Abstract
Among tuberculosis patients, timely diagnosis of human immunodeficiency virus (HIV) co-infection and early antiretroviral treatment are crucial, but are hampered by a myriad of individual and structural barriers. Community-based models to provide counseling and rapid HIV testing are few but offer promise. During November 2009–April 2010, community health workers offered and performed HIV counseling and testing by using the OraQuick Rapid HIV-1/2 Antibody Test to new tuberculosis cases in 22 Ministry of Health establishments and their household contacts (n = 130) in Lima, Peru. Refusal of HIV testing or study participation was low (4.7%). Intervention strengths included community-based approach with participant preference for testing site, use of a rapid, non-invasive test, and accompaniment to facilitate HIV care and family disclosure. We will expand the intervention under programmatic auspices for rapid community-based testing for new tuberculosis cases in high incidence establishments. Other potential target populations include contacts of HIV-positive persons and pregnant women.
Introduction
Tuberculosis (TB) control remains a challenge1 in part because of the convergence of infection with human immunodeficiency virus (HIV) and TB in countries with endemic TB.2,3 Tuberculosis is currently the leading cause of death among HIV-positive patients worldwide,1 with excess morbidity and mortality among co-infected individuals compared with HIV-negative TB cases and HIV-positive persons without active TB.4–9 Among co-infected persons, early HIV diagnosis is critical not only to ensure timely initiation of highly active antiretroviral therapy (HAART), but also to guide decisions about TB treatment regimens10–14 and to prevent further spread of infection. If one considers that the largest proportion of TB-related mortality occurs within the first two months of treatment, delays in HAART initiation can lead to worsening of illness and death.15
The Stop TB Working Group of the World Health Organization recommends that HIV testing and counseling be offered to all TB patients worldwide.1 However, in many resource-poor settings, a myriad of factors impede the implementation of these guidelines. A recent study based on interviews with community health workers (CHWs) and program managers in the Free State Province of South Africa, where testing of TB patients was low (46%) despite a 73% co-infection rate, identified individual factors and delivery-related factors.16 Individual factors included fear of HIV/acquired immunodeficiency syndrome (AIDS), TB-HIV co-infection, death, stigma, and perceived lack of confidentiality of HIV test results as impeding factors to testing. Delivery-related factors included staff shortages and high workload, and poor infrastructure to encourage testing and address barriers.16 A study in Uganda using qualitative data from patients and providers described the following barriers to universal HIV testing for TB patients: poor TB-HIV planning, coordination and leadership; inadequate provider knowledge, limited TB-HIV inter-clinic referral; high costs of services; and provider shortages amidst high patient loads.17 Aversion to traditional HIV testing caused by invasiveness of blood draws, fears of needles and/or blood, and lack of same-day results also negatively influence a person's decision to be tested.18
To address these challenges, strategies such as opt-out HIV counseling and testing using traditional testing methods by TB nurses in clinics have shown promise. In one study, this strategy was shown to significantly increase testing uptake (20.2% among intervention clinics versus 6.5% among control clinics; P = 0.009) in South Africa.19 However, in this study, most persons remained untested (79.3%) because TB staff failed to offer counseling. A similar approach of opt-out HIV testing in Zambia was somewhat more successful, but still low with HIV testing in 50% of TB patients.20
Rapid testing methods have also been shown to increase the number non-TB patients tested at point-of-care centers (e.g., as part of routine care, during urgent care or emergency department visits, and during labor) in resource-rich settings.21,22 Community-based models have been shown to extend the reach of rapid testing by targeting at-risk (e.g., drug users, homosexual and bisexual populations, and/or commercial sex workers) and hard to access populations.23–25 In a variety of resource-rich community settings, offering these services in mobile units has increased the rates of testing uptake among high-risk populations26,27 and the percentage of people receiving results along with post-test counseling.28 In a study conducted in Spain, the use of free rapid testing in urban areas identified a high (23.5%) HIV prevalence among men from Latin America who had not completed elementary school, a group that may be less likely to receive testing at a health clinic. However, one drawback was the inability to ensure confirmatory testing for 16.4% of persons with positive rapid test results.26 In another study, Seña and others provided home-based rapid oral HIV testing and pre-test counseling to 55.2% of 315 eligible Latino residents who used trained health promoters. However, of the 171 tests conducted, none of the results were positive for HIV; thus, it is unclear how confirmatory testing and referral would have worked.29
Data on community-based models using rapid test methods in resource-poor settings are limited and report mixed outcomes, but show promise. In an exploratory study, Baiden and others asked 403 persons in rural Ghana about HIV stigma and potential acceptance of home-based rapid HIV testing and the role of CHWs as counselors and/or rapid test givers. They reported that home-based voluntary counseling and testing performed by CHWs would be widely accepted (98.7%) and pointed to greater empathy and reach/familiarity offered by CHWs as key strengths.30 Lahuerta and others offered rapid blood-based finger prick HIV tests in Guatemala at sites identified by the community as frequented by transgender people, sex workers, and men who have sex with men (MSM) and compared data with tests given with similar protocols in sexually transmitted infection clinics. Of those tested, MSM tested in the mobile units were significantly less likely to have received a prior HIV test than those tested in the clinic, indicating community outreach testing reaches those more high-risk individuals who are less likely to seek formal clinical services.31 However, only 42% of HIV reactive mobile testers received their confirmatory results, compared with 65% at the health clinic. One challenge for community testing especially in resource-poor settings, as demonstrated by this study, is ensuring that confirmatory test results are discussed with patients, and that those who are confirmed positive connect with formal HIV services. The study of Lahuerta and others31 did not report rate of lost-to-follow-up, but recognized their inability to connect positive patients with the health system as a major shortcoming.
