian syndrome is one of the most intriguing unsolved puzzles related to the origins of modern humans. Standard explanations and models for cooperation and altruism—reciprocity, kin and group selection, and punishment—are not directly applicable to the emergence of egalitarian behavior in hierarchically organized groups that characterized the social life of our ancestors. Here I study an evolutionary model of group-living individuals competing for resources and reproductive success. In the model, the differences in fighting abilities lead to the emergence of hierarchies where stronger individuals take away resources from weaker individuals and, as a result, have higher reproductive success. First, I show that the logic of within-group competition implies under rather general conditions that each individual benefits if the transfer of the resource from a weaker group member to a stronger one is prevented. This effect is especially strong in small groups. Then I demonstrate that this effect can result in the evolution of a particular, genetically controlled psychology causing individuals to interfere in a bully–victim conflict on the side of the victim. A necessary condition is a high efficiency of coalitions in conflicts against the bullies. The egalitarian drive leads to a dramatic reduction in within-group inequality. Simultaneously it creates the conditions for the emergence of inequity aversion, empathy, compassion, and egalitarian moral values via the internalization of behavioral rules imposed by natural selection. It also promotes widespread cooperation via coalition formation.
Keywords: despotic, hawk–dove, bystander
Humans exhibit a strong egalitarian syndrome, i.e., the complex of cognitive perspectives, ethical principles, social norms, and individual and collective attitudes promoting equality (1–9). The universality of egalitarianism in mobile hunter-gatherers suggests that it is an ancient, evolved human pattern (2, 5, 6). Political egalitarianism of contemporary foragers is accomplished by a variety of cultural practices (leveling mechanisms) aiming at controlling overassertive, dominant, or very successful individuals who might wish to monopolize resources (3–5, 10). Although conscious, intentional choice of leveling behaviors can explain how egalitarianism is sustained (3–6), the question of how those cognitive and motivational processes evolved in the first place remains open (11).
In group-living organisms with strong social hierarchies, high-rank individuals get more and better resources than low-rank individuals (12). Although under certain conditions both monkeys and apes demonstrate some respect for ownership (13, 14), the dominant individuals can take food and other resources from subordinates especially if the value of the contested item is high, it can be easily taken over, and the owner’s rank is low (13, 15, 16). Similar behavior is observed in experiments with young children (17, 18). In hierarchical groups, resisting high-rank bullies alone is dangerous and unlikely to be successful. The only feasible way to successful resistance is via using help of other group members.
Helping behavior is a form of altruism that can evolve by kin or group selection or if there is reciprocity and/or punishment of noncooperators (19–21). However, the first three mechanisms ignore any social role asymmetry, so that help would be as likely directed toward the bully as toward the victim. The fourth mechanism does explicitly account for the social role (e.g., cooperator or not). However, in primates, policing and punishment are typically administered by the most dominant individuals (22–24), that is, by the bullies themselves. Therefore, although it can account for the maintenance of egalitarian behavior, the role of punishment in its origin is less clear. Helping behavior and its feasibility, dynamics, and patterns are studied by the theory of coalition and alliance formation (25–28). However, existing models typically assume that behavioral rules are fixed rather than evolvable (but see refs. 29 and 30) and that the number of interacting individuals is small (typically three, but see ref. 27) and do not consider explicitly the social role (e.g., bully or victim). Here I expand the theory of coalitions and alliances to study the evolutionary origins of egalitarian tendencies in a despotic society. Rather than focusing on a small number of fixed strategies (e.g., cooperate, defect, punish, etc), I study the evolution of genetically controlled situation-dependent behavioral rules used by individuals for making different decisions. This approach both accounts for the differences in individual personalities (31) and simultaneously describes how individual psychology (32) evolves by selection.
Models and Results
Owner–Bully Interactions.
I start by considering agonistic dyadic interactions within a group of n individuals ranked according to their strengths s1 > s2 … > sn. I assume that during their lifetime individuals come into possession of certain resource items of value b. Whenever one individual (referred to as “owner”) finds an item, another one (referred to as “bully”) may try to take it from the owner. These interactions can be described by a hawk–dove-type game (33) with two strategies: “display” (i.e., do not fight over the resource) and “escalate”. If the bully does not escalate, the owner keeps the item. If the bully escalates, the owner may give up without fighting. If both the owner and the bully escalate, a fight occurs. Whoever wins the fight, gets the resource item. I allow for differences in fighting costs between the winner (cw) and the loser (cl). I assume that the probability of winning a fight is an increasing S-shaped function of the ratio of the strengths of the two opponents and use a parameter σv to scale the steepness of this function. During the lifetime, each individual finds himself on average K times in each role (e.g., owner or bully). The overall amount of the resource accumulated, Ri, defines the reproductive success wi (e.g., the proportion of the group’s offspring fathered by the individual) according to a generalization of the Tullock contest success function,
 |
where f(R) is a monotonically increasing function and the sum is taken over all competitors. In the standard Tullock contest success function, which is extensively used in economics (34, 35) and evolutionary biology (36–40) and which I use in the simulations below, f(R) = Rβ. Parameter β measures the contest intensity: With β = 0 everybody gets an equal share; β = ∞ describes the “winner takes all” case.
