Abstract
Peripheral auditory neurons are tuned to single frequencies of sound. In the central auditory system, excitatory (or facilitatory) and inhibitory neural interactions take place at multiple levels and produce neurons with sharp level-tolerant frequency-tuning curves, neurons tuned to parameters other than frequency, cochleotopic (frequency) maps, which are different from the peripheral cochleotopic map, and computational maps. The mechanisms to create the response properties of these neurons have been considered to be solely caused by divergent and convergent projections of neurons in the ascending auditory system. The recent research on the corticofugal (descending) auditory system, however, indicates that the corticofugal system adjusts and improves auditory signal processing by modulating neural responses and maps. The corticofugal function consists of at least the following subfunctions. (i) Egocentric selection for short-term modulation of auditory signal processing according to auditory experience. Egocentric selection, based on focused positive feedback associated with widespread lateral inhibition, is mediated by the cortical neural net working together with the corticofugal system. (ii) Reorganization for long-term modulation of the processing of behaviorally relevant auditory signals. Reorganization is based on egocentric selection working together with nonauditory systems. (iii) Gain control based on overall excitatory, facilitatory, or inhibitory corticofugal modulation. Egocentric selection can be viewed as selective gain control. (iv) Shaping (or even creation) of response properties of neurons. Filter properties of neurons in the frequency, amplitude, time, and spatial domains can be sharpened by the corticofugal system. Sharpening of tuning is one of the functions of egocentric selection.
Keywords: auditory system, descending system, learning and memory, plasticity, tonotopic map
The central auditory system creates many physiologically distinct types of neurons for auditory signal processing. Their response properties have been interpreted to be produced by divergent and convergent interactions between neurons in the ascending auditory system. Until recently, the contribution of the descending (corticofugal) system to the shaping (or even creation) of their response properties has hardly been considered. Recent findings indicate that the corticofugal system plays important roles in shaping or even creating the response properties of central auditory neurons and in reorganizing cochleotopic (frequency) and computational (e.g., echo-delay) maps. Therefore, the understanding of the neural mechanisms for auditory signal processing is incomplete without the exploration of the functional roles of the corticofugal system. In this article, we first enumerate several types of neurons and computational maps created in the bat's central auditory system and then describe the anatomy and physiology of the corticofugal system.
Neurons Tuned to Acoustic Parameters Characterizing Biosonar Signals and Cochleotopic and Computational Maps
All peripheral neurons are tuned to single frequencies (best frequencies, BFs). In the central auditory system, excitatory, inhibitory, and facilitatory neural interactions take place at multiple levels and produce neurons with sharp level-tolerant (the width of a frequency tuning curve is narrow regardless of sound levels) frequency tuning curves (1) and also neurons tuned to specific values of parameters other than frequency. Some of these neurons apparently are related to the processing of biosonar signals. They are latency-constant, phasic on-responding neurons (2, 3); paradoxical latency-shift neurons (4); duration-tuned neurons (5); frequency modulation (FM)-sensitive or -specialized neurons (6–8); sharply frequency-tuned neurons showing level tolerancy (9, 10); FM or amplitude modulation (AM) rate-tuned neurons (11); constant frequency (CF)/CF and FM-FM combination-sensitive neurons (12); neurons tuned to particular combinations of frequency and amplitude (e.g., ref. 9); and binaural neurons (see ref. 13 for review). The biosonar signals of the mustached bat consist of CF and FM components. CF/CF and FM-FM neurons, respectively, are tuned to specific combinations of CF or FM components of the emitted pulse and its echo.
In the auditory cortex (AC) of the little brown bat, neurons with short best durations are located ventrally to those with long best durations. They may form a duration axis (14). In the AC of the mustached bat, CF/CF neurons are clustered in the CF/CF area and form frequency-vs.-frequency coordinates for the systematic representation of Doppler shift; FM-FM neurons are clustered in the FM-FM area and form an echo-delay axis for the systematic representation of target distance (see ref. 15 for review); and Doppler-shifted constant-frequency (DSCF) neurons are clustered in the DSCF area and form frequency-vs.-amplitude coordinates for the fine spatio-temporal representation of periodic frequency and amplitude modulations of echoes from flying insects (9). In the superior colliculus of the big brown bat, there is a space map, and some neurons are tuned to a sound source at a particular azimuth, elevation, and depth (16). No auditory space map has been found in the AC. Instead, it has been found that two types of binaural neurons (I-E and E-E) form binaural bands in the AC (mustached bats, ref. 17; cats, ref. 18), and that the best azimuth to excite neurons varies systematically along the frequency axis of the AC (mustached bats, ref. 19; big brown bats, ref. 20).
Neural mechanisms to create the response properties of neurons and the computational maps listed above have been explained by various mechanisms, such as inhibition, coincidence detection (facilitation), coincidence detection associated with delay lines, and disinhibition (1, 5–7, 15, 21–24). All investigators have assumed that the mechanisms are caused by divergent and convergent projections of neurons in the ascending auditory system. Because the neurons with the response properties listed above have been found in the subcortical auditory nuclei, they are expected to be under corticofugal modulation. Recent findings indicate that the corticofugal system plays important roles in shaping (or even creating) the response properties of central auditory neurons and in reorganizing the cochleotopic and computational maps.
The Corticofugal Auditory System: Anatomy
Neurons in the deep layers of the AC project to the medial geniculate body (MGB), inferior colliculus (IC), or subcollicular auditory nuclei (25–27). These corticofugal projections are tonotopically organized (27, 28). Corticothalamic fibers project only to the ipsilateral MGB and thalamic reticular nucleus (27, 29). However, corticocollicular fibers bilaterally project to the IC. The ipsilateral projection is much more extensive and topographically organized than the contralateral projection (26). Therefore, ipsilateral corticofugal modulation is expected to be much larger than contralateral corticofugal modulation in the IC and MGB and to be frequency-dependent. Corticofugal projections are bilateral to the subcollicular nuclei: superior olivary complex and cochlear nucleus (30). Corticofugal modulation is expected to take place even in the cochlea via olivocochlear neurons in the superior olivary complex. The central nucleus of the IC projects not only to the MGB and the superior colliculus, but also to medial olivocochlear neurons, which mostly project to contralateral cochlear outer hair cells. In general, olivocochlear neurons bilaterally project to the cochlea, although there are some differences in olivocochlear projections between species (see ref. 31 for review).
Because the corticofugal system forms multiple feedback loops, the exploration of corticofugal functions is ongoing at different levels of the auditory system. An obvious critical experiment to be performed is the selective inactivation of individual feedback loops without injuring the ascending auditory system. Such an experiment, however, appears to be impossible because of anatomical complexity.
Corticofugal Modulation of Auditory Signal Processing
Gain Control.