This finding might be a shortcoming for another study in Malawi that used non-laboratory health personnel to perform blood-based rapid testing on 10,819 participants using door-to-door recruitment that referred 815 HIV-positive patients to HIV services, but have no information about whether these patients made or attended a single appointment.32 Angotti and others explored retrieval of testing results in their comparative study in which testing was delivered by trained counselors in rural Malawi. In 2004, oral tests were offered in a tented public area and required participants to return several weeks later for results; in 2006, rapid finger prick tests were offered at the participant's choice of location, including home-based testing. In 2004, 91% of participants agreed to be tested, of whom 70% received their results. In 2006, 92% agreed to be tested and 98% received their results. Whether HIV-positive persons were able to connect with HIV services was not described.33
A third study in an island community on Lake Malawi offered rapid blood-based HIV testing and counseling to 1,030 adults and their spouses to determine whether the home-based provision of HIV testing and counseling had the potential to reduce socioeconomic-based HIV testing inequalities as compared with site-based testing. Facility-based HIV testing was available at no charge on the island. After successfully testing 582 participants in their homes and gathering income data, investigators discovered participants in the lower income quartile were 1.6 times more likely to accept a home-based test than the upper third quartiles. When the team controlled for sexual risk behavior and co-factors for HIV infection, the correlation grew stronger, suggesting that testing location choice may be essential to reaching the poorest fraction of society.25
In many resource-poor settings reaching high-risk populations less likely to attend clinics or hospitals, returning for test results and confirmatory testing, and initiating HAART are all major challenges to improving HIV/TB co-infection outcomes.34 Studies indicate that CHWs may be effective in addressing all these challenges.
Since 1998, Partners In Health has used CHWs to provide TB care to patients in their homes in rural Haiti.35 This community-based model was expanded to Peru in 1994 to provide directly observed therapy (DOT) and follow-up to multidrug-resistant TB patients.36,37 As of 2006, DOT and follow-up has been extended to TB-HIV co-infected patients in Lima.38
This report describes a pilot study in which trained CHWs offered home-based oral rapid HIV testing along with pre-test and post-test counseling to TB patients, followed by accompaniment to ensure confirmatory testing and follow-up HIV services. The aims of this strategy included 1) increasing HIV testing among TB patients by promoting a patient-centered approach in which CHWs provided non-invasive testing and communication of results during the same encounter; 2) task-shifting testing to CHWs to relieve clinic-based health professionals of this function; and 3) providing accompaniment to those diagnosed with HIV to ensure confirmatory testing, facilitating disclosure and assisting with the establishment of HIV care.16 To our knowledge, this is the first study in a resource-poor setting using CHWs to perform community-based rapid HIV testing to TB patients.
Methods
Study setting and routine care.
In Peru, where the DOTS strategy for TB worked to curb the incidence of TB in the 1990s, universal HIV testing of TB-infected patients remains elusive. In 2009, the Joint United Nations Program on HIV/AIDS (UNAIDS) and the World Health Organization estimated approximately 75,000 cases of HIV in Peru, with Lima and Callao containing 73% of the total cases.39 Since the introduction of government-sponsored HAART in 2004, TB/HIV co-infection rates have decreased, and although there are no recent data, co-infection rates are believed to have remained stable since 2006.40
Testing for HIV typically occurs at health centers per Ministry of Health standards. Tuberculosis providers recommend HIV testing to all new patients and inform the participant when and where voluntary counseling and testing is offered (usually in the same health center). At the center, a trained healthcare professional provides counseling and offers free HIV testing. Verbal informed consent for HIV testing is obtained by using a standardized protocol in which a summary of testing procedures, risks, and benefits is read to the patient, any questions or concerns are addressed, and the patient is asked to verbally confirm whether he or she wishes to pursue testing. The participant is allowed to accept or decline testing.
For initial screening for HIV, laboratories at Ministry of Health establishments perform enzyme-linked immunosorbent assay (ELISA) on blood. If testing occurs, the patient is given an appointment to return for results and post-test counseling. For positive results, a nurse helps the patient arrange for confirmatory HIV testing by using immunofluorescence testing at the closest clinic. If confirmed positive, the patient is given a referral to HIV services, and instructed to arrange an appointment at the nearest HIV treatment site. All patients confirmed to be HIV-positive are offered psychological counseling and peer support. Screening of contacts at risk of HIV is also recommended.
Study aims.
The primary objective of this pilot study was to assess the feasibility and acceptability of an HIV testing strategy for new TB patients and their household contacts by using the rapid oral HIV test administered by CHWs.
Study intervention.
