Abstract
Anemia affects one-quarter of the world's population, but its etiology remains poorly understood. We determined the prevalence of anemia and studied underlying risk factors in infants (6–23 months), young school-aged children (6–8 years), and young non-pregnant women (15–25 years) in south-central Côte d'Ivoire. Blood, stool, and urine samples were subjected to standardized, quality-controlled methods. We found high prevalence of anemia, malaria, inflammation, and deficiencies of iron, riboflavin, and vitamin A but low prevalence and intensities of soil-transmitted helminth and schistosome infections. Multivariate regression analysis revealed significant associations between anemia and Plasmodium falciparum for infants, inflammation for school-aged children, and cellular iron deficiency for both school-aged children and non-pregnant women. Women with riboflavin deficiency had significantly lower odds of anemia. Our findings call for interventions to protect infants from malaria, improved intake of dietary iron, better access to health care, and health education.
Introduction
It is currently estimated that anemia affects one-quarter of the world's population. Most of this burden occurs in developing countries, particularly among pre-school–aged children and women of reproductive age.1 Iron deficiency is recognized as the primary cause of anemia worldwide, but the etiology of anemia is multifactorial, including nutritional habits, bioavailability of micronutrients, parasitic infections (e.g., malaria and helminth infections), inflammation, and genetic factors.2 In addition to tiredness and impaired cognitive performance, the consequences of anemia include reduced educational achievement and work capacity, increased mortality and morbidity from infectious diseases, and poor pregnancy outcomes.3 In Côte d'Ivoire, previous studies estimated that more than 40% of the population is affected by anemia.4–6
The prevention and control of anemia is complex and depending on the setting, might require the implementation of a set of control measures. Iron fortification, long-lasting insecticidal nets (LLINs), intermittent preventive treatment (IPT) of malaria, and regular administration of anthelmintic drugs can be effective strategies to decrease the prevalence of anemia in developing countries.2,4,7 In Côte d'Ivoire, after one decade of sociopolitical unrest, armed conflict, and war,8 new efforts are getting underway to improve people's health and wellbeing. It is important to characterize the baseline situation of anemia in the most vulnerable population groups to serve as a benchmark for monitoring progress now that new control initiatives are being implemented.
Here, we report the results from a baseline cross-sectional survey carried out in the recently established Taabo health demographic surveillance system (Taabo HDSS) in south-central Côte d'Ivoire.9 The goal was to determine the prevalence of anemia and study the main risk factors for three population groups (i.e., infants, children at early school age, and young non-pregnant women). These findings have been instrumental for designing control interventions (e.g., community-based anthelmintic treatment and distribution of LLINs), and study participants will be followed longitudinally.
Materials And Methods
Ethical considerations.
The study protocol was approved by the institutional research commissions of the Swiss Tropical and Public Health Institute (Swiss TPH; reference no. FK 96) and ETH Zurich (reference no. EK 2009-N-19). Ethical approval was granted by the ethics committee of Basel (EKBB; reference no. 252/09) and Côte d'Ivoire (reference no. 1086 MSHP/CNER). Study investigators were covered by liability insurance (GNA Assurance, Abidjan, Côte d'Ivoire, policy no. 30105811010001). Village chiefs, participants, and parents/guardians of children were informed about the purpose and procedures of the study. Written informed consent (or fingerprints of illiterate people) was obtained from study participants and the parents/guardians of children. Participants accepted to take part in a longitudinal monitoring (five sampling time points; one time every 3–4 months), but they could withdraw from the study at any time without further obligations. Suspected clinical malaria (i.e., positive rapid diagnostic test [RDT] and tympanic temperature > 38°C), severe anemia (i.e., hemoglobin [Hb] < 8 g/dL according to the national cut-off defining severe anemia), and helminth infections were treated according to national guidelines.
Study setting.
The study area lies in the transition zone from rainforest to savannah in the V-Baoulé of south-central Côte d'Ivoire (Figure 1). There are two rainy seasons: a long one lasting from April to July and a shorter one in September and October. Villagers are mainly engaged in subsistence farming of cassava, plantains, and yams. Coffee and cacao are the predominant cash crops. Some men make a living from fishing in Lake Taabo.
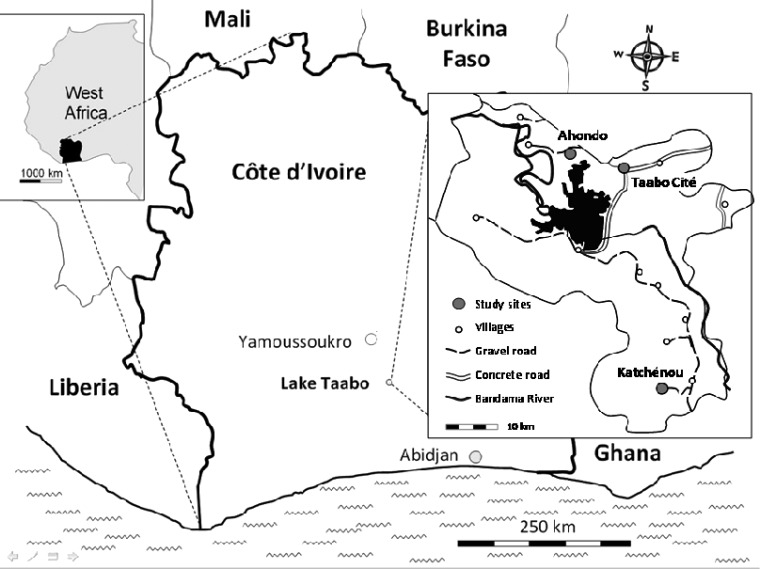
Figure 1.
Study sites. The study was embedded in the recently established Taabo HDSS located in south-central Côte d'Ivoire. Taabo HDSS covers the area around Lake Taabo. This survey was conducted in three settings: Taabo Cité, the only small town; Ahondo, 1 of 13 main villages in close proximity to Lake Taabo; and Katchénou, considered as 1 of over 100 small hamlets at the time of the survey and situated 55 km south of Taabo Cité. Modified from the work by Glinz and others.52
This study was carried out in the Taabo HDSS. The site covers the area around the manmade lake of Taabo impounded in the late 1970s.10 We purposely selected individuals from three settings that are representative of the main socioecological contexts in the Taabo HDSS: Taabo Cité (the only small town), Ahondo (1 of 13 main villages located in close proximity to Lake Taabo), and Katchénou (initially considered 1 of over 100 small hamlets and later, designated as a village). According to the April 2010 census, there were 6,813 people living in Taabo Cité. This small town is located approximately 170 km north-west of Abidjan. There is one small hospital with 12 beds. The populations in Ahondo and Katchénou are 2,230 and 693, respectively. Ahondo has a health dispensary and is situated 14 km west of Taabo Cité. Katchénou is located 55 km south of Taabo Cité, with no health facility at the time of the current study.
Sample size calculation.