Physiological data of corticofugal effects on MGB and IC neurons have been controversial: (i) only or predominantly inhibitory (32–38); (ii) only or predominantly excitatory or facilitative (39–41); or (iii) equally excitatory or inhibitory (42, 43). These data, regardless of the excitatory or inhibitory effect, indicate that one of the corticofugal functions can be nonspecific gain control. In the mustached bat, nonfocal inactivation of cortical auditory neurons, including neurons matched to recorded subcortical neurons, evokes a large reduction of the auditory responses of the subcortical neurons (44, 45). Matched means that electrically stimulated cortical neurons and recorded subcortical or cortical neurons are tuned to the same value of an acoustic parameter. Unmatched means that they are tuned to different values of an acoustic parameter. One of the corticofugal functions is amplification of the responses of subcortical neurons. Because there is a much larger number of corticofugal fibers than thalamocortical fibers, the corticofugal system should have much more elegant functions than simple gain control.
To study the functional roles of the corticofugal system, one should not ignore that corticofugal and subcortical neurons both are tuned to particular values of an acoustic parameter. Therefore, electrical stimulation or drug application for activation or inactivation should be highly focal except for the initial phase of corticofugal research, and corticofugal effects on subcortical neurons should be evaluated with regard to the relationship in tuning between stimulated or inactivated cortical neurons and recorded subcortical neurons. The recent research designed on this philosophy leads us to several findings in the mustached bat, Pteronotus parnellii parnellii, and the big brown bat, Eptesicus fuscus.
Egocentric Selection in the Mustached Bat.
In the mustached bat, DSCF neurons are extremely sharply tuned to frequencies at ≈61 kHz. They are specialized for processing frequency and/or amplitude modulated insect echoes. On the other hand, FM-FM neurons are combination-sensitive and are tuned to particular values of echo delays. They are specialized for processing target-distance information (see refs. 15 and 46 for review). To examine corticofugal modulation of the responses of thalamic and/or collicular DSCF or FM-FM neurons, the cortical DSCF (47, 48) or FM-FM area (49) was focally inactivated with 90 nl of 1.0% lidocaine or focally and repetitively activated with 100-nA, 0.2-ms long electric pulses delivered at a rate of 5/s for 7 min or 6.7/s for 6.7 min (ESar, repetitive electric stimulation of the AC).
DSCF Neurons.
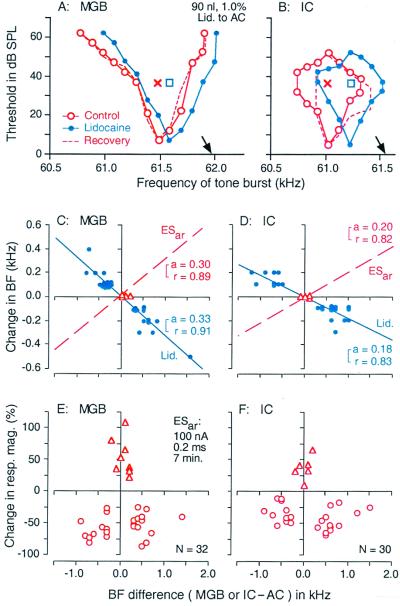
Cortical DSCF neurons, via the corticofugal system, mediate a highly focused positive feedback to augment the auditory responses at the BFs of matched thalamic or collicular DSCF neurons (hereafter, subcortical neurons). The BFs of these matched neurons are the same within ± 0.2 kHz as the BF of the electrically stimulated cortical neurons (hereafter, cortical BF). The positive feedback is very strong. Without it, the responses of subcortical neurons would be small. The positive feedback always is accompanied by widespread lateral inhibition, which suppresses the auditory responses at the BFs of unmatched subcortical DSCF neurons (hereafter, subcortical BF). The BFs of these subcortical neurons are different by more than 0.2 kHz, but not more than 2.0 kHz, from the cortical BF. Focal cortical inactivation with lidocaine evokes subcortical changes, which are exactly opposite to the above (Fig. 1 E and F).
Figure 1.
Corticofugal modulation of thalamic (MGB) and collicular (IC) neurons in the mustached bat. Changes in the frequency-tuning curves of thalamic (A) and collicular neurons (B) by a focal inactivation of cortical neurons with 90 nl of 0.1% lidocaine (Lid.). The BFs of the inactivated cortical neurons are indicated by the arrows. The curves were measured before (control; ○), during (●), and after (recovery; dashed lines) the cortical inactivation. The tuning curves and BFs shift toward the BFs of inactivated cortical neurons. Crosses and squares indicate the best amplitudes measured before and during the cortical inactivation, respectively. Changes in the BFs of thalamic (C) and collicular neurons (D), i.e., reorganization of the frequency map, evoked by a focal inactivation of cortical neurons with lidocaine. The abscissae represent the differences in BF between cortical (AC) and thalamic (MGB) or collicular (IC) neurons in the control condition. The cortical BF was ≈61.2 kHz. The triangles and filled circles represent the data obtained from matched and unmatched subcortical neurons, respectively. The regression lines (solid), their slopes (a), and correlation coefficients (r) are shown. The dashed lines in C and D, respectively, show the regression lines for the BF shifts of thalamic and collicular neurons evoked by a focal cortical activation with 0.2-ms, 100-nA electric pulses at a rate of 5/s for 7 min (ESar). The slopes and correlation coefficients of these dashed lines also are shown. The BF shift was symmetrical and centrifugal for ESar. (E and F) The abcissae are the same as those in C and D. The ordinates represent percent change in the response magnitude (number of impulses per tone burst) of thalamic (E) and collicular (F) neurons evoked by ESar. The triangles and circles, respectively represent percent changes in response magnitude of matched and unmatched subcortical neurons at the BFs of individual neurons in the control condition. To measure response magnitudes, tone bursts were set at the best amplitude of each neuron in the control condition. Changes in BF (C and D) and response magnitude (E and F) both are larger in the MGB than in the IC (47, 48).
Focal activation of cortical DSCF neurons modulates response magnitude of subcortical DSCF neurons, sharpens their frequency-tuning curves, and shifts the BFs and tuning curves of unmatched subcortical DSCF neurons away from the cortical BF. This centrifugal BF shift lasts up to 3 h, i.e., “recovers” in 3 h. Therefore, cortical neurons, via the corticofugal system, adjust and improve auditory information processing in the subcortical auditory nuclei. In other words, cortical neurons adjust and improve their own input. These corticofugal functions were named egocentric selection (49). The effects of egocentric selection are nearly two times larger for thalamic DSCF neurons than for collicular DSCF neurons (Fig. 1 C–F). In other words, cortical neurons adjust and improve their own inputs through multiple corticofugal feedback loops. Focal inactivation of cortical DSCF neurons results in corticofugal changes that are just opposite to those evoked by focal cortical activation (Fig. 1 A–D).
FM-FM Neurons.