During November 2009–April 2010, three CHWs trained in pre-test/post-test HIV counseling by Ministry of Health staff and in OraQuick rapid HIV test procedures by OraSure Technologies, Inc. (Bethlehem, PA) performed community-based oral rapid HIV testing by using the OraQuick Rapid HIV-1/2 Antibody Test. Similar to other rapid tests, OraQuik test provides sensitivity of 98.4–100% and specificity of 98.3–100%, and has proven its utility in detecting all HIV subtypes and recombinants common in developing countries.41 OraQuick was chosen because it delivered results within 20 minutes, enabling CHWs to incorporate some HIV education and pre-test counseling, did not require refrigerated storage, and decreased biohazards related to using finger prick or needles.
Individuals were eligible for the study if they were starting TB treatment, had no known HIV diagnosis, had no prior HIV test during the past six months, and were at least 18 years old. Incarcerated patients and those unable to obtain informed consent/assent because of cognitive, emotional, or neurologic impairment were excluded. In addition, we agreed to enroll household contacts of TB patients who solicited testing. Our target enrollment was determined by the number of HIV tests that could be purchased with available funds, i.e., 100 of which 7 were used for training and quality control.
Persons with TB were consecutively referred to our community-based team by health care providers in 22 health centers (chosen because of their high rates of TB). Health providers introduced patients to our team at the clinic. Patients were invited to provide informed consent to participate in the study, although study consent did not require consent to home visits and/or HIV testing. For those enrolled, CHWs arranged a follow-up meeting in the home or alternative location on the basis of the participant's preference.
During the home visit, CHWs informed participants of the purpose for their visit and that they could terminate the visit at any time. If the participant accepted, CHWs conducted an interview; delivered counseling per Peruvian Ministry of Health standards;42 provided details of the test, including accuracy and follow-up procedures; and offered to perform OraQuick test free of charge. If CHWs received verbal consent to test, they provided pre-test counseling and then administered the test, documenting the temperature and start and end time for the procedure to confirm adherence to testing protocols. During the 20–40 minute delay to achieve a result, CHWs asked participants with whom they would like to share results and gave a short educational session on HIV. After obtaining the result, CHW administered post-counseling and a structured interview on HIV risk behaviors. Documentation of test result was given to patients and their TB provider, upon permission from patient. To tests for possible false results, 6 patients (6.5% of total rapid tests) were chosen randomly to receive confirmatory tests at an HIV clinic using an ELISA. Of these tests, all ELISAs confirmed original rapid test results.
For any positive results, the CHW accompanied participants to obtain confirmatory HIV testing used by the Ministry of Health. The HIV-positive patients were encouraged to disclose their status to a partner, friend, or relative, facilitated by the same CHW. The CHW also ensured that close contacts (e.g. sexual partners, children) were tested for HIV. Ethics committee approval was obtained from Peruvian National Institute of Health and Brigham and Women's Hospital, Boston.
Data collection and analysis.
Data were collected by using standardized forms through chart review and interviews on sociodemographics, TB history, and HIV risk factors (using a modified version of the Risk Assessment Battery).43,44 We also documented rates of home visits, HIV testing and counseling (or reason for declining the test), and test results. For those with positive results, we recorded results and dates of confirmatory testing, CD4 and viral load, first HIV clinic visit, and antiretroviral treatment initiation. We calculated testing uptake (i.e., those who agreed to HIV testing) and protocol adherence (i.e., delivery of pre-test and post-test counseling for all those tested and appropriate adherence to timing and temperature for using the OraQuick test).
Data were entered into an Excel database (Microsoft, Redmond, WA) and analyzed by using SAS software (SAS Institute, Cary, NC). We summarized the distributions of key variables using frequencies and percentages, and means, medians, standard deviations, and skewness.
Results
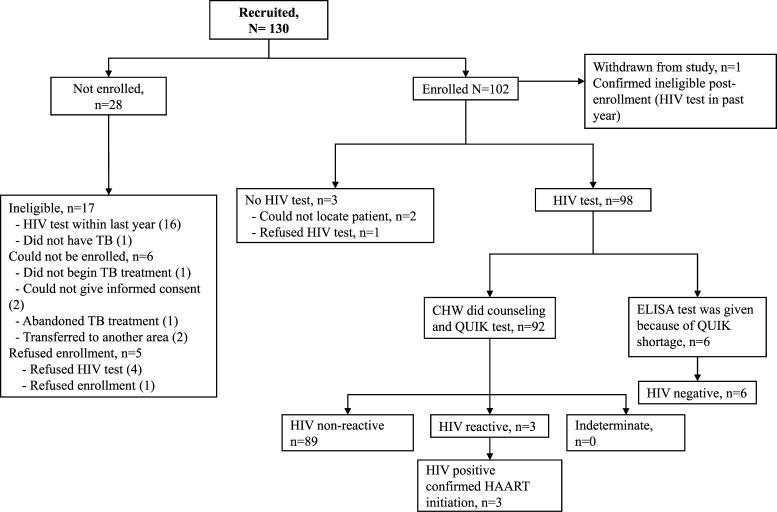
The flow diagram represents study recruitment and enrollment (Figure 1). Of 130 referred persons, 17 were deemed ineligible per study criteria: 16 had received an HIV test within the past year and one was subsequently found not to have TB. Six patients could not be enrolled because of factors beyond study control. Likewise, five refused enrollment (of whom four stated this was because they did not want HIV testing). Of the 102 persons enrolled, one person was found to be ineligible because of prior HIV testing. The remaining 101 persons comprised the cohort for this study. Of these persons, 94 (93.1%) had TB and 7 (6.9%) were household contacts. Median ± SD age was 30.5 ± 11.5 years. Of 94 persons with TB cases, 81 (88.0%) were in their first TB treatment regimen, one had multidrug-resistant TB, and participants were enrolled a median of 28 days into TB treatment (quartile 1 [Q1]–quartile 3 [Q3] = 11–48) (Table 1).
