Abstract
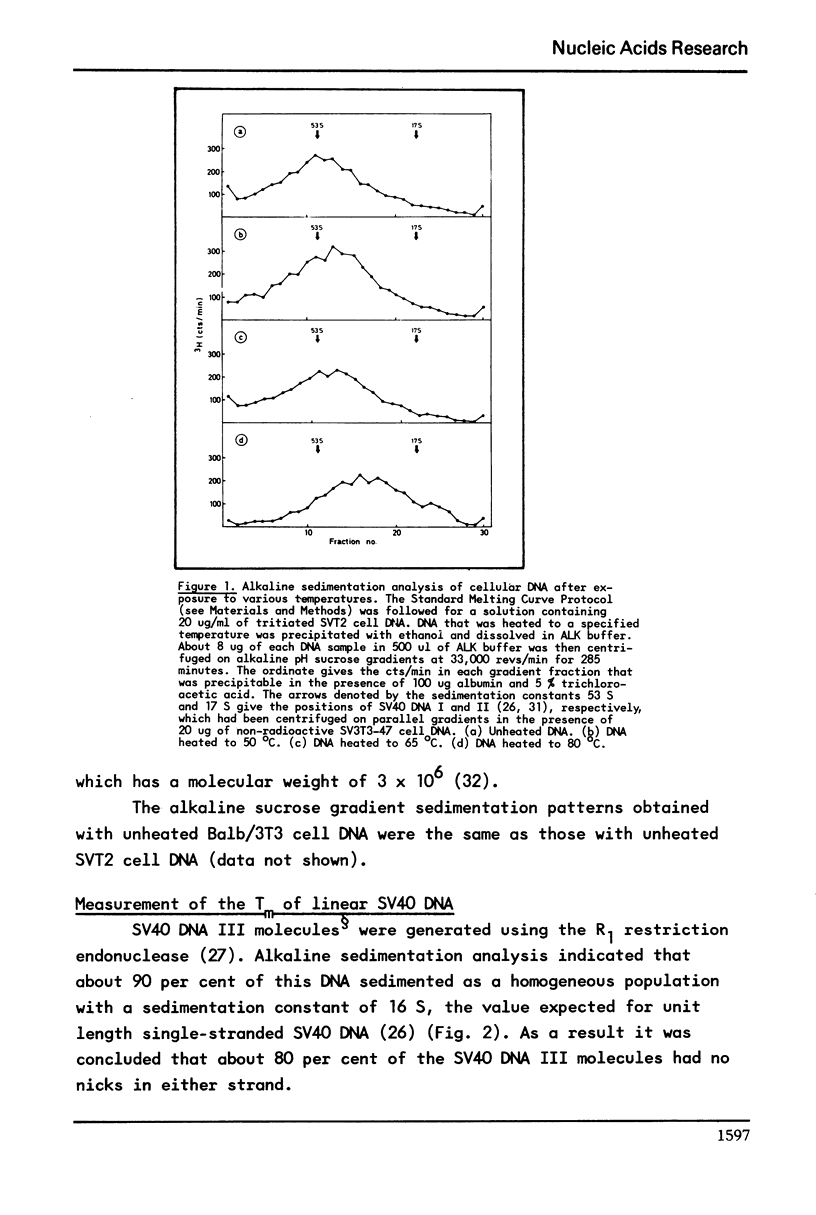
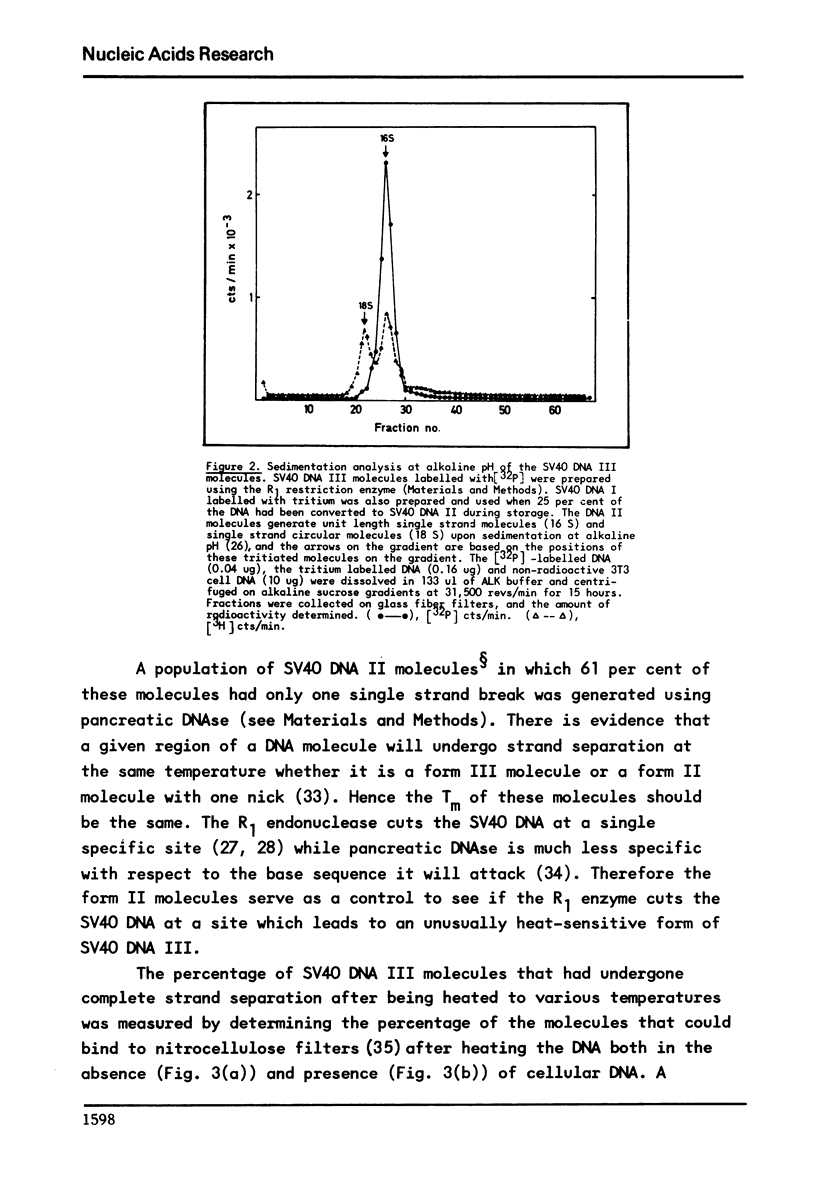
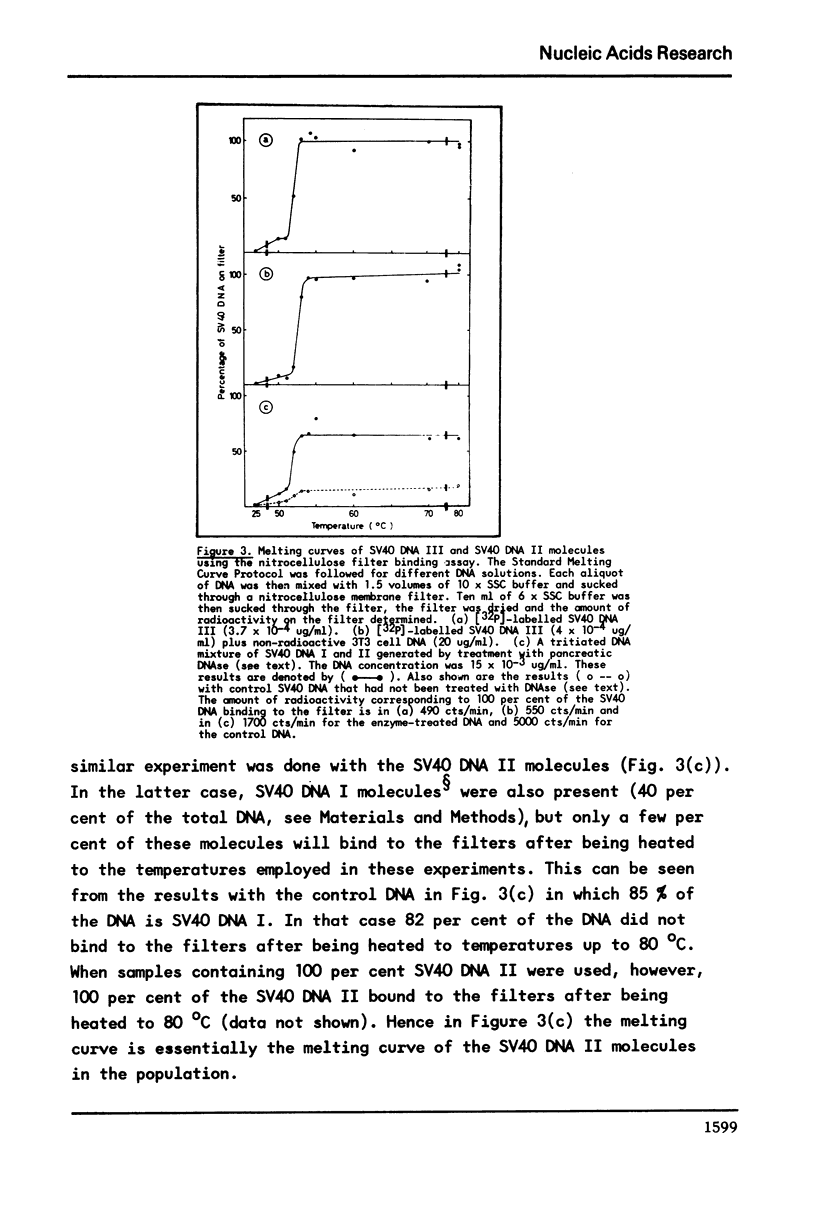
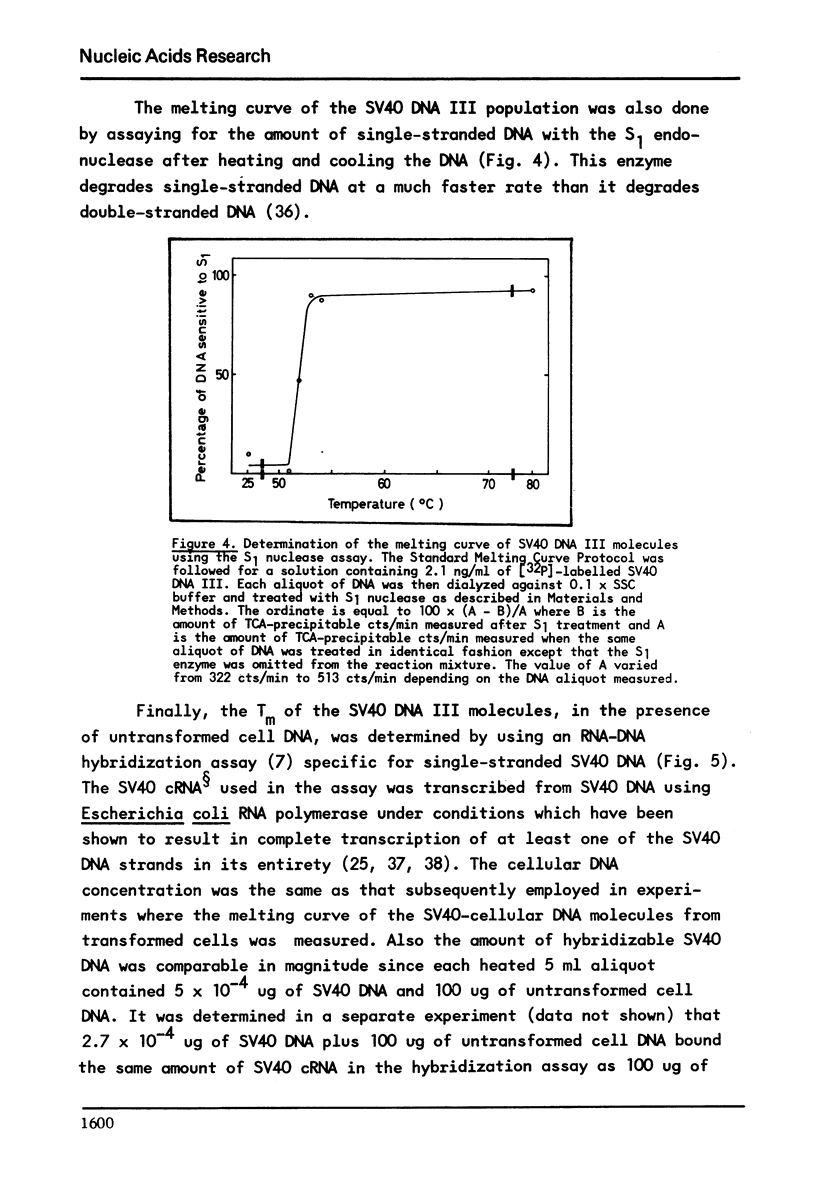
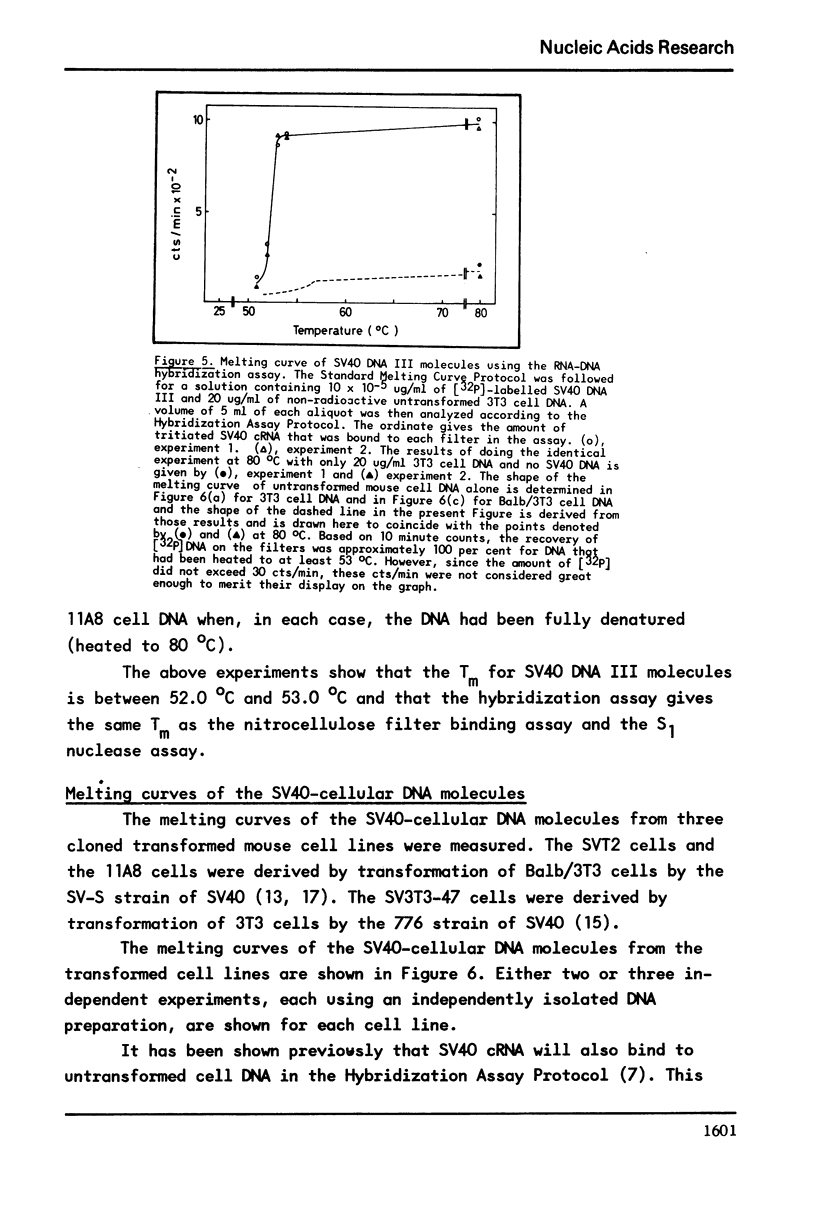
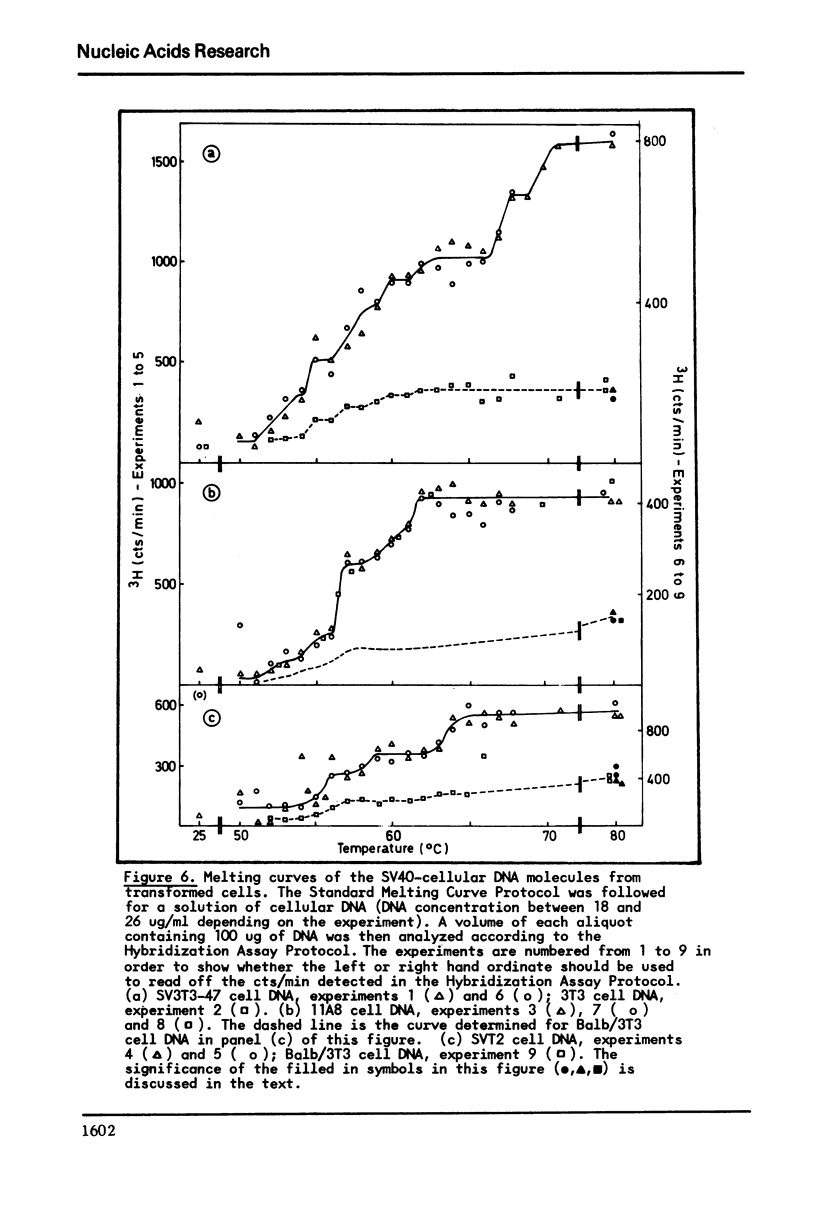
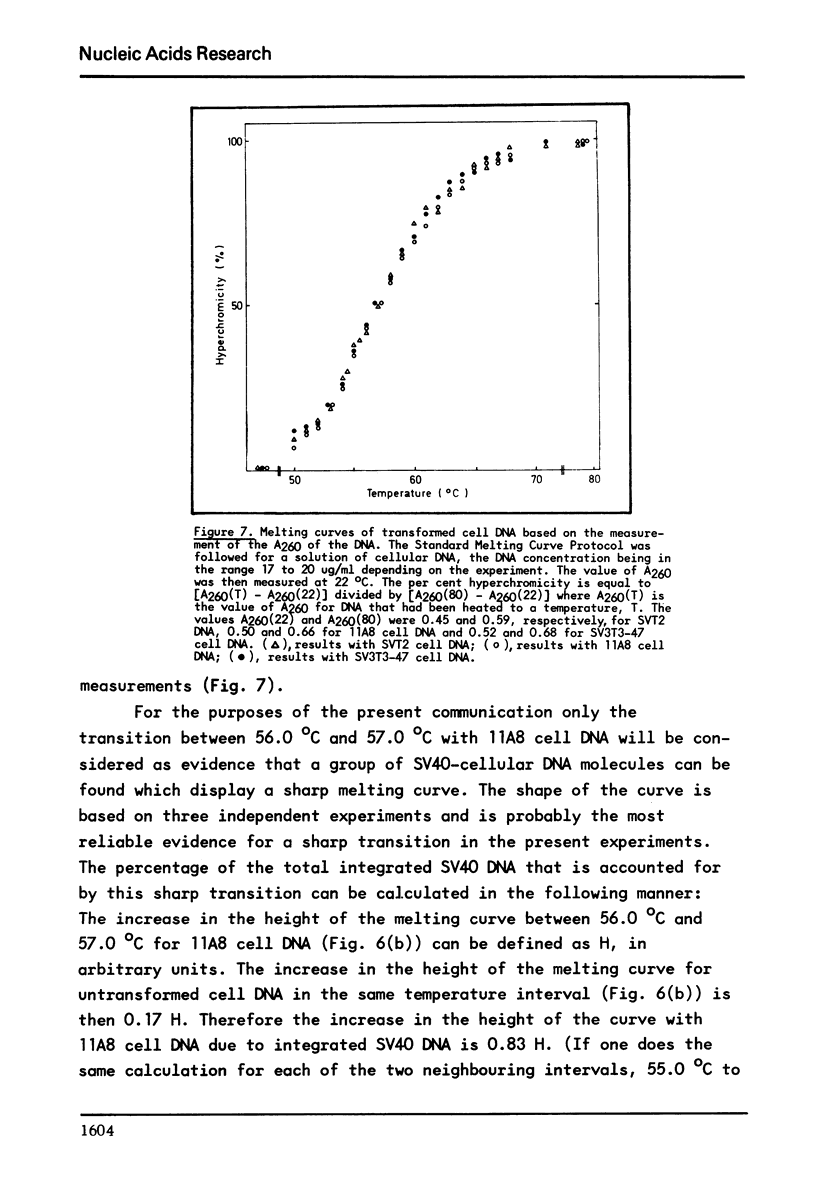
It has previously been demonstrated that the DNA molecules containing a genetic marker from one region of a bacterial genome undergo complete strand separation at temperatures usually different from the molecules containing a genetic marker from another