When cortical FM-FM neurons are electrically stimulated, exactly the same corticofugal effects as above were found on collicular FM-FM neurons, which are tuned to echo FM components after an emitted FM component with specific time delays corresponding to specific target distances. The best (echo) delay to excite them differs between neurons. Focal electrical stimulation of cortical FM-FM neurons facilitates the responses of matched collicular FM-FM neurons to a pair of FM sounds and sharpens their delay-tuning curves without shifting their best delays. The duration of facilitative response caused by the corticofugal positive feedback appears to be adjusted in the MGB by GABAergic inhibitory neurons in the thalamic reticular nucleus feedback (ref. 50; see ref. 51 for review). The matched collicular FM-FM neurons have the best delays which are within ± 0.4 ms of the best delay of the electrically stimulated cortical FM-FM neurons. On the other hand, the electrical stimulation of cortical FM-FM neurons suppresses the auditory responses at the best delays of unmatched collicular FM-FM neurons and sharpens and shifts their best delays away from the best delays of electrically stimulated cortical neurons. This centrifugal shift lasts up to 3 h. The unmatched collicular FM-FM neurons have best delays that are different by more than 0.4 ms from the best delay of the electrically stimulated cortical FM-FM neurons (49).
The corticofugal effects on subcortical DSCF and FM-FM neurons last long, so that it is hypothesized that egocentric selection is involved in the reorganization (plasticity) of the frequency and echo-delay maps of the central auditory system.
Corticofugal effects caused by positive feedback are stronger on subcortical FM-FM neurons than on subcortical DSCF neurons. Therefore, the processing of complex sounds by combination-sensitive neurons generally may depend on the corticofugal system more than does the processing by neurons primarily responding to single tones (44, 45).
Cochlear Hair Cells.
The corticofugal system probably modulates the activity of cochlear hair cells through inhibitory olivocochlear neurons (26, 27, 31). It has been proposed that olivocochlear fibers improve the discrimination of complex sounds (52), increase the signal-to-noise ratio (53), increase the dynamic range of intensity coding (54), mediate selective attention (55), control the gain (56), and reduce temporary threshold shift (57). The functional role of the corticofugal system in signal processing at the subcollicular nuclei and the cochlea remains to be explored.
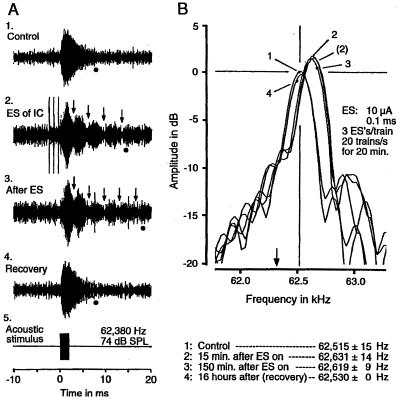
In the mustached bat, cochlear microphonic response (CM) recorded at the perilymphatic duct is sharply tuned to ≈61 kHz. Because of the sharp tuning, the CM evoked by a tone burst at ≈61 kHz shows a prominent off-response (CM-after), i.e., damped oscillations that occur at a fixed frequency (≈61 kHz) irrespective of the frequency of a stimulus tone burst (58). The focal electrical activation of the IC increases the resonance frequency and the duration of CM-after at ≈61 kHz (Fig. 2). Although the increase in the duration of CM-after suggests sharpening of the frequency tuning of outer hair cells, we don't yet know how our preliminary data are related to the modulation of auditory signal processing and whether focal activation of the cortical DSCF area representing ≈61 kHz evokes the same changes as those evoked by the focal activation of the IC.
Figure 2.
Colliculofugal modulation of cochlear hair cells in the mustached bat. Changes in microphonic response (CM) to a 2.0-ms tone pulse evoked by electrical stimulation (ES) of the contralateral collicular neurons tuned to ≈62.32 kHz (arrows in B). (A) Oscillograms of CMs. (B) Amplitude spectra of the after-potentials of CMs (CM-after) shown in A. 1–4 were, respectively recorded before (control condition), during (15 min after the beginning of ES), 150 min after, and 16 h after ES (recovery condition). In A, the dots and arrows indicate the end of CM-after and nodes of beat, respectively. The lengthening of CM-after by ES suggests sharpening of frequency tuning of hair cells. In B, the peaks of the amplitude spectra indicate the resonance frequencies, which shifted from 62,515 Hz to 62,631 Hz (see the list at the bottom). The acoustic stimulus shown in A5 was a 2.0-ms, 62,380-Hz tone pulse at 74 dB SPL. The parameters of ES are listed in B. The three vertical lines in A2 are stimulus artifacts. The CMs were recorded from the cochlear perilymphatic duct with a tungsten-wire electrode.
Egocentric Selection in the Big Brown Bat.
In the big brown bat, Jen et al. (38) found that electrical stimulation of the AC evoked either short latency facilitation (26%) or inhibition (74%) of collicular neurons regardless of whether they were matched or unmatched in BF with stimulated cortical neurons. They also found that the electric stimulation either augmented the auditory responses and broadened the frequency and spatial tuning curves of collicular neurons or suppressed the auditory responses and sharpened these tuning curves. The data obtained from the big brown bat by Suga and his coworkers (59–63), summarized below, are different from those obtained by Jen et al. (38), who used 0.1-ms long, 1.3- to 85-μA pulses for cortical stimulation. Suga and his coworkers used 0.2-ms long, 0.1-μA electric pulses for cortical stimulation.
Cortical neurons, via the corticofugal system, mediate focused positive feedback to augment the auditory responses at the BFs of matched collicular neurons without shifting their BFs and frequency tuning curves, as found in the mustached bat (Fig. 3 C and D). The BFs of matched neurons are within ± 0.5 kHz of the cortical BF.
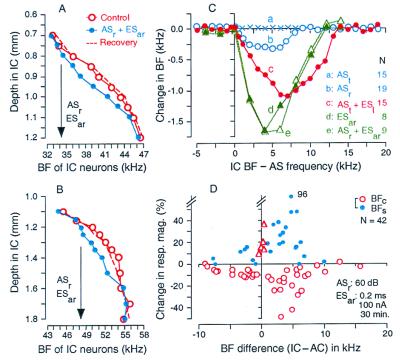
Figure 3.