Figure 1.
Study recruitment and enrollment, Lima, Peru.
Table 1.
Baseline characteristics and treatment history of 101 study participants, Lima, Peru*
| Characteristic | Value |
|---|---|
| Referral criteria | |
| TB patient | 94 (93.1) |
| Household contact | 7 (6.9) |
| Female | 41 (40.6) |
| Age, years | 30.5 ± 11.5 |
| Occupation (n = 101) | |
| Unemployed | 54 (53.5) |
| Used/professional | 17 (16.8) |
| Other | 30 (29.7) |
| Education (n = 82) | |
| Did not finish secondary | 26 (31.7) |
| Finished secondary | 56 (68.3) |
| Civil status (n = 97) | |
| Married or living together | 38 (39.2) |
| Single or not living with partner | 59 (60.8) |
| Baseline low BMI (< 18.5 cm/k2) (n = 93) | 11 (11.8) |
| Chronic hepatitis | 0 (0) |
| Drug addiction† | 27 (26.7) |
| Renal insufficiency | 0 (0) |
| Chronic diarrhea | 3 (3.0) |
| Alcohol abuse† | 32 (31.7) |
| Depression | 12 (11.9) |
| Diabetes | 3 (3.0) |
| Seizure disorder | 3 (3.0) |
| Treatment history (n = 92)‡ | |
| None | 81 (88.0) |
| Prior | 11 (12.0) |
| Documented MDR-TB (n = 94)‡ | 1 (1.1) |
| Days since first TB diagnosis (n = 94)‡ | 28 (11–48) |
Values are no. (%), mean ± SD, or median (quartile 1–quartile 3). TB = tuberculosis; BMI = body mass index; MDR = multidrug resistant.
Per patient self-report.
TB cases only.
As shown in Table 2, 56 (60.2%) participants had never previously been HIV tested. Of these participants, 25 (48.1%) said they were never offered a test, 20 (38.5%) did not perceive any risk, and seven (13.5%) gave other reasons (two did not want blood drawn, two could not afford the test, two were not sure they had HIV, and one did not see the test as a priority). Although 58 (57.4%) were minimally worried about possible exposure to HIV, 56 (55.4%) expressed concern about possibly getting HIV in the future. On the basis of self-report, drug addiction (26.7%) and alcohol abuse (31.7%) were common (Table 1), and two (2%) persons were intravenous drug users (IVDU), three (3.0%) persons had a prior sexually transmitted infection, four (4.0%) persons were sex workers, and five (5.1%) persons were bisexual, of whom two were MSM. Sixteen (17.6%) reported sex with more than two persons in the past six months and regular condom use was rare (7.2%) (Table 2).
Table 2.
Prior HIV testing and risk factors for 101 study participants, Lima, Peru*
| Characteristic | No. (%) |
|---|---|
| Previously tested for HIV (n = 93) | |
| Ever | 37 (39.8) |
| Never | 56 (60.2) |
| Reason for no prior HIV testing (n = 52) | |
| Never offered | 25 (48.1) |
| No perceived risk | 20 (38.5) |
| Did not want blood drawn | 2 (3.8) |
| Was not sure it was HIV | 2 (3.8) |
| Did not have means to pay | 2 (3.8) |
| When offered it was not a priority | 1 (1.9) |
| Concern you have been exposed to HIV? | |
| Not worried | 29 (28.7) |
| A little worried | 29 (28.7) |
| Somewhat worried | 11 (10.9) |
| Quite worried | 25 (24.8) |
| Very worried | 6 (5.9) |
| No information | 1 (1.0) |
| Concerned you could get HIV/AIDS in the future? | |
| Not worried | 7 (6.9) |
| A little worried | 23 (22.8) |
| Somewhat worried | 14 (13.9) |
| Quite worried | 30 (29.7) |
| Very worried | 26 (25.7) |
| No information | 1 (1.0) |
| Drank more than 4–5 drinks/day at some point in life | 37 (36.6) |
| Intravenous drug use | 2 (2.0) |
| Sexually transmitted infection | 3 (3.0) |
| Sex worker | 4 (4.0) |
| Blood transfusion | 0 (0) |
| Sexual preference (n = 99) | |
| Heterosexual | 94 (94.9) |
| Homosexual | 0 (0) |
| Bisexual (2 MSM) | 5 (5.1) |
| Sex partners (past 6 months) (n = 91) | |
| 0 | 20 (22.0) |
| 1 | 55 (60.4) |
| > 2 | 16 (17.6) |
| Sex for drugs (n = 98) | |
| Once per month or less | 2 (2.0) |
| Once per week | 1 (1.0) |
| Never | 95 (97.0) |
| Sex while drinking (n = 99) | |
| Once a month or less | 16 (16.2) |
| Several times a month | 6 (6.1) |
| Once a week | 4 (4.0) |
| Never | 72 (72.7) |
| Refuse response | 1 (1) |
| Sex with known HIV-positive person (n = 99) | 0 (0) |
| Contraceptives (current), (n = 98) | 16 (16.3) |
| Condom use (n = 97) | |
| All the time | 7 (7.2) |
| Not all the time | 15 (15.5) |
| Never | 75 (77.3) |
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; MSM = men who have sex with men.