The intended sample size at enrollment was 137 individuals in each of the three age groups, which would allow an accurate estimation of the prevalence of anemia in the selected groups with a 9% error margin.11 Confidence level was set to 95%, and the estimated values for the proportion of anemia in our population were 60% for infants and school-aged children and 40% for non-pregnant women.4–6 The sample size was calculated considering a drop-out rate of 20%.
Study design and participants.
We report the results from the baseline cross-sectional survey of a 14-month monitoring activity carried out in April of 2010 in the three study settings. The three age groups included were (1) infants aged 6–23 months, (2) children at early school age (6–8 years), and (3) young non-pregnant women aged 15–25 years. In Ahondo and Katchénou, all people within these age ranges were invited to participate. In Taabo Cité, to get a similar sample size, 120 infants, 90 school-aged children, and 90 young women were randomly selected from the existing database available at the Taabo HDSS.
Field and laboratory procedures.
One week before our cross-sectional survey, field enumerators went to all households selected for the study and explained the purpose, procedures, and potential risks and benefits of the planned work. On the evening before the first sampling day, each participant was given two plastic containers and invited to return filled containers with a fresh morning stool and a urine sample. On sampling day, the purpose of the study and the longer-term monitoring were again explained and questions by the people were answered. Those individuals who were interested in participating were asked to sign a written informed consent or give a fingerprint (illiterates), followed by stool and urine collection. Unique identification numbers were assigned to participating individuals, and these numbers were used in subsequent surveys.
Participants' height (to the nearest centimeter) and weight (to the nearest 0.5 kg) were recorded. Temperature was measured by a digital, battery-powered ear thermometer (to the nearest 0.1°C). Subsequently, finger-prick blood was collected from each participant. The presence of Plasmodium falciparum was determined using an RDT (ICT ML01 malaria Pf kit; ICT Diagnostics, Cape Town, South Africa). The determination of Plasmodium species was done with a thin blood film, and parasitemia assessed with a thick blood film. Hb quantification was done with a portable HemoCue Hb 301 device (HemoCue AB, Ängelholm, Sweden). Finally, a minimum of 5 mL venous blood was collected in heparin-coated tubes that were immediately put in a cool box containing ice.
Blood, stool, and urine samples were transferred to the Taabo hospital laboratory. Blood samples were centrifuged, aliquoted, and kept at –20°C before transfer to Abidjan and finally, to Switzerland. Duplicate Kato–Katz thick smears were prepared from each stool sample.12 Slides were allowed to clear for 30–45 minutes before examination under a microscope for the presence of Schistosoma mansoni and soil-transmitted helminth (Ascaris lumbricoides, hookworm, and Trichuris trichiura) eggs by laboratory technicians. The number of eggs was recorded for each helminth species separately. Urine samples were subjected to a filtration method.13 In brief, urine specimens were shaken, and 10 mL were gently pressed through a small-meshed filter. One drop of Lugol solution was added on the filter before the slides were quantitatively examined for S. haematobium eggs under a microscope by laboratory technicians. For quality control, 10% of the slides were reexamined by a senior technician; in case of discrepancies, the results were discussed with the technicians, and the corresponding slides were reexamined until agreement was found.
Venous blood examination.
Whole blood was screened for hemoglobinopathies within 1 week of blood sampling by using electrophoresis on cellulose acetate membranes.14 Riboflavin was measured by the erythrocyte glutathione reductase activity coefficient (EGRAC) assay using a modification of the method in the work by Dror and others.15 Previous validation studies done in our laboratories revealed inter- and intraassay coefficients of variation (CVs) of the EGRAC method of 3% and 4%, respectively. Although EGRAC cut-off values for defining riboflavin deficiency are still being debated, we used cut-off values > 1.4 to indicate clear deficiency.16 Ferritin, soluble transferrin receptor (sTfR), retinol binding protein (RBP), α1-acid glycoprotein (AGP), and C-reactive protein (CRP) were measured with a sandwich enzyme-linked immunosorbant assay (ELISA).17 Serum retinol (SR) was measured by high-pressure liquid chromatography (HPLC; Merck-Hitachi, Tokyo, Japan) according to the work by Tanumihardjo and others18 with reference material from the National Institute of Standards and Technology (Gaithersburg, MD).
Statistical analysis.
Parasitologic data were entered two times in Microsoft Access version 10.0 (2007 Microsoft Corporation). Serological data were entered in Microsoft Excel version 10.0 (2007 Microsoft Corporation). Double-entered datasets were compared using EpiInfo version 3.4.1 (Centers for Disease Control and Prevention, Atlanta, GA), and discrepancies were removed according to original records. Data were analyzed using STATA version 10 (StataCorp., College Station, TX). For multivariate logistic regression, only those individuals with complete data records were considered.
For each individual, the arithmetic mean of the helminth species-specific egg counts from the Kato–Katz thick smears was calculated and multiplied by a factor of 24 to obtain a standardized measure of infection intensity (i.e., eggs per gram of stool [EPG]).
Hb thresholds used to define anemia were 11.0 g/dL for infants aged 6–23 months, 11.5 g/dL for children aged 6–8 years, and 12.0 g/dL for non-pregnant women aged ≥ 15 years according to World Health Organization (WHO) guidelines.3Anemia was classified into moderate and severe anemia using Hb cut-offs of < 9.0 g/dL and < 7.0 g/dL, respectively. Storage iron depletion was defined as ferritin < 12 μg/L for infants without inflammation and < 15 μg/L for children and women without inflammation. For participants with AGP > 1 g/L or CRP > 10 mg, storage iron depletion was defined as ferritin < 30 μg/L.3 Cellular iron deficiency was defined as sTfR > 8.5 mg/L.19 Acute and chronic inflammations were, respectively, defined as CRP > 10 mg/L and AGP > 1 g/L. SR < 0.7 μmol/L indicated vitamin A deficiency (VAD).20 Because only 62 infants provided enough venous blood to quantify retinol through HPLC, RBP < 0.825 μmol/L was used as surrogate for VAD in infants.21 Because inflammation influences the concentration of many nutritional biomarkers,22,23 the estimated prevalence of micronutrient deficiencies was based exclusively on values from participants with CRP values ≤ 10 mg/L, excluding the 55 participants with acute inflammation.
Multivariate logistic regression was used for determining variables significantly associated with anemia using available WHO criteria. Odds ratios (ORs) were calculated, including 95% confidence intervals (CIs) and Wald test P values. Candidate explanatory variables for the multivariable logistic regression model were age, sex (for infants and school-aged children), school attendance (for children), type of activity (for women), soil-transmitted helminth, Schistosoma, and Plasmodium infection, storage iron depletion, cellular iron deficiency, riboflavin deficiency, VAD (based on retinol values for women and school-aged children and on RBP values for infants), acute inflammation, and chronic inflammation. A backward stepwise multivariate logistic regression with locality as random effect was computed for each age group, removing non-predicting covariates up to a significance level of 0.2. The remaining covariates were included into the final models, and the variables with a significant P value are reported.