I am interested in the situations when fights are rather costly relative to the benefit contested. In this case, if individuals have complete information about the conflict, the classical theory of evolutionarily stable strategies in asymmetric conflicts (33, 41) predicts that the conflicts will usually be settled without the actual fight but according to the ownership and/or strength asymmetry. With large differences in strengths, the stronger individual will escalate while the weaker one will display. With small differences in strengths, both the payoff-relevant (i.e., strength) and payoff-irrelevant (i.e., ownership) asymmetries can be used to settle the conflict without an actual fight (33).
Assuming that individuals have complete information is obviously unrealistic. I assume that individual decisions to escalate are made using imperfect information and a simple heuristic rule: Escalate if the perceived ratio of the opponent’s and one’s own strengths is smaller than a certain threshold. The larger the threshold, the more aggressive is the individual. The strengths are estimated with an error the magnitude of which is scaled by parameter σe. The escalation thresholds are different for the two roles (e.g., owner and bully) and are controlled genetically. Specifically I assume two unlinked additive diploid loci (with a continuum of alleles), one of which controls the average aggressiveness x (i.e., the average of the escalation thresholds for the two roles) and the other the ownership effect y (i.e., the ratio between the escalation thresholds in the roles of the owner and the bully). Trait x corresponds to the confidence trait in ref. 42. I assume that the escalation threshold in the role of the owner is not smaller than that in the role of the bully (e.g., because of a preexisting loss-aversion syndrome) (43) so that trait y ≥ 1.
What kind of “evolutionary psychology” will evolve in this system? I used numerical simulations describing a large number of groups each with n males and n females. Generations are discrete and nonoverlapping. Each female produces exactly one male and one female offspring; female offspring disperse randomly whereas male offspring stay in their natal groups. The results show that individuals evolve reduced aggressiveness so that the population average x < 1 and the average ownership effect is small (i.e., y is close to 1). This behavior is expected (33, 42). The escalation threshold equal to one means that the decision to escalate is based exclusively on the estimated probability of win. However, besides the probability of win, the corresponding costs and benefits are also important. With high benefits and low costs, increased aggressiveness (or overconfidence in terminology of ref. 42) will maximize the expected payoff. In contrast, with high costs as assumed here a reduced aggressiveness is expected so that each individual escalates only if he estimates that he is sufficiently stronger than the opponent. Increasing the conflict intensity (β) or the evaluation error (σe) makes individuals more aggressive whereas increasing group size (n) or costs (cw and cl) has opposite effects. The dominance structure emerging in such a population corresponds to a linear rank-resource relationship with weaker individuals usually giving up their resources without fighting. The inequality in resources results in strong inequality in reproductive success (Fig. S1). Describing the situations when only a few males father most of the group offspring (as happens in chimpanzees and other species living in hierarchically organized groups) (23, 44–47) requires one to assume that β is sufficiently larger than 1 (e.g., in the range 2–4) so that f(R) grows faster than linearly with R. For the rest of the paper, I make this assumption.
Egalitarian Drive.
In my model, natural selection optimizes individual behavior in possible dyadic interactions. However, the logic of competitive coexistence in groups implies that the outcome of each particular dyadic interaction has fitness consequences for all other group members (Methods). Specifically if function f(R) grows faster than linearly, then each cost-free (i.e., peaceful) transfer of resource from a “poorer” to a “wealthier” individual is detrimental for everybody else in the group. [If f(R) grows slower than linearly with R, the effect is reversed and each other group member benefits when a wealthier individual robs a poorer one.] From one’s perspective, one wants to maximize the amount of resource owned (which will increase the numerator of Eq. 1) and at the same time one wants everybody else to have an equal amount of resources (which will decrease the numerator of Eq. 1). This unappreciated feature of competitive coexistence in groups means that each observer of a conflict has an incentive in helping the poorer side (who, as a rule, is also the weaker). Is the egalitarian drive powerful enough to have evolutionary consequences, when helping is costly? To start attacking this question, I next generalize the model.