Corticofugal modulation of collicular (IC) neurons in the big brown bat. (A and B) Shifts in the BFs of collicular neurons (i.e., reorganization of the frequency map) were evoked by a 100-nA, 0.2-ms electrical stimulation of cortical auditory neurons (ESar) paired with a 20-ms, 60-dB SPL acoustic stimulus (ASr). ASr + ESar was delivered at a rate of 10/s for 30 min. BF depth curves were obtained in two dorso-ventral electrode penetrations (A and B) across the IC 30 min before (control; ○), 30 min after (●), and 2–3 h after (recovery; dashed lines) ASr + ESar. Because almost all data points obtained 2–3 h after ASr + ESar were the same as those obtained before the stimulation, they were expressed as a dashed curve without the individual data. The BFs of cortical neurons that were electrically stimulated are indicated by the arrows. (C) The amount of collicular BF shifts as a function of the difference between the BFs of collicular neurons and the frequency of acoustic stimulus (ASr or ASt) or the BF of electrically stimulated cortical neurons (ESar). ASr was a 20-ms, 50-dB SPL tone burst delivered at a rate of 10/s for 30 min. ASt was a 1.0-s train of tone bursts (10 ms each, 50 dB SPL, 33/s) delivered every 30 s for 30 min. ASt was delivered alone or followed by electric stimulation (50 ms, 0.15–0.57 mA) of the leg (ESl) with a 1.0-s gap as in trace conditioning. Each symbol indicates a mean of the data obtained from several electrode penetrations (N) across the IC. The standard error for each data point is not shown for simplicity. ASt alone evoked no BF shift (a), but ASr alone (b), ASt + ESl (c) or ESar alone (d) did. ASr + ESar evoked BF shifts that were similar to those evoked by ESar alone (e). (D) Changes in response magnitude (number of impulses per tone burst) of collicular neurons evoked by ASr + ESar are plotted as a function of the difference in BF between recorded collicular and electrically stimulated cortical neurons. The frequency of ASr was the same as the BF of the electrically stimulated cortical neurons. The triangles and circles (open and filled) represent the data obtained from matched and unmatched neurons, respectively. The open and filled circles, respectively represent the changes in response magnitude at the BFs in the control condition (BFc) and in the shifted condition (BFs). The data were obtained with stimulus tone bursts at 10 or 20 dB above minimum threshold of each neuron. The frequencies of ASt and ASr for curves a–c in C were 25.3 ± 7.84 kHz. The BFs of cortical neurons stimulated by ESar for curves d and e in C were 39.5 ± 9.57 kHz (59, 60).
The corticofugal effects on unmatched collicular neurons are inhibitory at their BFs and at frequencies higher than their BFs. However, the effects are facilitatory at the frequencies between the cortical and collicular BFs. The inhibitory and facilitative effects are larger on the high-frequency side than on the low-frequency side of the cortical BF (Fig. 3D). Because of these frequency-dependent corticofugal effects, the BFs and frequency-tuning curves of unmatched collicular neurons shift toward the cortical BF. This centripetal BF shift occurs predominantly for collicular neurons with BFs higher than the cortical BF. Therefore, the centripetal BF shift is asymmetrical (Fig. 3C d and e). Asymmetrical and centripetal BF shifts also can be evoked by acoustic stimuli [20-ms long, 50-dB sound pressure level (SPL) tone bursts delivered at a rate of 10/s for 30 min; Fig. 3Cb].
BF shifts mean the reorganization of the cochleotopic (frequency) map of the IC. This reorganization can be easily demonstrated by dorso-ventral penetrations of a recording electrode along the frequency axis of the IC. In such a penetration, BFs systematically become higher with the electrode depth (Fig. 3 A and B, ○). When cortical neurons with a particular BF (arrow in Fig. 3 A or B) are electrically stimulated, the BF-depth curve shifts toward the cortical BF. This shift occurs for collicular BFs, which are 0–12 kHz higher than the cortical BF (Fig. 3 A and B, ●). This reorganization of the frequency map results in the over-representation of the frequency equal to the cortical BF and the under-representation of frequencies that are 6–12 kHz higher than the cortical BF. Therefore, the contrast of the neural representation of the frequency of an acoustic stimulus is increased.
ESar also evokes BF shifts of cortical neurons located near the stimulation site that are very similar to collicular BF shifts. For example, centripetal BF shifts occur over 600-700 μm rostral to the electrically stimulated cortical neurons with a 30-kHz BF and over 500 μm caudal to these. The amount of BF shifts is asymmetrical and 3–4 times larger on the rostral (higher frequency) side of the stimulated cortical neurons than on the caudal (low frequency) side (62).
For 30-min ESar, collicular and cortical BF shifts develop up to ≈64% of the plateau within 2 min, reach a plateau at 30 min, and then recover ≈180 min after the cessation of ESar. The recovery of BF shift tends to be slightly slower in the AC than in the IC (see Fig. 5A a and b). The lengthening of ESar beyond 30 min, e.g., to 90 min, hardly increases the amount of BF shifts, but increases the duration of the plateau. The recovery time is nearly the same as that for the 30-min ESar. When the duration of ESar is shorter than 30 min, the BF shifts are small and recover quickly. For a 2-min ESar, the BF shifts are ≈64% of the plateau and recover in ≈42 min. BF shifts in the big brown bat are associated with sharpening of frequency-tuning curves of some neurons (62), as found in the mustached bat (47).
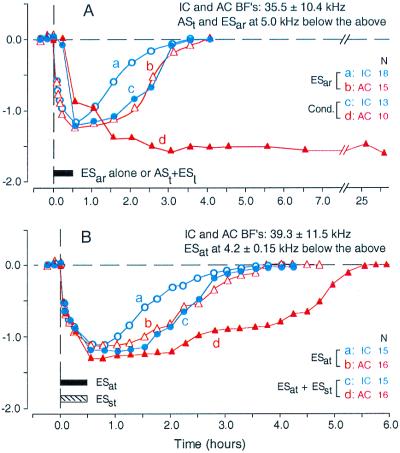
Figure 5.
Difference in time course of BF shift between collicular (IC) and cortical (AC) neurons evoked by focal cortical electrical stimulation or auditory conditioning in the big brown bat. (A) BF shifts were evoked by repetitive electric stimulation of the AC (ESar) (a and b) or the conditioning (cond.) (c and d). The conditioning consisted of a conditioned 1-s train of tone bursts (ASt) followed by an unconditioned electric leg stimulus (ESl). For ESar, cortical neurons showed a slightly slower BF recovery than did collicular neurons (a vs. b). For the conditioning, the BFs of cortical neurons slowly changed and did not recover within 1 day, but those of collicular neurons changed quickly and recovered as fast as those evoked by ESar (c vs. d) (61). (B) Collicular (a) and cortical BF shifts (b) evoked by trains of electric stimuli delivered to the AC (ESat) were augmented mainly in duration by electrical stimulation of the somatosensory cortex (ESst), as shown by c and d. ESat and ESst were delivered to mimic the conditioned (ASt) and unconditioned stimuli (ESl) (63). The mean BF of collicular and cortical neurons recorded was 35.5 ± 10.4 kHz for A and 39.3 ± 11.5 kHz for B. The frequencies of ASt were always 5.0 kHz lower than the recorded collicular and cortical BFs. The BFs of cortical neurons stimulated by ESar or ESat were 4.2 ± 0.15 kHz lower than the BFs of the recorded neurons. BF shifts were measured with tone bursts at 10 dB above minimum threshold of individual collicular or cortical neurons. N, number of neurons studied.
Corticofugal lateral inhibition of collicular or thalamic neurons may be based on (i) intrinsic cortical inhibition, which may adjust the amount of corticofugal positive feedback, (ii) intrinsic thalamic inhibition, (iii) inhibition by the thalamic reticular nucleus, and/or (iv) intrinsic collicular inhibition. Jen et al. (38) found that neurons in the external nucleus of the IC are excited by corticofugal fibers and in turn inhibit neurons in the central nucleus of the IC.