Results
Study cohort and compliance.
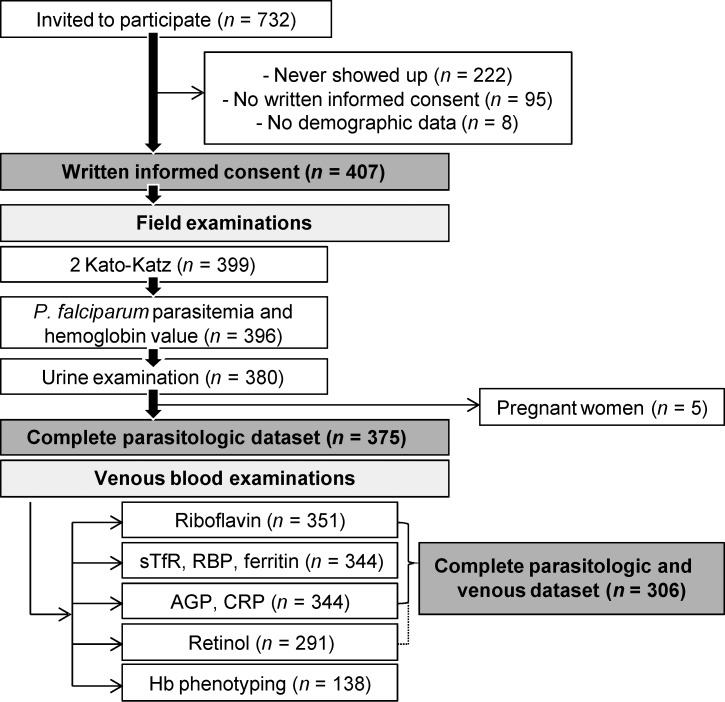
Overall, 732 individuals in Taabo Cité, Ahondo, and Katchénou were eligible and hence, invited to participate in the baseline cross-sectional survey (Figure 2). Written informed consent and stool and urine samples were provided by 407 individuals who were included in subsequent analyses. Complete parasitologic data were obtained for 375 individuals.
Figure 2.
Study participation and compliance. Diagram detailing the study participation of infants (6–23 months), school-aged children (6–8 years), and non-pregnant women (15–25 years) from Taabo Cité, Ahondo, and Katchénou in April of 2010. Individuals who provided at least one urine and/or one stool sample were considered for additional analyses. Blood samples from individuals with a complete parasitologic dataset were analyzed for nutrition parameters and inflammatory markers. Pregnant women were not considered for the final analyses. AGP = α1-acid glycoprotein; CRP = C-reactive protein; Hb = hemoglobin; RBP = retinol binding protein; sTfR = soluble transferrin receptor.
Venous blood samples obtained from individuals with complete parasitologic data were subjected to detailed laboratory work-up. However, because of insufficient quantities of blood, EGRAC could only be determined in 351 blood samples. ELISA and HPLC analyses were carried out using plasma samples from 344 and 291 individuals, respectively. A total of five women gave birth to a child within the following 9 months and hence, were excluded in additional analyses. Finally, 306 participants had complete data pertaining to parasitic infection, nutrition, and inflammation status. We intended to take one-third of the blood samples collected in each locality to assess the prevalence of hemoglobinopathies in our population. Hence, a subsample of 138 blood samples was used for Hb phenotyping.
Attrition analysis.
Given the low number of people with complete datasets (n = 306; 41.8%), an attrition analysis was performed for each age group to compare the characteristics of people who dropped out of the study with those people who had complete parasitologic and venous blood data. No significant difference was found for sex (infants and school-aged children) and median age (for school-aged children and women). Infants with complete data records were significantly older than those infants who dropped out (Wilcoxon rank sum test P = 0.008).
Population characteristics.
Population characteristics of participants with complete parasitologic data (n = 375), stratified by study setting, are presented in Table 1. More than 70% of the 6- to 8-year-old children were attending school in Taabo Cité, whereas one of two children did not attend school in Ahondo and Katchénou. Women in the three localities showed marked differences regarding educational attainment, occupation, number of children, and matrimonial status. Women were predominantly working outside the home, and less than 10% had more than three children, which was to be expected based on our inclusion criteria of age between 15 and 25 years.
Table 1.
Population characteristics (n = 375) according to Taabo HDSS data, stratified by study setting and age group
| Taabo Cité | Ahondo | Katchénou | Difference | |||||
|---|---|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | χ2 | P value | |
| Age group | ||||||||
| Infants (6–23 months) | 56 | 37.1 | 39 | 34.2 | 33 | 30.0 | ||
| Children (6–8 years) | 61 | 40.4 | 46 | 40.4 | 51 | 46.4 | ||
| Women (15–25 years) | 34 | 22.5 | 29 | 25.4 | 26 | 23.6 | 1.83 | 0.767 |
| Sex (female)* | 60 | 51.3 | 38 | 44.7 | 40 | 47.6 | 0.87 | 0.647 |
| Occupation | ||||||||
| Attending school† | 44 | 72.1 | 26 | 56.5 | 28 | 54.9 | 4.33 | 0.114 |
| Never attended school‡ | 8 | 22.9 | 13 | 41.9 | 18 | 71.4 | ||
| Pre-school‡ | 0 | 0 | 0 | 0 | 0 | 0.0 | ||
| Primary school‡ | 12 | 37.1 | 12 | 41.9 | 7 | 25.0 | ||
| Secondary school‡ | 14 | 40.0 | 1 | 6.5 | 1 | 3.6 | ||
| High school‡ | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Muslim school‡ | 0 | 0 | 3 | 9.7 | 0 | 0 | 30.54 | < 0.001 |
| Working‡ | 14 | 42.9 | 19 | 61.3 | 24 | 92.9 | ||
| Student‡ | 13 | 37.1 | 6 | 22.6 | 1 | 3.6 | ||
| Housekeeper‡ | 7 | 20.0 | 4 | 16.1 | 1 | 3.6 | 16.91 | 0.002 |
| Residence in the village | ||||||||
| < 6 months§ | 3 | 3.2 | 2 | 2.7 | 0 | 0 | ||
| 6 months to 1 year§ | 7 | 7.4 | 11 | 14.7 | 2 | 2.6 | ||
| > 1 year§ | 85 | 89.5 | 62 | 82.7 | 75 | 97.4 | 10.17 | 0.038 |
| Family | ||||||||
| No children‡ | 24 | 70.6 | 12 | 41.4 | 3 | 11.5 | ||
| 1–3 children‡ | 8 | 25.5 | 17 | 58.6 | 22 | 84.6 | ||
| > 3 children‡ | 2 | 5.9 | 0 | 0 | 1 | 2.9 | 24.1 | < 0.001 |
| Single‡ | 27 | 79.4 | 13 | 44.8 | 3 | 11.5 | ||
| With partner‡ | 3 | 8.8 | 8 | 27.6 | 14 | 53.9 | ||
| Married‡ | 3 | 8.8 | 8 | 27.6 | 9 | 34.6 | ||
| Other‡ | 1 | 2.9 | 0 | 0 | 0 | 0 | 31.27 | < 0.001 |
Considered for infants and children (n = 286).