Owner–Bully–Helper Interactions.
I assume that each owner–bully interaction is observed by a third individual (“bystander”) who may decide to help the victim. If an owner–helper coalition forms, its strength is defined as (27) 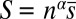 , where
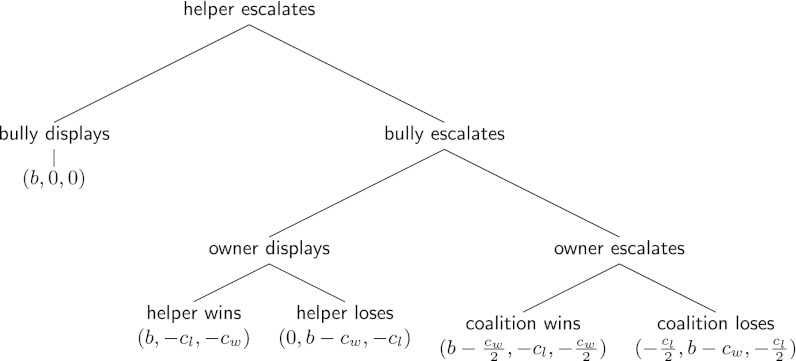
, where 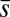 is the average strength of the coalition partners, n = 2 is the coalition size, and α is a positive parameter. (α = 1 means the coalition strength is determined additively; α > 1 means there is some synergy, so that the coalition is stronger than the sum of the strengths of its members.) This formulation follows the classical Lanchester laws used to describe military conflicts as well as conflicts in animals (48–50). The decision to help is based on the same heuristic rule (i.e., help if the perceived ratio of the opponent’s and the coalition’s strengths is sufficiently small), but the corresponding escalation threshold z is controlled by an independent third locus. In making his decision, the bystander assumes that the owner will escalate, which, however, is not guaranteed as the owner’s decision depends on his own escalation threshold and also is probabilistic. The conflict develops in the following order: First, the bully escalates against a single victim, then the bystander decides whether or not to help (by escalating against the bully), and then the bully and the owner simultaneously decide whether to back down or escalate, respectively, given the bystander’s decision. If the bystander does not escalate, the situation considered above ensues. The payoffs for the case when the helper does escalate are shown in Fig. 1. Note that in the case of a fight, the costs are assumed to be split equally between the coalition partners and that the helper’s payoff is never positive.
is the average strength of the coalition partners, n = 2 is the coalition size, and α is a positive parameter. (α = 1 means the coalition strength is determined additively; α > 1 means there is some synergy, so that the coalition is stronger than the sum of the strengths of its members.) This formulation follows the classical Lanchester laws used to describe military conflicts as well as conflicts in animals (48–50). The decision to help is based on the same heuristic rule (i.e., help if the perceived ratio of the opponent’s and the coalition’s strengths is sufficiently small), but the corresponding escalation threshold z is controlled by an independent third locus. In making his decision, the bystander assumes that the owner will escalate, which, however, is not guaranteed as the owner’s decision depends on his own escalation threshold and also is probabilistic. The conflict develops in the following order: First, the bully escalates against a single victim, then the bystander decides whether or not to help (by escalating against the bully), and then the bully and the owner simultaneously decide whether to back down or escalate, respectively, given the bystander’s decision. If the bystander does not escalate, the situation considered above ensues. The payoffs for the case when the helper does escalate are shown in Fig. 1. Note that in the case of a fight, the costs are assumed to be split equally between the coalition partners and that the helper’s payoff is never positive.
Fig. 1.
The payoffs to the owner, the bully, and the helper in different situations if the helper escalates.