Differences in Corticofugal Modulation Between Species and Between Ordinary and Specialized Areas
Egocentric selection has been found not only in the mustached bat (Fig. 1) and big brown bat (Fig. 3), but also in the cat (64), so that it may be a general function of the corticofugal system. Corticofugal positive feedback associated with lateral inhibition also has been found in the visual system (65). However, the effect of egocentric selection on the cochleotopic (frequency) map is different between different species of mammals and between ordinary and specialized areas of the AC of a single species.
Egocentric selection evokes centrifugal and symmetrical shifts of the BFs of DSCF neurons (Fig. 1) and the best delays of FM-FM neurons (49) of the mustached bat, but centripetal and asymmetrical BF shifts in the AC of the big brown bat (Fig. 3). BF shifts are centripetal and asymmetrical in the AC of the Mongolian gerbil (Meriones unguiculatus) and are centripetal and somewhat symmetrical in the posterior division of the AC of the mustached bat (66). The range and amount of the BFs shifted by ESar are also different from species to species and between the ordinary and specialized areas of the AC.
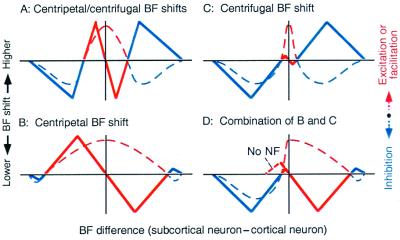
Corticofugal excitation or facilitation and inhibition appear to evoke centripetal and centrifugal BF shifts of unmatched subcortical neurons, respectively (Fig. 4A). If the excitatory effect is strong and widespread to neighboring unmatched neurons and negative feedback is weak, it may evoke prominent centripetal BF shifts (Fig. 4B). On the contrary, if the excitatory effect is highly focused to matched neurons and the inhibitory effect is strong and widespread to neighboring unmatched neurons, it may evoke prominent centrifugal BF shift (Fig. 4C). The corticofugal excitatory and inhibitory effects shown in Fig. 4 B and C may be combined in different ways (Fig. 4D). For example, if the excitatory effect is strong and widespread on the high-frequency side and there is neither excitatory nor inhibitory effect on the low-frequency side, it may evoke asymmetrical centripetal BF shift (Fig. 4D Right).
Figure 4.
The hypothesis to explain centripetal (solid red lines) and centrifugal BF shifts (solid blue lines) of subcortical neurons evoked by focal electrical stimulation of the auditory cortex. BF shifts (ordinates) are plotted as a function of difference in BF between recorded subcortical and activated cortical neurons (abscissae). The directions of BF shifts are hypothesized to depend on corticofugal excitation or facilitation (dashed red lines) and inhibition (dashed blue lines). NF, negative feedback. See the text.
Neural representation of auditory signals in an ordinary AC appears to be improved by centripetal BF shifts, which result in over-representation of a particular value of a parameter characterizing a given acoustic stimulus. The area for over-representation is always bordered with the area or areas for under-representation. On the other hand, a specialized AC such as the DSCF and FM-FM areas over-represent particular values of a parameter in a narrow range in the natural condition. Therefore, further improvement for signal processing is performed by increasing the contrast in neural representation by centrifugal shifts in tuning curves. Asymmetrical centripetal BF shifts appear to be related to the asymmetrical shape of a frequency-tuning curve, as previously discussed (59). Regardless of types of BF shifts, tuning curves of neurons are sharpened by egocentric selection (47, 62).
Corticofugal Modulation and Plasticity of the Auditory System: Physiology and Behavior
The response properties of neurons and the sensory maps in a sensory cortex and subcortical sensory nuclei can be changed by conditioning, learning of a discrimination task, or focal cortical electrical stimulation (see refs. 67–69 for review). The importance of the corticofugal system to evoke plasticity in the central sensory system had not been considered until very recently (in the auditory system, refs. 47, 49, 51, and 59; in the somatosensory system, refs. 70 and 71). Gao and Suga (60, 61) exposed the big brown bat to various stimuli and obtained data indicating the importance of the corticofugal system in cortical plasticity. Their data and conclusions are summarized below.
Collicular and cortical neurons show asymmetrical and centripetal BF shifts not only for focal electrical stimulation of the AC (Fig. 3Cd), but also for a repetitive delivery of 20-ms long, 50-dB SPL tone bursts at a rate of 10/s for 30 min (ASr, repetitive acoustic stimuli) (Fig. 3Cb). These findings indicate that egocentric selection is an intrinsic mechanism for the reorganization of the central auditory system.
A 1.0-s long train of 10-ms long, 50-dB SPL tone bursts at a burst rate of 33/s (ASt, train of acoustic stimuli), delivered alone to the animal every 30 s for 30 min, does not evoke BF shift (Fig. 3Ca). However, when ASt is delivered as a conditioned stimulus followed by an unconditioned electric leg stimulation (ESl), large BF shifts are evoked that are also asymmetrical and centripetal (Fig. 3Cc). Neither ESl alone nor ASt delivered after ESl (backward conditioning) evokes BF shifts. Because ASt alone is behaviorally irrelevant, the data indicate that when an acoustic stimulus becomes behaviorally relevant to the animal, it evokes, via egocentric selection, considerable plasticity in the central auditory system, and that behavioral relevance is determined by the auditory and nonauditory systems, through associative learning.
The AC and the somatosensory cortex are both necessary for the BF shifts in the IC (60) and AC (61) caused by the conditioning, i.e., by associative learning. Electrical stimulation of the somatosensory cortex augments the collicular and cortical BF shifts evoked by the electric stimulation of the AC (63). Therefore, one of the nonauditory systems described above is the somatosensory cortex activated by the unconditioned leg stimulation. Another nonauditory system to be considered is the cholinergic basal forebrain, because its involvement in the plasticity of the AC has been demonstrated by Bakin and Weinberger (72) and Kilgard and Merzenich (73, 74).
Cortical and collicular BF shifts evoked by 30-min ESar are nearly the same in amount and recovery time (Fig. 5A a and b). However, those evoked by a 30-min conditioning session were quite different from each other (Fig. 5A c and d). Namely, the collicular BF shift is largest at the end of conditioning, and it is larger than the cortical BF shift within 45 min after the conditioning. The collicular BF shift recovers 180 min after the conditioning, just like that evoked by ESar (compare c with a in Fig. 5A). On the other hand, the cortical BF shift gradually increases after the conditioning, reaches a plateau at the time when the collicular BF shift almost recovers, and then stays over many hours (Fig. 5Ad). This is quite different from the cortical BF shift evoked by ESar (Fig. 5Ab). When the second conditioning session is given to the animal after the recovery of the collicular BF shift, it evokes the collicular BF shift, which is almost the same as that evoked by the first, and the cortical BF shift, which gradually increases over 3 h to a new plateau. However, the second conditioning session given to the animal at the beginning of the recovery phase of the collicular BF shift hardly changes the cortical BF shift, but prolongs the collicular BF shift. These observations indicate: (i) the collicular BF shift is not at all a consequence of the cortical BF shift, (ii) it always precedes the cortical BF shift, and (iii) its increasing phase is more related to the large cortical BF shift than its decreasing phase. The collicular BF shift appears to boost the cortical BF shift (61). Hereafter, we call plasticity lasting up to 3 h short term and plasticity lasting longer than 3 h long term.