Considered for children only (n = 158).
Considered for women only (n = 89).
Considered for children and women (n = 247).
Prevalence of anemia, stratified by age and setting.
The overall prevalence of anemia among participants with complete parasitologic data was 58.4%, with no difference in the prevalence and severity of anemia according to study setting. As shown in Tables 2 and 3, infants had the highest prevalence of anemia (78.1%). Among them, 61%, 32%, and 7% had mild, moderate, and severe anemia. In school-aged children and women, slightly less than 50% were anemic. There was no case of severe anemia in children, and only one woman suffered from severe anemia.
Table 2.
Prevalence and severity of anemia (n = 375)
| Infants (n = 128) | Children (n = 158) | Women (n = 89) | ||||
|---|---|---|---|---|---|---|
| Cases | Percent | Cases | Percent | Cases | Percent | |
| Anemia* | 100 | 78.1 | 74 | 46.8 | 45 | 47.9 |
| Mild | 61 | 61.0 | 70 | 94.6 | 42 | 93.3 |
| Moderate† | 32 | 32.0 | 4 | 5.4 | 2 | 4.4 |
| Severe‡ | 7 | 7.0 | 0 | 0 | 1 | 2.2 |
Anemia is defined as Hb < 12 g/dL for women, Hb < 11.5 g/dL for children, and Hb < 11 g/dL for infants.
Moderate anemia is defined as Hb < 9 g/dL.
Severe anemia is defined as Hb < 7 g/dL.
Table 3.
Prevalence and severity of anemia (n = 375)
| Taabo Cité (n = 151) | Ahondo (n = 114) | Katchénou (n = 110) | ||||
|---|---|---|---|---|---|---|
| Cases | Percent | Cases | Percent | Cases | Percent | |
| Anemia* | 76 | 50.3 | 72 | 63.2 | 71 | 64.6 |
| Mild | 58 | 76.3 | 60 | 83.3 | 55 | 77.6 |
| Moderate† | 17 | 22.4 | 9 | 12.5 | 12 | 16.9 |
| Severe‡ | 1 | 1.3 | 3 | 4.2 | 4 | 5.6 |
Anemia is defined as Hb < 12 g/dL for women, Hb < 11.5 g/dL for children, and Hb < 11 g/dL for infants.
Moderate anemia is defined as Hb < 9 g/dL.
Severe anemia is defined as Hb < 7 g/dL.
Prevalence of parasitic infections, micronutrient deficiencies, and hemoglobinopathies.
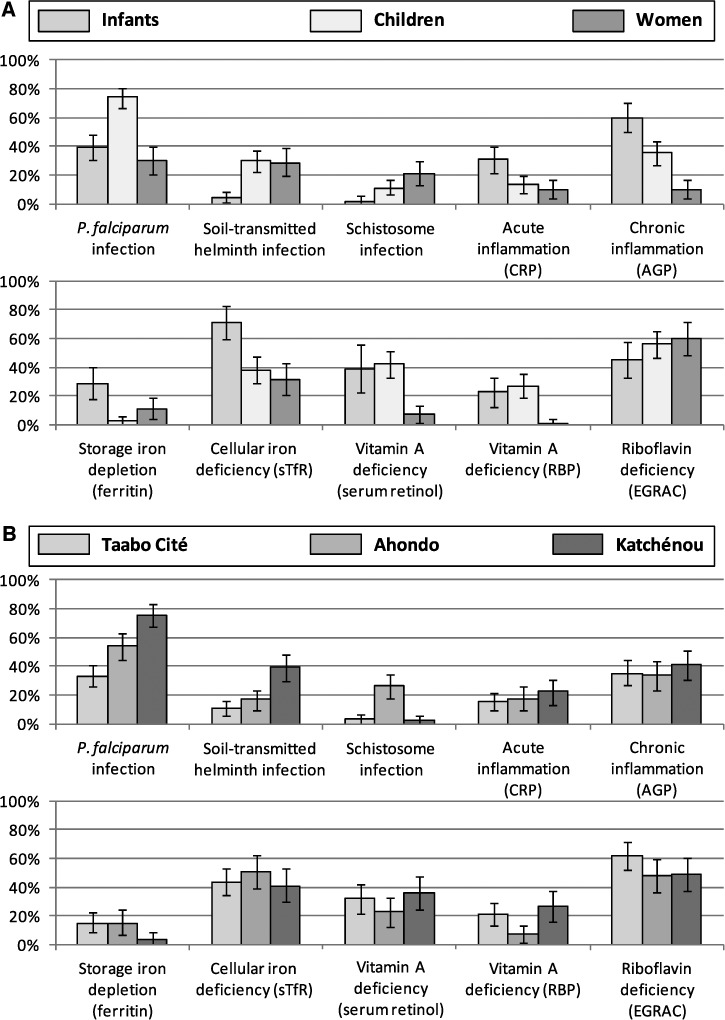
The prevalence of parasitic infection, inflammation, and micronutrient deficiency in each setting, stratified by age, is presented in Figure 3. P. falciparum was highly prevalent throughout. However, RDT results revealed a significantly higher prevalence in Katchénou (75%) compared with Ahondo (54%) and Taabo Cité (33%; χ2 = 45.90, P < 0.001). The highest prevalence of Plasmodium infection was found in school-aged children (74%), whereas the respective prevalence in infants (39%) and non-pregnant women (30%) was considerably lower (χ2 = 56.06, P < 0.001). Among participants found positive for P. falciparum, 27% of the infants, 9% of the school-aged children, and 7% of the non-pregnant women harbored > 5,000 parasites/μL of blood.
Figure 3.
(A) Prevalence of P. falciparum, soil-transmitted helminth, and schistosome infection (n = 375), acute inflammation (n = 251), chronic inflammation (n = 251), storage iron depletion (n = 251), cellular iron deficiency (n = 251), VAD (based on SR values; n = 221), VAD (based on RBP values; n = 251), and riboflavin deficiency (n = 251), stratified by age group. (B) Prevalence of P. falciparum, soil-transmitted helminth, and schistosome infection (n = 375), acute inflammation (n = 251), chronic inflammation (n = 251), storage iron depletion (n = 251), cellular iron deficiency (n = 251), VAD (based on SR values; n = 221), VAD (based on RBP values; n = 251), and riboflavin deficiency (n = 251), stratified by locality. The prevalence of micronutrient deficiency is calculated for participants with complete parasitologic datasets but without acute inflammation (CRP ≤ 10 mg/L) in the Taabo HDSS in April of 2010. AGP = α1-acid glycoprotein; CRP = C-reactive protein; EGRAC = erythrocyte glutathione reductase activity coefficient; sTfR = soluble transferrin receptor.