Acceptance of rapid HIV tests among TB patients.
Acceptance of the test was high among participants: 98 (97.0%) accepted HIV testing (Table 3). If we considered all eligible persons (including the 12 persons who did not enroll in the study), then testing uptake occurred in 98 (87.5%) of 112 persons. Uptake was greater than expected. We had originally estimated that we would need to enroll 200 patients to complete our goal of 90 tests, but we achieved the goal with only 94 enrollees. Because test uptake was greater than expected, we used conventional methods for six people after the rapid oral test was used up.
Table 3.
Operational characteristics of intervention for 101 study participants, Lima, Peru*
| Characteristic | Value |
|---|---|
| Total no. encounters per participant | 1.95 ± 1.62 |
| Site of initial encounter | |
| Health establishment | 78 (77.2) |
| Home | 18 (17.8) |
| Park | 2 (2.0) |
| Other | 3 (3.0) |
| Education on HIV and TB provided | 100 (99.0) |
| Offered HIV testing | 101 (100) |
| Initially declined OraQuik testing | 30 (29.7) |
| Preferred alternative day | 14 (46.7) |
| Preferred alternative testing site | 15 (50.0) |
| At home | 9 (60.0) |
| At health establishment | 5 (33.3) |
| Other site | 1 (6.7) |
| Did not approve of rapid test method | 0 (0) |
| Did not want testing at all | 1 (3.3) |
| Accepted and underwent HIV testing | 98 (97.0) |
| Patients given QUIK (n = 92) | |
| OraQuik counseling pre-test | 92 (100) |
| OraQuik counseling post-test | 92 (100) |
| CHW correctly recorded time and duration of test (n = 92) | 92 (100) |
| Site of OraQuik test (n = 92) | |
| Health establishment | 54 (58.7) |
| Home | 31 (33.7) |
| Park | 3 (3.3) |
| Other | 4 (4.3) |
| No. household contacts present during OraQuik test (n = 91) | |
| 0 | 60 (65.9) |
| 1 | 23 (25.3) |
| ≥ 2 | 8 (8.8) |
| Results communicated to personal contact (n = 49) | |
| Partner | 15 (30.7) |
| Sibling | 10 (20.4) |
| Mother | 11 (22.5) |
| Child | 3 (6.1) |
| Relative or other | 5 (10.2) |
| Friend | 1 (2.0) |
| Rehabilitation center personnel | 3 (6.1) |
| Religious leader | 1 (2.0) |
| Results communicated to health provider (n = 96) | 96 (100) |
Values are mean ± SD or no. (%). HIV = human immunodeficiency virus; TB = tuberculosis; CHW = community health worker.
Feasibility of rapid HIV testing under study conditions.
The CHWs had a mean ± SD of 1.95 ± 1.62 encounters per patient. Thirty persons (29.7%) deferred testing during the first encounter, usually because of a preference for a different site (15) or different day (14). Of these persons, 28 (93.3%) were successfully tested during a follow-up visit. In terms of preference for testing site, 54 (58.7%) chose to receive their test in their health establishment, 31 (33.7%) opted for testing at home, 3 (3.3%) were tested in a park, and 4 (4.4%) were tested in another location (i.e., place of work or public place).
All persons gave permission to the CHW to provide a copy of the test result to the TB Program for their patient chart. All results were communicated to the health provider of total participants, including 100% among HIV-positive patients.
Adherence to the testing protocol by CHWs was high. The CHWs delivered pre-test and post-test counseling to all those who received OraQuick testing. Duration and temperature for Oraquick testing was appropriately documented in all cases, resulting in 100% adherence to the OraQuick protocol (Table 3). The CHWs accompanied persons who had positive results to ensure optimal care.
Three (3%) of the tested persons were given a diagnosis of seropositivity by using the OraQuick test. All three participants were followed-up through HAART initiation. On average, for these three patients, CHWs facilitated confirmatory testing within 5 days (Q1–Q3 = 1–7 days), disclosure of their diagnosis to at least one family member in all cases, referral to HIV services in 19 days (Q1–Q3 = 7–35 days), and initiation of HAART in 57 days (Q1–Q3 = 46–57 days).
Discussion
Our study suggests that a strategy in which CHWs administer a non-invasive rapid HIV test at patient's preferred location can achieve near-universal screening among high-risk populations, exceeding HIV screening through conventional strategies of voluntary counseling and testing at health establishments.21–23,31 We identified HIV in 3% of our study cohort, compared with approximately 0.4% (Q1–Q3 = 0.3–0.5%) among adults ≥ 15 years of age nationwide.45 This relatively high rate suggests that TB patients might represent an important HIV risk group in this region, and an easily targetable population for intensive HIV screening campaigns.