Stimulation of the basal forebrain with 0.2-ms, 100-μA electric pulses immediately before and/or during (but not after) ESar augments the collicular and cortical BF shifts evoked by ESar, i.e., by the AC and the corticofugal system. The cortical BF shift becomes long-lasting, but the lengthening the recovery time of the collicular BF shift is small (63).
By 1990, a number of important findings on learning and memory had been made: (i) Acetylcholine plays an important role in learning and memory (see ref. 75 for review). (ii) The cholinergic basal nucleus of the forebrain projects diffusely and widely to the cerebral cortex (see ref. 76 for review). (iii) The basal forebrain plays an important role in learning and memory (77–79). (iv) The basal forebrain receives an input from the amygdala, which is necessary for the acquisition of conditioned response (80–82). (v) The amygdala receives an input from thalamic nuclei (83). Weinberger (see refs. 69 and 83 for review) pointed out the importance of the amygdala and cholinergic basal forebrain for plasticity of the AC evoked by fear conditioning. The basal forebrain undoubtedly plays an important role in the plasticity of the AC (72–74).
In the big brown bat, an acetylcholine application to the AC during auditory conditioning augments both collicular and cortical BF shifts, which were barely evoked by a 15-min conditioning session. An atropine application to the AC during the 30-min conditioning session completely abolishes the cortical BF shift and reduces the collicular BF shift (84). These observations indicate that the cholinergic system can augment the plasticity evoked by the AC and the corticofugal system and that the collicular BF shift can be evoked without the cortical BF shift. These observations suggest that the subcortical short-term change caused by egocentric selection, with the help of acetylcholine, boosts the cortical change into a long-term change.
Working Hypothesis
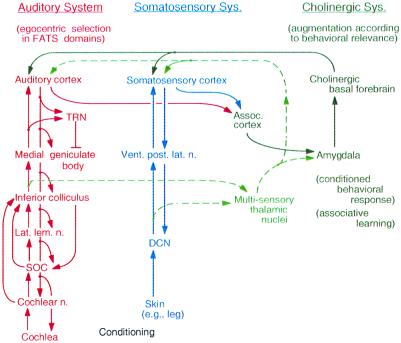
We propose the following working hypothesis of cortical plasticity. When behaviorally irrelevant acoustic stimuli are delivered to an animal, auditory signals representing the stimuli ascend from the cochlea to the AC. Then, the AC and the corticofugal system perform egocentric selection, which is a small and short-term modulation of subcortical signal processing. Accordingly, the small and short-term cortical change is evoked (Fig. 6 Left). When the acoustic stimuli are paired with electric leg stimulation, the auditory and somatosensory signals ascend from the periphery to the auditory and somatosensory cortices, respectively (Fig. 6 Center), and then to the amygdala through association cortices. These signals are associated in the amygdala, which is essential for evoking conditioned behavioral response. Therefore, the acoustic stimuli become behaviorally relevant to the animal. The amygdala sends the “associated” signal to the cholinergic basal forebrain, which increases the cortical acetylcholine level (Fig. 6 Right). Then, the change in the AC is augmented. Accordingly, egocentric selection is augmented, and the subcortical change becomes larger, so that the cortical change becomes larger and long term. This positive feedback loop is controlled by inhibition mediated by the thalamic reticular nucleus.
Figure 6.
Block diagram to explain our working hypothesis for the adjustment and improvement of auditory signal processing according to associative learning. DCN, dorsal column nuclei in the spinal cord. FATS, frequency, amplitude, time, and space. SOC, superior olivary complex. TRN, thalamic reticular nucleus. See the text.
The dashed arrows in Fig. 6 indicate pathways through the multisensory thalamic nuclei that have been considered to be essential for cortical plasticity by Weinberger (see refs. 69 and 83 for review). These pathways appeared to be quite reasonable to us. However, the importance of these pathways is doubtful because of the following data obtained from the big brown bat (61). (i) Inactivation or activation of the somatosensory cortex, respectively abolishes or augments cortical and collicular BF shifts evoked by fear conditioning. (ii) Collicular changes evoked by the corticofugal system precede cortical changes. (iii) The AC and corticofugal system have an intrinsic mechanism for cortical and subcortical plastic changes, which are highly specific to the parameters characterizing an acoustic stimulus. (iv) Fear conditioning evokes plasticity in the IC, which is the nucleus one step below the thalamus. In other words, the multisensory thalamic nuclei are not the first place where the plasticity caused by the conditioning is evoked.
Multiple Functions of the Corticofugal System for Hearing
The corticofugal function consists of at least the following subfunctions.
Egocentric Selection for Short-Term Adjustment and Improvement of Auditory Signal Processing According to Auditory Experience.
Egocentric selection is performed by the AC and the corticofugal system. It is based on focused positive feedback associated with lateral inhibition. Egocentric selection adjusts and improves cortical neurons' own input, so that it is similar to the function of corticofugal feedback in the visual system (85).
Reorganization for Long-Term Adjustment and Improvement of Signal Processing.
Such reorganization is based on egocentric selection working together with nonauditory systems.
Gain Control.
Overall facilitative or inhibitory corticofugal modulation indicate this function. Egocentric selection can be viewed as selective gain control.
Shaping (or Even Creating) Response Properties of Neurons, in Particular, of Combination-Sensitive Neurons.
Filter properties of neurons in the frequency (37, 38, 47, 62), amplitude, time (49), and spatial domains (38) can be sharpened by the corticofugal system. Sharpening of tuning is one of the functions of egocentric selection. The creation of combination sensitivity through interactions between the ascending and descending systems (45) must be a particularly important function for processing behaviorally relevant complex sounds. In the visual system, response properties of thalamic neurons become complex because of corticofugal feedback (86).
Binding of the Different Features of Auditory Signals.
The problem of binding has been extensively studied in the visual system, but not in the auditory system. In the visual system, the corticofugal system evokes feature-linked synchronized discharges in the thalamic neurons (86, 87).
Stabilization of Thalamic Auditory Responses Via the Thalamic Reticular Nucleus.
The thalamic reticular nucleus receives axon collaterals from both ascending thalamo-cortical fibers and descending cortico-thalamic fibers. Corticofugal positive feedback has a high gain, so that ringing would be evoked if it is not incorporated with inhibition through the thalamic reticular nucleus (see ref. 51 for review). If the thalamic reticular nucleus does not operate properly, long-lasting discharges, perhaps responsible for tinnitus, would be produced. Cooling of the AC had “complex” effects on the auditory responses of MGB and reticular nuclear neurons, so that Villa et al. (41) proposed that the reticular nucleus takes a role as an adaptive filter.
Attentional Modulation of Auditory Signal Processing.