The overall prevalence of infection with any helminth species was 13% in Taabo Cité, 33% in Ahondo, and 41% in Katchénou. In the latter setting, 53% of school-aged children and women were found infected. S. haematobium was mainly encountered in Ahondo, where 40% of school-aged children and women had a positive urine filtration result. Of the 102 participants positive for any helminth species, only 2 participants presented with a hookworm infection of heavy intensity (≥ 4,000 EPG), and 5 participants presented with a heavy S. haematobium infection (≥ 50 eggs/10 mL of urine). All other helminth infections were of light or moderate intensity.
The prevalence of acute inflammation (17%), as indicated by elevated CRP, was moderate in all study areas and all age groups. Interestingly, the prevalence of chronic infection or inflammation, as indicated by high AGP values (37%), was the highest in infants (60%), despite a considerably higher prevalence of P. falciparum parasitemia and helminth infection in school-aged children.
Around 15% of participants from both Taabo Cité and Ahondo showed storage iron depletion, whereas 4% of the study population in Katchénou was concerned by this shortage. Infants constituted the group at highest risk of cellular iron deficiency (71%; χ2 = 29.03, P < 0.001) and storage iron depletion (29%; χ2 = 32.15, P < 0.001). Considerably fewer school-aged children suffered from cellular iron deficiency (38%), and only 3% showed reduced storage iron. The respective percentages of young women considered iron-deficient and iron-depleted were 34% and 12%, respectively.
Regardless of the choice of the marker, the repartition of VAD was similar in the three settings. Infants and young school-aged children were the most concerned by VAD. Based on HPLC measures, VAD was found in 31% of all study participants, whereas RBP determined a percentage of 19%. RBP showed a specificity of 94% and a sensitivity of 55% compared with retinol measures done with HPLC, and both methods correlated well (Spearman's ρ = 0.72). Two-thirds of the women suffered from riboflavin deficiency with an EGRAC > 1.4, suggesting clear deficiency, and altogether, more than 50% of the participants showed reduced riboflavin values. Hb phenotypes determined through electrophoresis revealed that 84.3% carried adult Hb (HbAA), 7.1% carried a C allele (HbAC), 7.9% carried an S allele (HbAS), and 0.8% had sickle cell anemia (HbSS).
Risk factors for anemia stratified by age.
Table 4 summarizes the statistically significant (P < 0.05) risk factors for anemia, as determined by multivariable logistic regression, stratified by age. Locality was included as a random effect in the models. In infants, P. falciparum was the only significant risk factor for anemia (OR = 8.08, 95% CI = 1.69–38.63). Cellular iron deficiency was a risk factor of anemia in both school-aged children and young non-pregnant women. Moreover, school-aged children with chronic inflammation were more likely to have anemia than children without chronic inflammation (OR = 3.58, 95% CI = 1.56–8.25). In young women, riboflavin deficiency was associated with a lower odds of anemia (OR = 0.29, 95% CI = 0.10–0.87).
Table 4.
Risk factors significantly associated with anemia in infants, school-aged children, and young non-pregnant women living in Taabo Cité, Ahondo, and Katchénou in April of 2010 as determined with multivariable logistic regression with locality as random effect (n = 306)
| Risk factor | OR | 95% CI | Wald test P value |
|---|---|---|---|
| Infants (n = 95)* | |||
| P. falciparum | 8.08 | 1.69–38.63 | 0.009 |
| School-aged children (n = 133)† | |||
| Chronic inflammation‡ | 3.58 | 1.56–8.25 | 0.003 |
| Cellular iron deficiency | 2.26 | 1.02–5.02 | 0.045 |
| Young women (n = 78)§ | |||
| Cellular iron deficiency | 6.00 | 1.75–20.59 | 0.004 |
| Riboflavin deficiency | 0.29 | 0.10–0.87 | 0.027 |
| Occupation of housekeeper | 0.15 | 0.03–0.78 | 0.038 |
The original model included the following explanatory variables: age, sex, cellular iron deficiency, storage iron depletion, riboflavin deficiency, VAD, chronic inflammation, acute inflammation, P. falciparum, schistosomiasis, and soil-transmitted helminthiasis. Step-wise backward multivariate logistic regression was performed keeping only explanatory variables with P values < 0.2. OR was adjusted for chronic inflammation and storage iron depletion.
The original model included age, sex, school attendance, cellular iron deficiency, storage iron depletion, riboflavin deficiency, VAD, chronic inflammation, acute inflammation, P. falciparum, schistosomiasis, and soil-transmitted helminthiasis. OR was adjusted for soil-transmitted helminthiasis and school attendance.
Defined as AGP > 1 g/L.
The original model included age, occupation, chronic inflammation, acute inflammation, P. falciparum, soil-transmitted helminthiasis, schistosomiasis, cellular iron deficiency, storage iron depletion, retinol deficiency, and riboflavin deficiency. OR was adjusted for schistosomiasis and occupation.
According to WHO criteria, anemia is Hb < 12 g/dL for non-pregnant women, Hb < 11.5 g/dL for children, and Hb < 11 g/dL for infants.
Discussion
Data from the current cross-sectional survey on anemia and underlying causes revealed that 78% of infants and around 50% of school-aged children and non-pregnant women were anemic in two rural and one urban settings of south-central Côte d'Ivoire. Our findings, therefore, underscore that anemia is an important public health issue in Côte d'Ivoire.6 The identification of risk factors for the three specific age classes studied here (i.e., infants aged 6–23 months, children aged 6–8 years, and non-pregnant women aged 15–25 years) confirmed the multiple and complex etiology of anemia in a typical, primarily rural setting of sub-Saharan Africa.2 We found specific risk factors for anemia depending on age, which calls for the development and implementation of an integrated approach targeting these vulnerable groups.
The main limitations of the study presented here are as follows. First, the overall compliance was low; from the 732 people initially invited to participate, less than 50% had complete data records. Particularly, young women showed low compliance (28%). Reasons for the low compliance are numerous: the challenge to motivate people to participate, the difficulty of collecting urine and stool samples from infants, and people's hesitation to provide venous blood, particularly in areas where human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is present. Moreover, the collection of sufficiently large volumes of venous blood that were necessary for the battery of tests used here proved difficult, particularly for infants. Second, the choice of our study settings was a combination of representativeness and operational feasibility. Because our longitudinal monitoring would last for more than 1 year, with visits one time every 3–4 months, the accessibility of the chosen localities throughout the study period was a mandatory criterion. Third, there are some shortcomings regarding the methods used for appraisal of micronutrient deficiencies, such as indirect estimation of the riboflavin status and non-universally recognized cut-off values for sTfR.3
Our results indicate that S. haematobium and soil-transmitted helminth infections are present in all three study settings but with low overall prevalence and infection intensities. Hence, compared with previous research and thanks to local control efforts implemented in the Taabo district, major improvements regarding S. haematobium endemicity have been achieved.10,24,25 Of note, surveys done in farming communities require the collection of urine samples in the early morning, because farmers leave their homes early and only return in the late afternoon after having worked in the fields. However, S. haematobium egg output is highest around midday, and hence, the true prevalence of S. haematobium is likely to have been underestimated.26 With regard to soil-transmitted helminths, hookworm was the predominant species. Because only one stool specimen was subjected to duplicate Kato–Katz thick smear examination, it is conceivable that the reported prevalence of soil-transmitted helminths is a considerable underestimation of the true infection prevalence.27–30 This issue is important, because it might have reduced the likelihood of documenting a significant relationship between helminth infection and anemia.