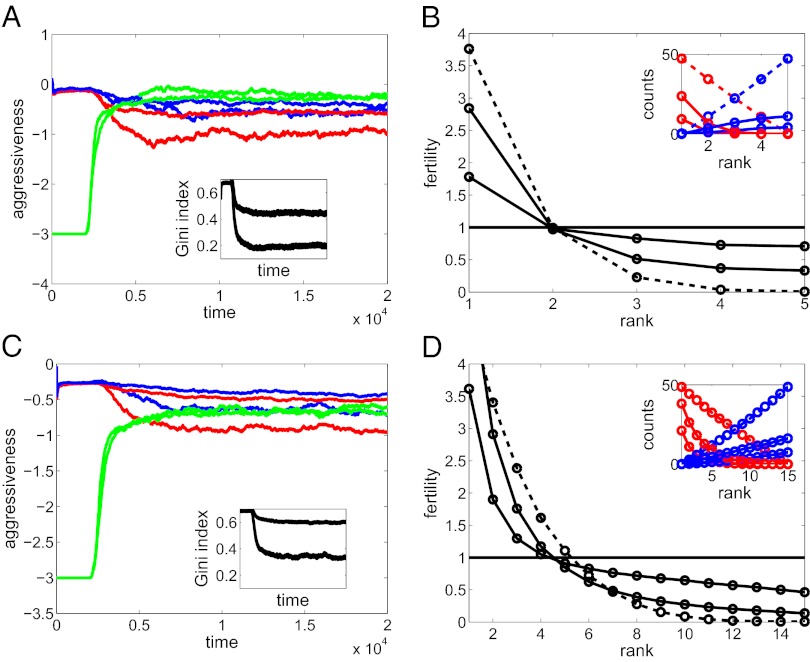
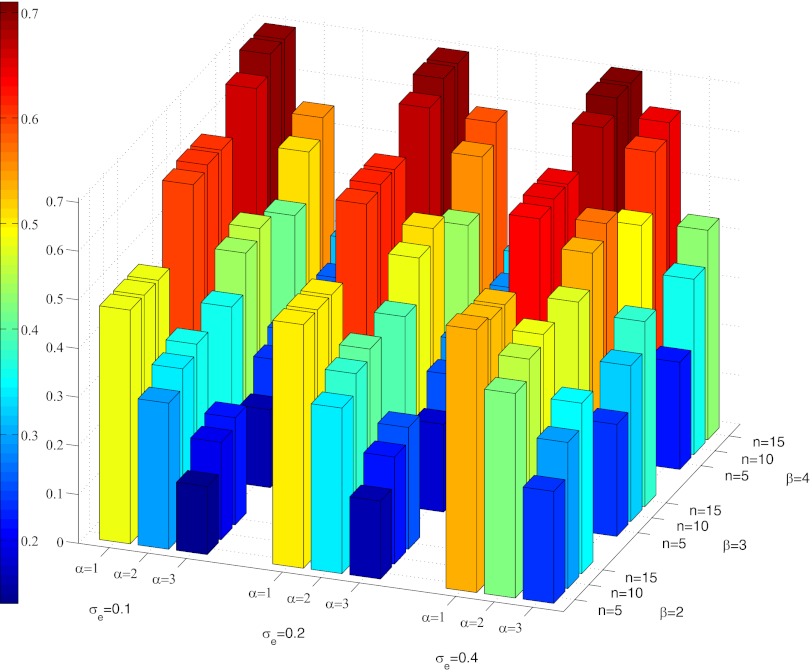
I performed a very large number of numerical simulations for different parameter values and initial conditions. The results show that under some conditions helping behavior does evolve, resulting in a significant reduction in within-group inequality (Figs. 2 and 3, Figs. S2–S7, and Table S1). The most important requirement is a strong synergy between the strengths of coalition partners in their effects on the strength of the coalition. Specifically, helping behavior has minimum phenotypic effects with α = 1 but with α = 3 there can be a two- to threefold decrease in the Gini index of inequality (defined as half the relative mean difference) (51). Strong helping is associated with strong ownership effect. The effects are strongest in smaller groups (small n) with strong preexisting hierarchies (large β). The more reliable strength evaluation is (small σe), the more likely helping behavior. The costs of fighting (cw and cl) have weak negative effects. The groups never achieve complete equality and their strongest members continue to have fitness advantages. However, there is a significant reduction in the number of successful bullying acts. The changes happen relatively fast on the timescale of thousands of generations. The evolved helping psychology can be summarized as “help if helping is feasible.” The evolved escalation thresholds are similar for all three roles. Bullies remain opportunistic and are prevented from bulling only by the active opposition of the helpers.
Fig. 2.
Evolutionary dynamics and their effects. (A and C) Escalation thresholds in the role of victim (blue), bully (red), and helper (green) on the logarithmic scale. (Insets) The Gini index of inequality. (B and D) Average fertility of males of different rank. (Insets) The average number of times each individual lost the resource to a bully (increasing blue curves) and took the resource from an owner (decreasing red curves). Two sets of curves are shown, corresponding to α = 2 (less helping and equality) and α = 3 (more helping and equality). For the first 2,000 generations the helper aggressiveness was fixed at −3 and no evolution of helping was allowed. The dashed lines in B and D show the corresponding curves at generation 2,000 and solid curves are computed for the final generation. n = 5 in A and B; n = 15 in C and D. Other parameters: c = 8, β = 4, γ = σe = 0.2, σv = 0.4.
Fig. 3.