In cats, visual attention to a mouse reduces auditory responses of the dorsal cochlear nucleus (88) and a visual discrimination task reduces auditory nerve responses to clicks (89). In humans, visual attention reduces auditory nerve responses (90) and otoacoustic emissions evoked by a click (91). The corticofugal system probably mediates attentional modulation of auditory signal processing.
Low-Frequency Modulation of Brain Rhythm.
The corticofugal system transmits slow oscillatory changes in cortical activity to the thalamic visual nucleus. This slow oscillation (0.6–1.0 Hz) interacts with spindles (7–14 Hz) generated in the thalamus, modulates neural excitability, and produces different brain rhythms characterizing various behavioral states (see ref. 92 for review).
Acknowledgments
We thank Drs. S. P. Dear, K. K. Ohlemiller, J. J. Wenstrup, and Mr. N. Laleman for their comments on this paper. This work has been supported by a research grant from the National Institute on Deafness and Other Communicative Disorders (DC 00175).
Abbreviations
- AC
auditory cortex
- ASr
repetitive acoustic stimuli
- ASt
train of acoustic stimuli
- BF
best frequency
- CF
constant frequency
- CM
cochlear microphonic response
- DSCF
Doppler-shifted constant frequency
- ESar
repetitive electric stimulation of the AC
- ESl
electric stimulation of the leg
- FM
frequency modulation
- IC
inferior colliculus
- MGB
medial geniculate body
- SPL
sound pressure level
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Suga N, Zhang Y, Yan J. J Neurophysiol. 1997;77:2098–2114. doi: 10.1152/jn.1997.77.4.2098. [DOI] [PubMed] [Google Scholar]
- 2.Suga N, Schlegel P. J Acoust Soc Am. 1973;54:174–190. doi: 10.1121/1.1913561. [DOI] [PubMed] [Google Scholar]
- 3.Covey E, Casseday J H. J Neurosci. 1991;11:3456–3470. doi: 10.1523/JNEUROSCI.11-11-03456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan W E. J Neurophysiol. 1982;48:1011–1132. doi: 10.1152/jn.1982.48.4.1011. [DOI] [PubMed] [Google Scholar]
- 5.Casseday J H, Ehrlich D, Covey E. Science. 1994;264:847–850. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- 6.Suga N. J Physiol (London) 1965;181:671–700. doi: 10.1113/jphysiol.1965.sp007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suga N. J Physiol (London) 1969;200:555–574. doi: 10.1113/jphysiol.1969.sp008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuzessery Z M. J Neurophysiol. 1994;72:1061–1079. doi: 10.1152/jn.1994.72.3.1061. [DOI] [PubMed] [Google Scholar]
- 9.Suga N, Manabe T. J Neurophysiol. 1982;47:225–255. doi: 10.1152/jn.1982.47.2.225. [DOI] [PubMed] [Google Scholar]
- 10.Haplea S, Covey E, Casseday J H. J Comp Physiol A. 1994;174:671–683. doi: 10.1007/BF00192716. [DOI] [PubMed] [Google Scholar]
- 11.Casseday J H, Covey E, Grothe B. J Neurophysiol. 1997;77:1595–1605. doi: 10.1152/jn.1997.77.3.1595. [DOI] [PubMed] [Google Scholar]
- 12.Suga N, O'Neill W E, Kujirai K, Manabe T. J Neurophysiol. 1983;49:1573–1626. doi: 10.1152/jn.1983.49.6.1573. [DOI] [PubMed] [Google Scholar]
- 13.Irvine D R F. In: The Mammalian Auditory Pathway: Neurophysiology. Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 153–231. [Google Scholar]
- 14.Galazyuk A V, Feng A S. J Comp Physiol A. 1997;180:301–311. doi: 10.1007/s003590050050. [DOI] [PubMed] [Google Scholar]
- 15.Suga N. In: The Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1994. pp. 295–318. [Google Scholar]
- 16.Valentine D E, Moss C F. J Neurosci. 1997;17:1720–1733. doi: 10.1523/JNEUROSCI.17-05-01720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manabe T, Suga N, Ostwald J. Science. 1978;200:339–342. doi: 10.1126/science.635594. [DOI] [PubMed] [Google Scholar]
- 18.Imig T J, Adrián H O. Brain Res. 1977;138:241–257. doi: 10.1016/0006-8993(77)90743-0. [DOI] [PubMed] [Google Scholar]
- 19.Kujirai K, Suga N. Auris Nasus Larynx (Tokyo) 1983;10:9–24. doi: 10.1016/s0385-8146(83)80024-8. [DOI] [PubMed] [Google Scholar]
- 20.Jen P H-S, Sun X D, Lin P J J. J Comp Physiol. 1989;165:1–14. doi: 10.1007/BF00613794. [DOI] [PubMed] [Google Scholar]
- 21.Suga N, Tsuzuki K. J Neurophysiol. 1985;53:1109–1145. doi: 10.1152/jn.1985.53.4.1109. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Pollak G D, Resler C. J Neurophysiol. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- 23.Fuzessery Z M, Hall J C. J Neurophysiol. 1996;76:1059–1053. doi: 10.1152/jn.1996.76.2.1059. [DOI] [PubMed] [Google Scholar]
- 24.Jen P H-S, Feng R B. J Comp Physiol A. 1999;184:185–194. doi: 10.1007/s003590050317. [DOI] [PubMed] [Google Scholar]
- 25.Kelly J P, Wong D. Brain Res. 1981;212:1–15. doi: 10.1016/0006-8993(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 26.Saldana E, Feliciano M, Mugnaini E. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Huffman R F, Henson O W., Jr Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- 28.Malmierca M S, Le Beau F E N, Rees A. Hear Res. 1996;93:167–180. doi: 10.1016/0378-5955(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 29.Ojima H. Cereb Cortex. 1994;6:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- 30.Feliciano M, Saldana E, Mugnaini E. Aud Neurosci. 1995;1:287–308. [Google Scholar]
- 31.Warr W B. In: The Mammalian Auditory Pathway: Neuroanatomy. Webster D B, Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 410–448. [Google Scholar]
- 32.Desmedt J E, Mechelse K. Proc Soc Exp Biol. 1958;99:772–775. doi: 10.3181/00379727-99-24496. [DOI] [PubMed] [Google Scholar]
- 33.Massopust L C, Jr, Ordy J M. Exp Neurol. 1962;6:465–477. doi: 10.1016/0014-4886(62)90072-9. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Yanagisawa K, Kanzaki J, Katsuki Y. Exp Brain Res. 1966;2:302–317. doi: 10.1007/BF00234776. [DOI] [PubMed] [Google Scholar]
- 35.Amato G, La Grutta V, Enia F. Arch Sci Biol. 1969;53:291–313. [PubMed] [Google Scholar]
- 36.Sun X, Jen P H S, Sun D, Zhang S. Brain Res. 1989;495:1–8. doi: 10.1016/0006-8993(89)91212-2. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Chen Q, Jen P H. Neurosci Lett. 1996;212:131–134. doi: 10.1016/0304-3940(96)12788-9. [DOI] [PubMed] [Google Scholar]