Our patient-centered approach and use of a non-invasive rapid test may have contributed to high uptake of HIV testing. Many patients who initially declined testing subsequently accepted testing at a later time and different place. These data underline the importance of accommodating patient preferences, including testing in the place designated by the participant, administration by a CHW (who was perceived as a trustworthy community member), and use of a free non-invasive test. Interestingly, more than half of tests were conducted in health establishments. An alternative strategy could be for healthcare personnel to offer rapid oral testing in the health care facility, involving CHWs only in cases in which the patient declined center-based testing, could not be located, or needed accompaniment through HAART initiation.
In addition, our pilot study demonstrated that community-based accompaniment if conducted correctly ensures that any persons who have positive test results for HIV are not lost to follow-up before receiving HIV care. One key to the study's success in this regard is that the field team prioritized patient follow-up. This follow-up was especially challenging when patients manifested interest in testing but were not home or were not in conditions where a confidential test could be given. In one case, a patient who had a positive rapid test result told the study team that the patient had received the confirmatory result, but in a follow-up home visit confessed that the patient had been to the clinic but became afraid and left without the results. Patient accompaniment provided an added benefit throughout the participant's life in the study.
Barriers to returning for test results, obtaining confirmatory testing, establishing HIV care, and initiating HAART include distance to health facilities, poverty (costs associated for travel to appointments or paying required for follow-up and treatment), low health literacy (lack of information about how HIV is transmitted, extended HIV survival rates caused by improvements in care), fear/stigma, and inadequate health infrastructure. In our study, CHWs accompanied all test-positive patients to laboratories for procedures and medical appointments to ensure successful HAART initiation. Furthermore, CHWs facilitated disclosure to a family member and provided critical emotional support to the patient and family member. For all three HIV cases, the disclosure occurred smoothly without any sign of rejection or conflict on the part of the family member.
Three serious concerns about CHWs administering rapid HIV tests that we considered before our pilot study were stigma, confidentiality, and technical ability to correctly administer the test.30 All CHWs received prior training on confidentiality and HIV-related stigma, and only approached participants in areas and at times that were deemed acceptable. We received no complaints of CHW conduct. In addition, clear training and procedure protocols, including documentation of testing procedures and quality control procedures, enabled us to ensure that CHWs provided quality counseling and rapid HIV testing in the field. A perfect level of adherence based on time and temperature documentation was also achieved.
One potential drawback is that we only offered testing to TB contacts who spontaneously solicited the test; in that sense, we were unable to come to conclusive results about contact testing. No contacts that we tested were found to be infected with HIV. Because access to household contacts, including family members and partners, has surfaced as one of the strengths of home-based HIV testing,46 future studies should focus contact testing on high-risk household contacts and include in-depth qualitative data to understand HIV knowledge and factors influencing testing acceptance. Furthermore, as shown by our population's self-reported low use of condoms (77.3% reported never using condoms during sexual intercourse), future studies and interventions should also investigate cultural and socioeconomic factors influencing sexual practices and sexual risk behavior. Where feasible, a secondary intervention of providing condoms and educating individuals on appropriate condom use should be offered.
Our results suggest that outreach HIV testing by CHWs to TB patients by using a non-invasive rapid test in the community can overcome many barriers to universal HIV testing. We propose future programmatic application of community-based OraQuick testing to achieve universal HIV testing of TB cases in high TB-incidence establishments. We believe that this model can be replicated in other resource-poor settings through identification of high TB-incidence establishments and potential expansion to other hard-to-reach populations, including contacts of HIV-positive persons, pregnant women, and children.
ACKNOWLEDGMENTS
We thank Orasure Technologies, Inc. for facilitating purchase and delivery of OraQuick tests to Lima, and for training community health workers on its administration.
Footnotes
Authors' addresses: Adrianne K. Nelson and Adolfo Caldas, Division of Global Health Equity, Brigham and Women's Hospital, Boston, MA, E-mails: aknelson@partners.org and adolfo.caldas@childrens.harvard.edu. Jose Luis Sebastian, Christina Magan, and Gustavo Rosell, Ministry of Health, Peru, National HIV Program, Lima, Peru, E-mails: jlsebastianm@gmail.com, cmagan@minsa.gob.pe, and grosell@minsa.gob.pe. Mirabel Muñoz, Jose Yamanija, Judith Saldivar, Betty Espiritu, and Jaime Bayona, Socios en Salud, HIV Program, Lima, Peru, E-mails: mmunoz_ses@pih.org, jyamanija_ses@pih.org, jsaldivar_ses@pih.org, bespiritu_ses@pih.org, and jbayona_SES@pih.org. Cesar Bonilla, Ministry of Health, National Strategy for TB Control, Lima, Peru, E-mail: cesarbon@yahoo.es. Oswaldo Jave, Dos de Mayo National Hospital, National TB Program, Lima, Peru, E-mail: osjave@amauta.rcp.net.pe. Sonya Shin, Division of Social Medicine, Brigham and Women's Hospital, Boston, MA, E-mail: sshin@partners.org.