Effects of α, β, n, and σe on the Gini inequality index for fertilities at generation 20,000. Other parameters: c = 4, γ = 0.2, σv = 0.4.
Discussion
Animals living in a group have common interests such as defense from predators and acquisition and defense of various resources (including mating opportunities) from competitors that include conspecifics. These common interests, however, do not necessarily mean an elimination or a significant reduction of competition between group members. A variation between individuals in their fighting abilities (which is always present due to various environmental, genetic, developmental, and stochastic factors) implies that some of them can take resources from others by force. Then natural selection is expected to drive the evolution of a particular psychology with stronger individuals attempting to rob weaker individuals with the latter giving up resources without fighting back. A result is the emergence of group hierarchies in which resources are appropriated in a very nonequal way with high-rank bullies usurping a disproportionally large share (12). The more limited are the subordinates’ options outside the group, the stronger the expected degree of despotism (52).
Resisting high-rank bullies alone is costly and unlikely to be successful. However, the same forces that shape the emergence of highly despotic groups dialectically create conditions for the evolution of counterdominant coalitionary behavior and psychology (11). As I have shown above, in such groups seeking personal benefits can lead to a particular other-regarding preference: All others should be more equal. A necessary condition for this preference is that the share of group reproduction obtained by a high-rank bully grows faster than his share of the group resource (increasing marginal efficiency). A way to fulfill the preference is to help the weak against the strong, even at a cost. When everybody acts to enforce equality among all other members of the group, a group-level equality develops. In the model studied here, universal, genetically controlled inequity aversion evolves as a result of each person promoting beneficial to himself (i.e., self-centered) equality among all other individuals within the context of within-group competition. This evolution could have been the force that drove the egalitarian transition in our lineage. Once the tendencies for egalitarianism (or pair bonding) (40) are well grounded in genes, they can be elaborated and augmented by cultural norms.
Was the transition to helping behavior and a more egalitarian social structure a relatively sudden or a protracted process? This question is difficult to answer. One can speculate, however, on the reasons for its onset that also offer some insights on why it did not happen in other animals. In terms of the model studied, these reasons could be an increased efficiency of coalitionary aggression (larger α) and decreased uncertainty in evaluating fighting skills of group members (smaller σe). Both could have followed the evolution of better cognitive abilities (53, 54) and the development of better coordination skills and weapons as a result of cooperative big-game hunting.
There are several additional factors not considered here that are expected to promote the effect described. In particular, allowing for multiple helpers would increase the effectiveness and decrease the cost of helping. The presence of winner–loser effects (i.e., a correlation between past and future performance in fights) (55) creates additional motivation for helping (because defeating the bully decreases the chance he will successfully attack the helper in the future). Differential group fertility (assuming that groups with fewer internal conflicts will produce more offspring) will accelerate spreading the genes for helping across the whole population.
A few additional comments are in order. In the mechanism advanced here, bullying behavior is the reason for the evolution of helping. However, bullying tendencies remain present; they are not expressed only because of counterdominant helping. It works because it explores the concurrence of the interests of many in the face of the exploitation by a few. The helping benefits are direct but delayed. In the end, it is pure selfish tendencies that could drive the emergence of helping behavior, empathy, and moral values. I was focusing on helping the “poor” against the aggression of the “wealthy”. However, the general mechanism should operate any time one individual can facilitate the transfer of resources from a dominant to a weaker individual. The mechanism studied here is very powerful in that it does not require relatedness, group selection, reciprocity, or reputation. It also promotes widespread cooperation via the formation of coalitions, which in humans occur at many different levels (ranging from within-family to between-nation states) and represent the most dominant factor in social interactions that has shaped human history (56–58).
The origins of moral values have intrigued scholars for millennia. Darwin saw human morality as derived from animal “social instincts” (59) that transform to a “moral sense or conscience as soon as … intellectual powers become … well developed” (ref. 59, p. 8). In a modern perspective, viewing human conscience as a mere by-product of intelligence is an oversimplification. Boehm (6) convincingly argues that additional processes and factors such as moralistic punishment, internalization of culturally enforced norms, symbolic language and gossiping, and social selection for altruism and self-restraint applied by groups to its members need to be considered. That notwithstanding, identifying evolutionary roots for and the dynamics of genetically controlled egalitarian social instincts is a necessary step in getting a better understanding of the origins of a uniquely human sense of right and wrong.
Methods
Egalitarian Drive.