- 38.Jen P H-S, Chen Q C, Sun X D. J Comp Physiol. 1998;183:683–697. doi: 10.1007/s003590050291. [DOI] [PubMed] [Google Scholar]
- 39.Andersen P, Junge K, Sveen O. Brain Behav Evol. 1972;6:170–184. doi: 10.1159/000123705. [DOI] [PubMed] [Google Scholar]
- 40.Orman S S, Humphrey G L. Exp Brain Res. 1981;42:475–482. doi: 10.1007/BF00237512. [DOI] [PubMed] [Google Scholar]
- 41.Villa A E, Rouiller E M, Simm G M, Zurita P, de Ribaupierre Y, de Ribaupierre F. Exp Brain Res. 1991;86:506–517. doi: 10.1007/BF00230524. [DOI] [PubMed] [Google Scholar]
- 42.Ryugo D K, Weinberger N M. Exp Neurol. 1976;51:377–391. doi: 10.1016/0014-4886(76)90262-4. [DOI] [PubMed] [Google Scholar]
- 43.Syka J, Popelar J. Neurosci Lett. 1984;51:235–240. doi: 10.1016/0304-3940(84)90557-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Suga N. J Neurophysiol. 1997;78:3489–3492. doi: 10.1152/jn.1997.78.6.3489. [DOI] [PubMed] [Google Scholar]
- 45.Yan J, Suga N. J Neurophysiol. 1999;81:817–824. doi: 10.1152/jn.1999.81.2.817. [DOI] [PubMed] [Google Scholar]
- 46.Suga N. Sci Am. 1990;262:60–68. doi: 10.1038/scientificamerican0690-60. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Suga N, Yan J. Nature (London) 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Suga N. J Neurophysiol. 2000;84:325–333. doi: 10.1152/jn.2000.84.1.325. [DOI] [PubMed] [Google Scholar]
- 49.Yan J, Suga N. Science. 1996;273:1100–1103. doi: 10.1126/science.273.5278.1100. [DOI] [PubMed] [Google Scholar]
- 50.Butman J A. Ph.D. thesis. St. Louis: Washington University; 1992. [Google Scholar]
- 51.Suga N, Butman J A, Teng H, Yan J, Olsen J F. In: Active Hearing. Flock A, Ottoson D, Ulfendahl M, editors. London: Elsevier; 1995. pp. 13–30. [Google Scholar]
- 52.Dewson J H. J Neurophysiol. 1968;31:122–130. doi: 10.1152/jn.1968.31.1.122. [DOI] [PubMed] [Google Scholar]
- 53.Kawase T, Delgutte B, Liberman M C. J Neurophysiol. 1993;70:2533–2549. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- 54.Geisler C D. J Acoust Soc Am. 1974;56:1910–1902. doi: 10.1121/1.1903533. [DOI] [PubMed] [Google Scholar]
- 55.Oatman L C, Anderson B W. Exp Neurol. 1977;57:200–211. doi: 10.1016/0014-4886(77)90057-7. [DOI] [PubMed] [Google Scholar]
- 56.Siegel J H, Kim D O. Hear Res. 1982;6:171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- 57.Rajan R. Brain Res. 1990;506:192–204. doi: 10.1016/0006-8993(90)91251-b. [DOI] [PubMed] [Google Scholar]
- 58.Suga N, Jen P H-S. J Exp Biol. 1977;69:207–232. doi: 10.1242/jeb.69.1.207. [DOI] [PubMed] [Google Scholar]
- 59.Yan W, Suga N. Nat Neurosci. 1998;1:54–58. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- 60.Gao E, Suga N. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao E, Suga N. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowdhury S, Suga N. J Neurophysiol. 2000;83:1856–1863. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- 63.Ma X, Suga N. Assoc. Res. Otolaryngol. Abstr. 2000. p. 259. , no. 899. [Google Scholar]
- 64.He J F. J Neurophysiol. 1997;77:896–908. doi: 10.1152/jn.1997.77.2.896. [DOI] [PubMed] [Google Scholar]
- 65.Tsumoto T, Creutzfeldt O D, Legendy C R. Exp Brain Res. 1978;32:345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- 66.Sakai, M & Suga, N. (2000) Soc. Neurosci. Abstr., in press.
- 67.Irvine D R F, Rajan R. Clin Exp Pharmacol Physiol. 1996;23:939–947. doi: 10.1111/j.1440-1681.1996.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 68.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 69.Weinberger N M. Neurobiol Learning Memory. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- 70.Ergenzinger E R, Glasier M M, Hahm J O, Pons T P. Nat Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- 71.Krupa D J, Ghazanfar A A, Nicolelis M A L. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakin J S, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 74.Kilgard M P, Merzenich M M. Nat Neurosci. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartus R T, Dean R L, III, Beer B, Lipps A S. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 76.Johnston M V, McKinney M, Coyle J T. Proc Natl Acad Sci USA. 1979;76:5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartus R T, Flicker C, Dean R L, Pontecorvo M, Figueiredo J C, Fisher S K. Pharmacol Biochem Behav. 1985;23:125–135. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 78.Rigdon G C, Pirch J H. J Neurosci. 1986;6:2535–2542. doi: 10.1523/JNEUROSCI.06-09-02535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wozniak D F, Stewart G R, Finger S, Olney J W, Cozzari C. Nerobiol Aging. 1989;10:173–179. doi: 10.1016/0197-4580(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 80.Krettek J E, Price J L. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 81.Price J L, Amaral D G. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peinado-Manzano A. Behav Brain Res. 1988;29:61–71. doi: 10.1016/0166-4328(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 83.Weinberger N M. Concepts Neurosci. 1990;1:91–123. [Google Scholar]
- 84.Ji, W., Suga, N. & Gao, E. (2000) Soc. Neurosci. Abstr., in press.
- 85.Murphy P C, Duckett S G, Sillito A M. Science. 1999;286:1552–1554. doi: 10.1126/science.286.5444.1552. [DOI] [PubMed] [Google Scholar]
- 86.Sillito A M, Jone H E, Gerstein G L, West D C. Nature (London) 1994;369:479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- 87.Gray C M, König P, Engel A K, Singer W. Nature (London) 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 88.Hernandez-Peon R, Scherrer H, Jouvet M. Science. 1956;123:331–332. doi: 10.1126/science.123.3191.331. [DOI] [PubMed] [Google Scholar]
- 89.Oatman L C. Exp Neurol. 1971;32:341–356. doi: 10.1016/0014-4886(71)90003-3. [DOI] [PubMed] [Google Scholar]
- 90.Lukas J H. Psychophysiology. 1980;17:444–452. doi: 10.1111/j.1469-8986.1980.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 91.Puel J L, Bonfils P, Pujol R. Brain Res. 1988;447:380–383. doi: 10.1016/0006-8993(88)91144-4. [DOI] [PubMed] [Google Scholar]
- 92.Steriade M. Trends Neurosci. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]