References
- 1.TB/HIV Working Group of the Global Partnership to Stop TB, WHO . Guidelines for HIV Surveillance among Tuberculosis Patients. Second edition. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Obermeyer Z, Abbott-Klafter J, Murray CJ. Has the DOT strategy improved case finding or treatment success? An empirical assessment. PLoS ONE. 2008;3:e1721. doi: 10.1371/journal.pone.0001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet. 2002;359:775–780. doi: 10.1016/s0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Garcia Mde L, Ponce-De-Leon A, Garcia-Sancho MC, Ferreyra-Reyes L, Palacios-Martinez M, Fuentes J, Kato-Maeda M, Bobadilla M, Small P, Sifuentes-Osornio J. Tuberculosis-related deaths within a well-functioning DOTS control program. Emerg Infect Dis. 2002;8:1327–1333. doi: 10.3201/eid0811.020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, Mugerwa RD, Ellner JJ. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5:225–232. [PubMed] [Google Scholar]
- 7.Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 8.Nunn P, Brindle R, Carpenter L, Odhiambo J, Wasunna K, Newnham R, Githui W, Gathua S, Omwega M, McAdam K. Cohort study of human immunodeficiency virus infection in patients with tuberculosis in Nairobi, Kenya. Analysis of early (6-month) mortality. Am Rev Respir Dis. 1992;146:849–854. doi: 10.1164/ajrccm/146.4.849. [DOI] [PubMed] [Google Scholar]
- 9.Kawai V, Soto G, Gilman RH, Bautista CT, Caviedes L, Huaroto L, Ticona E, Ortiz J, Tovar M, Chavez V, Rodriguez R, Escombe AR, Evans CA. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg. 2006;75:1027–1033. [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Munsiff SS, Driver CR, Sackoff J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin Infect Dis. 2005;41:83–91. doi: 10.1086/430377. [DOI] [PubMed] [Google Scholar]
- 11.Burman W, Benator D, Vernon A, Khan A, Jones B, Silva C, Lahart C, Weis S, King B, Mangura B, Weiner M, El-Sadr W. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173:350–356. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 12.Center for Disease Control. Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin-based regimens. MMWR Morb Mortal Wkly Rep. 2002;51:214–215. [PubMed] [Google Scholar]
- 13.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, Hosseinipour MC, Lalloo U, Veloso VG, Some FF, Kumarasamy N, Padayatchi N, Santos B, Reid S, Hakim J, Mohapi L, Mugyenyi P, Sanchez J, Lama J, Pape J, Sanchez A, Asmelash A, Moko E, Sawe F, Andersen J, Sanne I. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, Kim C, Lak KK, Hak C, Dim B, Sin CI, Sun S, Guillard B, Sar B, Vong S, Fernandez M, Fox L, Delfraissy JF, Goldfeld AE. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heunis JC, Wouters E, Norton W, Engelbrecht M, Kigozi N, Sharma A, Ragin C. Patient- and delivery-level factors related to acceptance of HIV counseling and testing services among tuberculosis patients in South Africa: a qualitative study with community health workers and program managers. Implement Sci. 2011;6:27. doi: 10.1186/1748-5908-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okot-Chono R, Mugisha F, Adatu F, Madraa E, Dlodlo R, Fujiwara P. Health system barriers affecting the implementation of collaborative TB-HIV services in Uganda. Int J Tuberc Lung Dis. 2009;13:955–961. [PubMed] [Google Scholar]
- 18.Holm-Hansen C, Nyombi B, Nyindo M. Saliva-based HIV testing among secondary school students in Tanzania using the OraQuick rapid HIV1/2 antibody assay. Ann N Y Acad Sci. 2007;1098:461–466. doi: 10.1196/annals.1384.036. [DOI] [PubMed] [Google Scholar]
- 19.Pope DS, Deluca AN, Kali P, Hausler H, Sheard C, Hoosain E, Chaudhary MA, Celentano DD, Chaisson RE. A cluster-randomized trial of provider-initiated (opt-out) HIV counseling and testing of tuberculosis patients in South Africa. J Acquir Immune Defic Syndr. 2008;48:190–195. doi: 10.1097/QAI.0b013e3181775926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Provider-initiated HIV testing and counseling of TB patients–Livingstone District, Zambia, September 2004–December 2006. MMWR Morb Mortal Wkly Rep. 2008;57:285–289. [PubMed] [Google Scholar]
- 21.Kassler WJ, Alwano-Edyegu MG, Marum E, Biryahwaho B, Kataaha P, Dillon B. Rapid HIV testing with same-day results: a field trial in Uganda. Int J STD AIDS. 1998;9:134–138. doi: 10.1258/0956462981921882. [DOI] [PubMed] [Google Scholar]
- 22.Myers JJ, Modica C, Dufour MS, Bernstein C, McNamara K. Routine rapid HIV screening in six community health centers serving populations at risk. J Gen Intern Med. 2009;24:1269–1274. doi: 10.1007/s11606-009-1070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diserens EA, Bodenmann P, N'Garambe C, Ansermet-Pagot A, Vannotti M, Masserey E, Cavassini M. Clients of sex workers in Switzerland: it makes sense to counsel and propose rapid test for HIV on the street, a preliminary report. BMC Infect Dis. 2010;10:74. doi: 10.1186/1471-2334-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts KJ, Grusky O, Swanson AN. Outcomes of blood and oral fluid rapid HIV testing: a literature review, 2000–2006. AIDS Patient Care STDS. 2007;21:621–637. doi: 10.1089/apc.2006.0196. [DOI] [PubMed] [Google Scholar]