region of the genome. The experiments also showed that if a group of molecules undergo complete strand separation over a narrow temperature range, of the order of one to three degrees, it is highly likely that they all come from one region of the bacterial genome. The purpose of the work reported here was to establish appropriate procedures for doing a similar analysis of the DNA molecules containing the integrated SV40 DNA in transformed mouse cells. One result of interest is that in the 11A8 cell line about 40 per cent of the integrated SV40 DNA detectable by an RNA-DNA hybridization assay can be accounted for by a group of molecules which undergo complete strand separation within a 1.0 degree C interval.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G., Ehrlich S. D., Thiery J. P. The specificity of deoxyribonucleases and their use in nucleotide sequence studies. Nat New Biol. 1973 Nov 14;246(150):36–40. doi: 10.1038/newbio246036a0. [DOI] [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- Fried A. H., Sokol F. Synthesis in vitro by bacterial RNA-polymerase of simian virus 40-specific RNA: multiple transcription of the DNA template into a continuous polyribonucleotide. J Gen Virol. 1972 Oct;17(1):69–79. doi: 10.1099/0022-1317-17-1-69. [DOI] [PubMed] [Google Scholar]
- GANESAN A. T., LEDERBERG J. PHYSICAL AND BIOLOGICAL STUDIES ON TRANSFORMING DNA. J Mol Biol. 1964 Sep;9:683–695. doi: 10.1016/s0022-2836(64)80175-3. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. On the factors controlling the reversibility of DNA denaturation. J Mol Biol. 1962 Jun;4:467–487. doi: 10.1016/s0022-2836(62)80103-x. [DOI] [PubMed] [Google Scholar]
- GINOZA W., ZIMM B. H. Mechanisms of inactivation of deoxyribonucleic acids by heat. Proc Natl Acad Sci U S A. 1961 May 15;47:639–652. doi: 10.1073/pnas.47.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILD W. R. Evidence for intramolecular heterogeneity in pneumococcal DNA. J Mol Biol. 1963 Mar;6:214–229. doi: 10.1016/s0022-2836(63)80071-6. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. M., Guild W. R. A differential solvent effect on thermal stability of genetic markers in DNA. J Mol Biol. 1966 Oct;20(3):497–503. doi: 10.1016/0022-2836(66)90005-2. [DOI] [PubMed] [Google Scholar]
- ROGER M., HOTCHKISS R. D. Selective heat inactivation of pneumococcal transforming deoxyribonucleate. Proc Natl Acad Sci U S A. 1961 May 15;47:653–669. doi: 10.1073/pnas.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- SUBIRANA J. A. THE IRREVERSIBLE DENATURATION OF BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Biochim Biophys Acta. 1965 May 11;103:13–24. doi: 10.1016/0005-2787(65)90538-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]