Consider the fitness of a focal individual i before (wi) and after (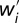 ) a transfer of δ > 0 units of the resource from individual ω to individual κ. Using Eq. 1,
) a transfer of δ > 0 units of the resource from individual ω to individual κ. Using Eq. 1,
where the sums are over all individuals except for κ and ω. From here, the transfer decreases the fitness of the focal individual (i.e., 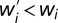 ) if
) if
which can be rearranged as
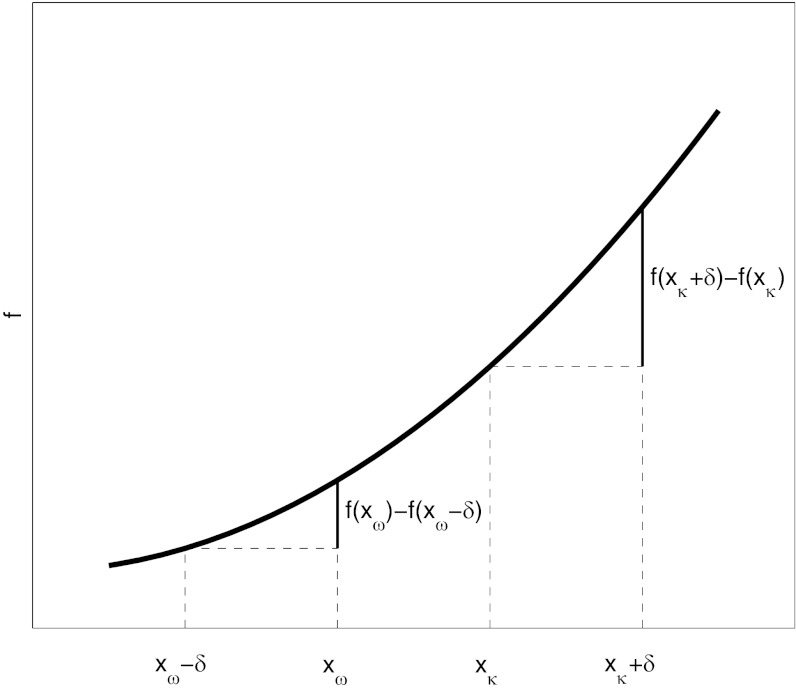
A simple graphical argument (Fig. 4) shows that this inequality is satisfied if f grows faster than linearly with x provided that xκ > xω − δ. The fitness loss 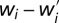 is inversely proportional to the group size n. It should be clear that if f grows slower than linearly, then the focal individual benefits when resources are transferred from a poorer to a richer group member.
is inversely proportional to the group size n. It should be clear that if f grows slower than linearly, then the focal individual benefits when resources are transferred from a poorer to a richer group member.
Fig. 4.
A graphical proof that for any function f(x) increasing faster than linearly, any δ > 0 and any xκ > xω − δ, f(xκ + δ) − f(xκ) > f(xω) − f(xω − δ).
Numerical Model Implementation.
Individual strengths si are chosen randomly and independently from a lognormal distribution with mean and SD equal to 1. Individual i escalates against opponent j if the ratio sjej/(siei) is smaller than the escalation threshold in a given role (e.g., owner or bully). The evaluation errors e are chosen randomly and independently from a lognormal distribution with mean 1 and standard variance σe. A similar rule is used for escalating when an individual is a part of or against a coalition, with an appropriate change in the strength term. It was convenient to measure the escalation thresholds on a logarithmic scale and to define the probability of winning by the lognormal cumulative distribution function (of the ratio of the opponents’ strengths) with mean 0 and SD σv.
Parameters.
The following is a list of parameter values varied in simulations: the number of males n = 5, 10, 15; coalition synergy α = 1, 2, 3; contest intensity β = 2, 3, 4; the average cost (c = (cw + cl)/2) of a fight c = 2, 4, 8; the ratio γ = (cw/cl) of costs for winner and loser γ = 0.1, 0.2, 0.4; strength evaluation error σe = 0.1, 0.2, 0.4; and contest unpredictability σv = 0.2, 0.4, 0.8. The average number of interactions in each role K = 50. The mutation rate per gene per generation was 10−3, and the effects of mutations were chosen from a normal distribution 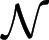 (0,0.5). There were 200 groups. I ran 10 simulations for each of 37 combinations of parameters.
(0,0.5). There were 200 groups. I ran 10 simulations for each of 37 combinations of parameters.
Supplementary Material
Acknowledgments
I thank B. M. Auerbach, C. Boehm, A. Kramer, M. Mesterton-Gibbons, B. O’Meara, and the reviewers for comments.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. P.J.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201718109/-/DCSupplemental.