A previous study conducted in villages in close proximity to the Taabo HDSS revealed that the administration of anthelmintic drugs (albendazole plus praziquantel) to school-aged children two times with a 3-month interval significantly increased Hb concentration by 0.24 g/dL.4 A recent metaanalysis revealed that S. haematobium and hookworm infections were significantly associated with anemia in children < 4 years old and that up to 10% of anemic cases could be adverted by treating helminth infections.31 The current data suggest that helminth infection was not a significant risk factor for anemia for any of the three age groups investigated. Nevertheless, because albendazole and ivermectin have been administered at the population level after the baseline cross-sectional survey reported here, the results from subsequent surveys will shed new light on the long-term effect of deworming campaigns against anemia.
The present survey was conducted shortly before the rainy season. Interestingly, the prevalence of P. falciparum infection was slightly higher than what has been reported before for school-aged children in the same area.4 Our data confirm that malaria is still highly endemic in Côte d'Ivoire, perhaps explained by the prolonged sociopolitical crises,8 and the overall low coverage rate of proven interventions such as LLINs.32 Although malaria has a wide range of clinical outcomes, malaria-related anemia is one of the leading causes of death, particularly in children.33 In our study, infection with P. falciparum was the only risk factor significantly associated with anemia in infants. Our data also suggest that anemia of chronic disease (ACD), also phrased anemia of inflammation, plays a role in the process of anemia for children aged 6–8 years. Interestingly, only 14% and 36% of school-aged children had an elevated CRP and AGP, respectively, despite a considerably higher prevalence of Plasmodium parasitemia. This discrepancy may, at least partly, be explained by the generally low parasitemia found in this age group, and it indicates that afebrile malaria is not necessarily associated with increased AGP and CRP. These observations were reversed in infants; although slightly less than 40% had a positive RDT, 60% showed elevated AGP values. This result may be explained by other illnesses that can increase inflammatory markers in children during their first years of life. Intestinal and urinary tract infections, respiratory diseases, hepatitis, measles, HIV, and small injuries are among factors that affect the young child and could increase inflammatory markers.
The body of evidence pertaining to cellular iron deficiency and its major contribution to anemia is compelling.3,34 Clearly, other micronutrients, such as vitamins A and B12 and folate, are important factors in the pathophysiology of anemia, although we still lack intervention studies that confirm and quantify these associations.35–38 Our study confirms that iron-deficiency anemia is prevalent in south-central Côte d'Ivoire.6,39 However, our results also emphasize the lack of robust markers for estimating iron deficiency in populations where malaria and more generally, inflammation are widespread. Both ferritin and sTfR showed a significant correlation with AGP and CRP. sTfR weakly but significantly correlated with CRP (Spearman's ρ = 0.26, P < 0.01) contrary to previous studies that showed that the specificity and sensitivity of sTfR in estimating iron deficiency remained, even in areas endemic for malaria, unaffected by the acute-phase response.6,40
Although we found the highest prevalence of iron deficiency and iron depletion in infants, these parameters were not identified as significant risk factors for anemia in this age group. Chronic inflammation was the only variable significantly negatively associated with anemia in infants free of malaria. It has been shown that iron fortification can prevent anemia in infants free of malaria,41 and a recent meta-analysis showed that iron supplementation is recommended in areas where malaria is endemic when regular malaria surveillance and treatment services are provided.42 Of note, sTfR is a marker of cellular iron demand, and there are mid-to-moderate forms of iron deficiency where anemia is absent. These forms of iron deficiency often lead to anemia if they remain untreated. This observation is reflected in the school-aged children for whom iron deficiency becomes an important risk factor. Iron fortification or supplementation coupled with the distribution of LLINs and IPT of malaria in infants would be effective strategies to prevent the development of iron-deficiency anemia in our population. Moreover, although the prevalence of iron deficiency was slightly lower in young women, it represents a significant risk factor for this age group, confirming the body of evidence about the burden of iron deficiency in women of childbearing age.
Riboflavin deficiency was very common in the study populations. Indeed, 60% of the women, one-half of the group of 6- to 8-year-old children, and more than 40% of infants showed riboflavin deficiency. These results are not surprising considering the low intake of dairy products in our study population. Although there is only little data on the riboflavin status of populations from sub-Saharan Africa, other studies suggest that this deficiency may be common in this part of the world.43–46 Riboflavin supplementation has been shown to improve hematological status alone or combined with iron in deficient populations.47,48 Interestingly, our study is the first that showed lower odds of anemia for young women with riboflavin deficiency. One hypothesis is that riboflavin deficiency protects from malaria and indirectly improves hematological parameters.49,50 Nevertheless, multivariate regression analysis and Wilcoxon rank-sum tests revealed that riboflavin deficiency and EGRAC values were associated with neither malaria prevalence nor Plasmodium parasitemia in women. Moreover, riboflavin deficiency was also significantly negatively associated with anemia in women free of malaria, suggesting another mechanism. It is also worth noting that EGRAC is a method with results that rely on the activity of EGR. Thus, even in populations where dairy food is not consumed daily, caution is indicated with the interpretation of abnormal EGRACs as indicators of true nutritional riboflavin deficiency. A previous study done in neighboring villages showed that daily intake of riboflavin was insufficient in children,46 suggesting that at least a part of the low EGRAC values observed in our population is because of a true riboflavin deficiency.
We found that one-third of children were vitamin A-deficient, confirming previous findings from a neighboring locality.51 RBP showed a rather low sensitivity (55%) but good specificity (94%) in the detection of VAD. However, the definition of VAD according to RBP or serum retinol did not modify the results of our multivariate logistic regression in school-aged children and young women. Moreover, within the 62 infants for whom vitamin A status was also assessed by HPLC, this parameter was not correlated to Hb. Although vitamin A supplementation has shown a positive effect on hematological status of children in areas free of malaria,36 VAD was not a significant risk factor for anemia in participants free of malaria.