- 25.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Fuente L, Delgado J, Hoyos J, Belza MJ, Alvarez J, Gutierrez J, Neira-Leon M, Suarez M. Increasing early diagnosis of HIV through rapid testing in a street outreach program in Spain. AIDS Patient Care STDS. 2009;23:e9. doi: 10.1089/apc.2009.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellen JM, Bonu S, Arruda JS, Ward MA, Vogel R. Comparison of clients of a mobile health van and a traditional STD clinic. J Acquir Immune Defic Syndr. 2003;32:388–393. doi: 10.1097/00126334-200304010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Guenter D, Greer J, Barbara A, Robinson G, Roberts J, Browne G. Rapid point-of-care HIV testing in community-based anonymous testing program: a valuable alternative to conventional testing. AIDS Pateint Care STDS. 2008;22:195–204. doi: 10.1089/apc.2007.0137. [DOI] [PubMed] [Google Scholar]
- 29.Seña AC, Hammer JP, Wilson K, Zeveloff A, Gamble J. Feasibility and acceptability of door-to-door rapid HIV testing among Latino immigrants and their HIV risk factors in North Carolina. AIDS Patient Care STDS. 2010;24:165–173. doi: 10.1089/apc.2009.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baiden F, Akanlu G, Hodgson A, Akweongo P, Debpuur C, Binka F. Using lay counsellors to promote community-based voluntary counselling and HIV testing in rural northern Ghana: a baseline survey on community acceptance and stigma. J Biosoc Sci. 2007;39:721–733. doi: 10.1017/S0021932006001829. [DOI] [PubMed] [Google Scholar]
- 31.Lahuerta M, Sabido M, Giardina F, Hernandez G, Palacios JF, Ortiz R, Fernandez VH, Casabona J. Comparison of users of an HIV/syphilis screening community-based mobile van and traditional voluntary counselling and testing sites in Guatemala. Sex Transm Infect. 2011;87:136–140. doi: 10.1136/sti.2010.043067. [DOI] [PubMed] [Google Scholar]
- 32.Molesworth AM, Ndhlovu R, Banda E, Saul J, Ngwira B, Glynn JR, Crampin AC, French N. High accuracy of home-based community rapid HIV testing in rural Malawi. J Acquir Immune Defic Syndr. 2010;55:625–630. doi: 10.1097/QAI.0b013e3181f98628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angotti N, Bula A, Gaydosh L, Kimchi EZ, Thornton RL, Yeatman SE. Increasing the acceptability of HIV counseling and testing with three C's: convenience, confidentiality and credibility. Soc Sci Med. 2009;68:2263–2270. doi: 10.1016/j.socscimed.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009;6:217–223. doi: 10.1007/s11904-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farmer P, Robin S, Ramilus SL, Kim JY. Tuberculosis, poverty, and “compliance”: lessons from rural Haiti. Semin Respir Infect. 1991;6:254–260. [PubMed] [Google Scholar]
- 36.Mitnick C, Bayona J, Palacios E, Shin S, Furin J, Alcantara F, Sanchez E, Sarria M, Becerra M, Fawzi MC, Kapiga S, Neuberg D, Maguire JH, Kim JY, Farmer P. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 37.Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59:1529–1539. doi: 10.1016/j.socscimed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H. Antiretroviral therapy in resource-poor settings: decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43((Suppl 1)):S123–S126. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- 39.UNAIDS . Lucha Contra VIH/SIDA. Lima, Peru: UNAIDS; 2011. [Google Scholar]
- 40.Alliance IHA. Peru: 24/24 Country. Special on Via Libre. Lima, Peru: Alliance IHA, ed. Special on Via Libre; 2011. [Google Scholar]
- 41.Holguín A, Gutiérrez M, Portocarrero N, Rivas P, Baquero M. Performance of OraQuick Advance® Rapid HIV-1/2 Antibody Test for detection of antibodies in oral fluid and serum/plasma in HIV-1+ subjects carrying different HIV-1 subtypes and recombinant variants. J Clin Virol. 2009;45:150–152. doi: 10.1016/j.jcv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Ministry of Health . Documento Técnico: Consejería en ITS/VIH y SIDA. Lima, Peru: Gobierno de Peru; 2008. [Google Scholar]
- 43.Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, Woody GE. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res Hum Retroviruses. 1994;10((Suppl 2)):S281–S283. [PubMed] [Google Scholar]
- 44.Samet JH, Krupitsky EM, Cheng DM, Raj A, Egorova VY, Levenson S, Meli S, Bridden C, Verbitskaya EV, Kamb ML, Zvartau EE. Mitigating risky sexual behaviors among Russian narcology hospital patients: the PREVENT (Partnership to Reduce the Epidemic via Engagement in Narcology Treatment) randomized controlled trial. Addiction. 2008;103:1474–1483. doi: 10.1111/j.1360-0443.2008.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UNAIDS Peru . HIV and AIDS Estimates. Lima, Peru: UNAIDS; 2009. [Google Scholar]
- 46.Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, Gupta S, Grosskurth H, Jaffar S, Coutinho A, Bunnell R. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr. 2010;55:245–252. doi: 10.1097/QAI.0b013e3181e9e069. [DOI] [PubMed] [Google Scholar]