References
- 1.Zupanov J. Egalitarizam i industrializam [Egalitarianism and industrialism] Sociologija. 1970;12:5–45. Croatian. [Google Scholar]
- 2.Knauft BB. Violence and sociality in human evolution. Curr Anthropol. 1991;32:391–428. [Google Scholar]
- 3.Boehm C. Egalitarian behavior and reverse dominance hierarchy. Curr Anthropol. 1993;34:227–254. [Google Scholar]
- 4.Boehm C. 1997. Impact of the human egalitarian syndrome on Darwinian selection mechanics. Am Nat 150:S100–S121.
- 5.Boehm C. Hierarchy in the Forest. The Evolution of Egalitarian Behavior. Cambridge, MA: Harvard Univ Press; 1999. [Google Scholar]
- 6.Boehm C. Moral Origins: Social Selection and the Evolution of Virtue, Altruism, and Shame. New York: Basic Books; 2012. [Google Scholar]
- 7.Dawes CT, Fowler JH, Johnson T, McElreath R, Smirnov O. Egalitarian motives in humans. Nature. 2007;446:794–796. doi: 10.1038/nature05651. [DOI] [PubMed] [Google Scholar]
- 8.Fehr E, Bernhard H, Rockenbach B. Egalitarianism in young children. Nature. 2008;454:1079–1083. doi: 10.1038/nature07155. [DOI] [PubMed] [Google Scholar]
- 9.Tricomi E, Rangel A, Camerer CF, O’Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463:1089–1091. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- 10.Shultziner D, et al. The causes and scope of political egalitarianism during the Last Glacial: A multi-disciplinary perspective. Biol Philos. 2010;25:319–346. [Google Scholar]
- 11.Erdal D, Whiten A. On human egalitarianism: An evolutionary product of Machiavellian status escalation? Curr Anthropol. 1994;35:175–178. [Google Scholar]
- 12.Ellis L. Dominance and reproductive success among nonhuman animals - cross-species comparison. Ethol Sociobiol. 1995;16:257–333. [Google Scholar]
- 13.Thierry B, Wunderlich D, Gueth C. Possession and transfer of objects in a group of brown capuchins (Cebus apella) Behaviour. 1989;110:294–305. [Google Scholar]
- 14.Kummer H, Cords M. 1991. Cues of ownership in long-tailed macaques, Macaca fascicularis. Anim Behav 42:529–549.
- 15.Sigg H, Falett J. Experiments on respect of possession and property in hamadryas baboons (Papio hamadryas) Anim Behav. 1995;33:978–984. [Google Scholar]
- 16.Boesch C, Boesch H. Hunting behavior of wild chimpanzees in the Taï National Park. Am J Phys Anthropol. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. [DOI] [PubMed] [Google Scholar]
- 17.Bakeman R, Brownlee JR. In: Peer Relationships and Social Skills in Childhood. Rubin KH, Ross HS, editors. New York: Springer; 1982. pp. 99–112. [Google Scholar]
- 18.Weigel RM. The application of evolutionary models to the study of decisions by children during object possession conflicts. Ethol Sociobiol. 1984;5:229–238. [Google Scholar]
- 19.Frank S. Foundations of Social Evolution. Princeton: Princeton Univ Press; 1998. [Google Scholar]
- 20.Nowak M. 2006. Evolutionary Dynamics (Harvard Univ Press, Cambridge, MA)
- 21.McElreath R, Boyd R. Mathematical Models of Social Evolution. A Guide for the Perplexed. Chicago: Chicago Univ Press; 2007. [Google Scholar]
- 22.Clutton-Brock TH, Parker GA. Punishment in animal societies. Nature. 1995;373:209–216. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- 23.de Waal FB. Primates—a natural heritage of conflict resolution. Science. 2000;289:586–590. doi: 10.1126/science.289.5479.586. [DOI] [PubMed] [Google Scholar]
- 24.Flack JC, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. Am Nat. 2005;165:E126–E139. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- 25.Harcourt AH, de Waal FBM. 1992. Coalitions and Alliances in Humans and Other Animals (Oxford Univ Press, Oxford)
- 26.van Schaik CP, Pandit SA, Vodel ER. 2006. Cooperation in Primates and Humans eds Kappeler PM, van Schaik CP (Springer, Berlin), pp 151–171.