We conclude that, although anemia is multifactorial, our study revealed that there are specific risk factors for specific age groups. Infants are primarily affected by malarial anemia; older children are primarily affected by anemia of inflammation and/or iron-deficiency anemia. The latter is also the main risk factor of anemia in young non-pregnant women. Our study, moreover, brought to light that deficiencies in iron and riboflavin can have an opposite association with anemia. Taken together, our observations call for interventions targeting malaria, and the large-scale distribution of LLINs to mothers of preschool-aged children should be done without delay. Additionally, dietary improvements must be explored. In view of the importance of anemia of inflammation in school-aged children, regular deworming and community-led total sanitation and health education to improve sustainability should be considered. After the current baseline cross-sectional survey completed in April of 2010, interventions have been launched, but there were interruptions because of the post-election crisis in late 2010/early 2011. The results communicated here will serve as a benchmark for longitudinal monitoring, including the impact of armed conflict and war.
ACKNOWLEDGMENTS
We are grateful to Prof. Bassirou Bonfoh, Director-General of the Centre Suisse de Recherches Scientifiques en Côte d'Ivoire, Mr. Koné Siaka, Mr. Louis Botti, Mr. Fabian Zouzou, and all other collaborators of the Taabo health and demographic surveillance system for their support and facilitation of the study. Many thanks go to Mr. Mahamadou Traoré, Mr. Laurent K. Lohourignon, Mr. Meledje D. G. Rameau, Mr. N'Cho Monsan, Mr. Brou A. Sostène, Mr. Guy D. Raphaël, and Mr. Laurent K. Valian for their quality work in the field and the bench. We also thank Mr. Christophe Zeder and Mr. Adam Krzystek for their expertise and advice about the different methods used to analyze blood samples in Switzerland and Mrs. Alice Aebischer for her help during the analytical work. Last but not least, we would like to thank all the study participants for their commitment and willingness to collaborate and the five field workers, Mrs. Caroline Brou, Mrs. Sandrine N'Guetta, Mr. Kouamé Y. Mathurin, Mr. Kouamé N'Gbin, and Mr. Kouakou Lucien, without whom this study would not have been possible.
Footnotes
Financial support: This work was supported by the Swiss National Science Foundation (project no. IZ70Z0_123900). Start-up funding for the establishment and running of the Taabo HDSS has been granted by Fairmed. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Aurélie A. Righetti and Jürg Utzinger, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute and University of Basel, Basel, Switzerland, E-mails: aurelie.righetti@unibas.ch and juerg.utzinger@unibas.ch. Ahou-Yah G. Koua and Sébastien Niamké, Laboratoire de Biotechnologies, Unité de Formation et de Recherche Biosciences, Université de Cocody, Abidjan, Côte d'Ivoire, E-mails: kouayahgisele@yahoo.fr and niamkes@yahoo.fr. Lukas G. Adiossan, Hôpital Général de Taabo, Taabo Cité, Côte d'Ivoire, E-mail: adiossanlukas@yahoo.fr. Dominik Glinz, Richard F. Hurrell, and Rita Wegmüller, Laboratory of Human Nutrition, Institute of Food, Nutrition, and Health, ETH Zurich, Zurich, Switzerland, E-mails: dominik.glinz@hest.ethz.ch, richard.hurrell@hest.ethz.ch, and rita.wegmueller@hest.ethz.ch. Eliézer K. N'Goran, Laboratoire de Zoologie Biologie Animale, Unité de Formation et de Recherche Biosciences, Université de Cocody, Abidjan, Côte d'Ivoire, E-mail: eliezerngoran@yahoo.fr.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51. [PubMed] [Google Scholar]
- 3.WHO/UNICEF/UNU . Iron Deficiency Anemia: Assessment, Prevention and Control. Geneva: World Health Organization; 2001. [Google Scholar]
- 4.Rohner F, Zimmermann MB, Amon RJ, Vounatsou P, Tschannen AB, N'Goran EK, Nindjin C, Cacou MC, Te-Bonle MD, Aka H, Sess DE, Utzinger J, Hurrell RF. In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in Ivorian children, only anthelmintic treatment shows modest benefit. J Nutr. 2010;140:635–641. doi: 10.3945/jn.109.114256. [DOI] [PubMed] [Google Scholar]
- 5.Wegmüller R, Camara F, Zimmermann MB, Adou P, Hurrell RF. Salt dual-fortified with iodine and micronized ground ferric pyrophosphate affects iron status but not hemoglobin in children in Côte d'Ivoire. J Nutr. 2006;136:1814–1820. doi: 10.1093/jn/136.7.1814. [DOI] [PubMed] [Google Scholar]
- 6.Staubli Asobayire F, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Côte d'Ivoire. Am J Clin Nutr. 2001;74:776–782. doi: 10.1093/ajcn/74.6.776. [DOI] [PubMed] [Google Scholar]
- 7.ter Kuile FO, Terlouw DJ, Kariuki SK, Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68:68–77. [PubMed] [Google Scholar]
- 8.Bonfoh B, Raso G, Koné I, Dao D, Girardin O, Cissé G, Zinsstag J, Utzinger J, Tanner M. Research in a war zone. Nature. 2011;474:569–571. doi: 10.1038/474569a. [DOI] [PubMed] [Google Scholar]
- 9.Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, Hatz C, Kern WV, N'Goran EK, Utzinger J. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis. 2011;5:e1292. doi: 10.1371/journal.pntd.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.N'Goran EK, Diabate S, Utzinger J, Sellin B. Changes in human schistosomiasis levels after the construction of two large hydroelectric dams in central Côte d'Ivoire. Bull World Health Organ. 1997;75:541–545. [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Adequacy of Sample Size in Health Studies. New York: John Wiley & Sons; 1990. [Google Scholar]
- 12.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 13.WHO . Basic Laboratory Methods in Medical Parasitology. Geneva: World Health Organization; 1991. [Google Scholar]
- 14.Schneider RG. Differentiation of electrophoretically similar hemoglobins—such as S, D, G, and P; or A2, C, E, and O—by electrophoresis of the globin chains. Clin Chem. 1974;20:1111–1115. [PubMed] [Google Scholar]
- 15.Dror Y, Stern F, Komarnitsky M. Optimal and stable conditions for the determination of erythrocyte glutathione reductase activation coefficient to evaluate riboflavin status. Int J Vitam Nutr Res. 1994;64:257–262. [PubMed] [Google Scholar]
- 16.Sauberlich H. Vitamin B-2. Laboratory Tests for the Assessment of Nutritional Status. 2nd Ed. Boca Raton, FL: CRC Press; 1999. pp. 55–69. [Google Scholar]
- 17.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 18.Tanumihardjo SA, Permaesih D, Muhilal Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. Eur J Clin Nutr. 2004;58:1223–1230. doi: 10.1038/sj.ejcn.1601953. [DOI] [PubMed] [Google Scholar]