- 27.Gavrilets S, Duenez-Guzman EA, Vose MD. Dynamics of alliance formation and the egalitarian revolution. PLoS ONE. 2008;3:e3293. doi: 10.1371/journal.pone.0003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesterton-Gibbons M, Gavrilets S, Gravner J, Akçay E. Models of coalition or alliance formation. J Theor Biol. 2011;274:187–204. doi: 10.1016/j.jtbi.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Mesterton-Gibbons M, Sherratt TN. Coalition formation: A game-theoretic analysis. Behav Ecol. 2007;18:277–286. [Google Scholar]
- 30.Mesterton-Gibbons M, Sherratt TN. Neighbor intervention: A game-theoretic model. J Theor Biol. 2009;256:263–275. doi: 10.1016/j.jtbi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Nettle D. The evolution of personality variation in humans and other animals. Am Psychol. 2006;61:622–631. doi: 10.1037/0003-066X.61.6.622. [DOI] [PubMed] [Google Scholar]
- 32.Tooby J, Cosmides L. In: The Handbook of Evolutionary Psychology. Buss DM, editor. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 33.Hammerstein P. The role of asymmetries in animal contests. Anim Behav. 1981;29:193–205. [Google Scholar]
- 34.Tullock G. In: Toward a Theory of the Rent-Seeking Society. Buchanan JM, Tollison RD, Tullock G, editors. College Station, TX: Texas A&M University; 1980. pp. 97–112. [Google Scholar]
- 35.Konrad K. Strategy and Dynamics in Contests. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 36.Hawkes K, Rogers AR, Charnov EL. The male’s dilemma: Increased offspring production is more paternity to steal. Evol Ecol. 1995;9:662–677. [Google Scholar]
- 37.Kokko H, Morrell LJ. 2005. Mate guarding, male attractiveness, and paternity under social monogamy. Behav Ecol 16:724–731.
- 38.Reeve HK, Hölldobler B. The emergence of a superorganism through intergroup competition. Proc Natl Acad Sci USA. 2007;104:9736–9740. doi: 10.1073/pnas.0703466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley PH, Hwan Baik K. Variable valuations and voluntarism under group selection: An evolutionary public goods game. J Theor Biol. 2010;265:238–244. doi: 10.1016/j.jtbi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Gavrilets S. Human origins and the transition from promiscuity to pair-bonding. Proc Natl Acad Sci USA. 2012;109:9923–9928. doi: 10.1073/pnas.1200717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maynard Smith J. Evolution and the Theory of Games. Cambridge, UK: Cambridge Univ Press; 1982. [Google Scholar]
- 42.Johnson DD, Fowler JH. The evolution of overconfidence. Nature. 2011;477:317–320. doi: 10.1038/nature10384. [DOI] [PubMed] [Google Scholar]
- 43.Kahneman D, Knetsch JL, Thaler RH. The endowment effect, loss aversion, and status quo bias. J Econ Perspect. 1991;5:193–206. [Google Scholar]
- 44.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Belknap Press; 1986. [Google Scholar]
- 45.van Schaik CP, Pandit SA, Vodel ER. A model for within-group coalitionary aggression among males. Behav Ecol Sociobiol. 2004;57:101–109. [Google Scholar]
- 46.Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: From mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- 47.Boesch C, Kohou G, Néné H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï forest. Am J Phys Anthropol. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- 48.Kingman JFC. Stochastic aspects of Lanchester’s theory of warfare. J Appl Probab. 2002;39:455–465. [Google Scholar]
- 49.Helmold RL. Osipov: The ‘Russian Lanchester’. Eur J Oper Res. 1993;65:278–288. [Google Scholar]
- 50.Wilson ML, Britton NF, Franks NR. Chimpanzees and the mathematics of battle. Proc Biol Sci. 2002;269:1107–1112. doi: 10.1098/rspb.2001.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gini C. In: Memorie di Metodologia Statistica [Memoirs of Statistical Methodology] Pizetti E, Salvemini T, editors. Rome: Libreria Eredi Virgilio Vesc; 1955. Italian. [Google Scholar]
- 52.Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Anim Behav. 1983;31:667–682. [Google Scholar]
- 53.Byrne RW, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford, UK: Clarendon; 1988. [Google Scholar]
- 54.Gavrilets S, Vose A. The dynamics of Machiavellian intelligence. Proc Natl Acad Sci USA. 2006;103:16823–16828. doi: 10.1073/pnas.0601428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 56.Wright HT. Recent research on the origin of the state. Annu Rev Anthropol. 1977;6:379–397. [Google Scholar]
- 57.Johnson AW, Earle T. The Evolution of Human Societies. From Foraging Group to Agrarian State. Stanford, CA: Stanford Univ Press; 1987. [Google Scholar]
- 58.Turchin P. Historical Dynamics: Why States Rise and Fall. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 59.de Waal F. The Age of Empathy: Nature’s Lessons for a Kinder Society. New York: Harmony Books; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.