- 19.Cook JD, Skikne BS, Baynes RD. Serum transferrin receptor. Annu Rev Med. 1993;44:63–74. doi: 10.1146/annurev.me.44.020193.000431. [DOI] [PubMed] [Google Scholar]
- 20.WHO . Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [Google Scholar]
- 21.Gorstein JL, Dary O, Pongtorn, Shell-Duncan B, Quick T, Wasanwisut E. Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr. 2008;11:513–520. doi: 10.1017/S1368980007000821. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800:760–769. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet. 2003;362:2052–2058. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- 24.N'Goran EK, Utzinger J, N'Guessan AN, Müller I, Zamblé K, Lohourignon KL, Traoré M, Sosthène BA, Lengeler C, Tanner M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d'Ivoire. Trop Med Int Health. 2001;6:817–825. doi: 10.1046/j.1365-3156.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- 25.Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]
- 26.WHO . Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization; 1998. [Google Scholar]
- 27.Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31:3044–3045. doi: 10.1128/jcm.31.11.3044-3045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utzinger J, Booth M, N'Goran EK, Müller I, Tanner M, Lengeler C. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology. 2001;122:537–544. doi: 10.1017/s0031182001007752. [DOI] [PubMed] [Google Scholar]
- 29.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopp S, Rinaldi L, Khamis IS, Stothard JR, Rollinson D, Maurelli MP, Steinmann P, Marti H, Cringoli G, Utzinger J. A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans R Soc Trop Med Hyg. 2009;103:347–354. doi: 10.1016/j.trstmh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Magalhães RJ, Clements ACA. Mapping the risk of anaemia in preschool-age children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med. 2011;8:e1000438. doi: 10.1371/journal.pmed.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noor AM, Mutheu JJ, Tatem AJ, Hay SI, Snow RW. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373:58–67. doi: 10.1016/S0140-6736(08)61596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64((1–2 Suppl)):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 34.Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24:S99–S103. doi: 10.1177/15648265030244S206. [DOI] [PubMed] [Google Scholar]
- 35.Haider BA, Bhutta ZA. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in developing countries. Cochrane Database Syst Rev. 2011;10:CD006980. doi: 10.1002/14651858.CD006980.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann MB, Biebinger R, Rohner F, Dib A, Zeder C, Hurrell RF, Chaouki N. Vitamin A supplementation in children with poor vitamin A and iron status increases erythropoietin and hemoglobin concentrations without changing total body iron. Am J Clin Nutr. 2006;84:580–586. doi: 10.1093/ajcn/84.3.580. [DOI] [PubMed] [Google Scholar]
- 37.Scott JM. Nutritional anemia: B-vitamins. In: Kraemer K, Zimmermann MB, editors. Nutritional Anemia. Basel, Switzerland: Sight and Life Press; 2007. pp. 111–132. [Google Scholar]
- 38.West KP, Jr, Gernand AD, Sommer A. In: Vitamin A in nutritional anemia. Nutritional Anemia. Kraemer K, Zimmermann MB, editors. Basel, Switzerland: Sight and Life Press; 2007. pp. 133–154. [Google Scholar]
- 39.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, Tjalsma H, Wiegerinck E, Tanno T, Hurrell RF, Hounhouigan J, Zimmermann MB. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92:1385–1392. doi: 10.3945/ajcn.2010.30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roodenburg AJ, West CE, Beguin Y, Van Dijk JE, Van Eijk HG, Marx JJ, Beynen AC. Indicators of erythrocyte formation and degradation in rats with either vitamin A or iron deficiency. J Nutr Biochem. 2000;11:223–230. doi: 10.1016/s0955-2863(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 41.Walter T, Dallman PR, Pizarro F, Velozo L, Pena G, Bartholmey SJ, Hertrampf E, Olivares M, Letelier A, Arredondo M. Effectiveness of iron-fortified infant cereal in prevention of iron deficiency anemia. Pediatrics. 1993;91:976–982. [PubMed] [Google Scholar]
- 42.Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011;10:CD006589. doi: 10.1002/14651858.CD006589.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Faber M, Jogessar VB, Benade AJ. Nutritional status and dietary intakes of children aged 2–5 years and their caregivers in a rural South African community. Int J Food Sci Nutr. 2001;52:401–411. doi: 10.1080/09637480120078285. [DOI] [PubMed] [Google Scholar]
- 44.Abrams SA, Mushi A, Hilmers DC, Griffin IJ, Davila P, Allen L. A multinutrient-fortified beverage enhances the nutritional status of children in Botswana. J Nutr. 2003;133:1834–1840. doi: 10.1093/jn/133.6.1834. [DOI] [PubMed] [Google Scholar]
- 45.Siekmann JH, Allen LH, Bwibo NO, Demment MW, Murphy SP, Neumann CG. Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B-12 is the only detectable micronutrient response to meat or milk supplementation. J Nutr. 2003;133:3972S–3980S. doi: 10.1093/jn/133.11.3972S. [DOI] [PubMed] [Google Scholar]
- 46.Rohner F, Zimmermann MB, Wegmüller R, Tschannen AB, Hurrell RF. Mild riboflavin deficiency is highly prevalent in school-age children but does not increase risk for anaemia in Côte d'Ivoire. Br J Nutr. 2007;97:970–976. doi: 10.1017/S0007114507665180. [DOI] [PubMed] [Google Scholar]
- 47.Powers HJ, Hill MH, Mushtaq S, Dainty JR, Majsak-Newman G, Williams EA. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM) Am J Clin Nutr. 2011;93:1274–1284. doi: 10.3945/ajcn.110.008409. [DOI] [PubMed] [Google Scholar]
- 48.Powers HJ, Bates CJ, Prentice AM, Lamb WH, Jepson M, Bowman H. The relative effectiveness of iron and iron with riboflavin in correcting a microcytic anaemia in men and children in rural Gambia. Hum Nutr Clin Nutr. 1983;37:413–425. [PubMed] [Google Scholar]
- 49.Kaikai P, Thurnham DI. The influence of riboflavin deficiency on Plasmodium berghei infection in rats. Trans R Soc Trop Med Hyg. 1983;77:680–686. doi: 10.1016/0035-9203(83)90204-3. [DOI] [PubMed] [Google Scholar]
- 50.Thurnham DI, Oppenheimer SJ, Bull R. Riboflavin status and malaria in infants in Papua New Guinea. Trans R Soc Trop Med Hyg. 1983;77:423–424. doi: 10.1016/0035-9203(83)90180-3. [DOI] [PubMed] [Google Scholar]
- 51.Yapi HF, Ahiboh H, Ago K, Ake M, Monnet D. Protein profile and vitamin A in children of school age in Ivory Coast. Ann Biol Clin (Paris) 2005;63:291–295. [PubMed] [Google Scholar]
- 52.Glinz D, N'Guessan NA, Utzinger J, N'Goran EK. High prevalence of Strongyloides stercoralis among school children in rural Côte d'Ivoire. J Parasitol. 2010;96:431–433. doi: 10.1645/GE-2294.1. [DOI] [PubMed] [Google Scholar]