Abstract
We investigated the occurrence of Leishmania infantum chagasi in Didelphis albiventris opossums at a wild animal rehabilitation center in the city of Campo Grande, Brazil. A total of 54 opossums were tested for L. i. chagasi infection in peripheral blood and bone marrow samples. The samples were analyzed by direct examination, culturing in a specific medium, and polymerase chain reaction–restriction fragment length polymorphism. Leishmania i. chagasi DNA was detected by polymerase chain reaction–restriction fragment length polymorphism in 11 (20.37%) animals. A total of 81.81% of positive opossums were captured in areas of known visceral leishmaniasis transmission. These results suggest a role for D. albiventris in the urban transmission of visceral leishmaniasis.
Leishmaniasis is a disease caused by protozoa. A large number of Leishmania species have been characterized as parasites of wild animals and are less frequently found in domesticated animals.1,2 A number of studies have reported high infection rates among domestic dogs, indicating that these animals are major reservoirs for visceral leishmaniasis (VL).1–3 Other animals have also been investigated with regard to their role in the transmission of this disease, with major attention given to synanthropic animals, especially opossums (Didelphis spp.).2,4,5
There is evidence that the presence of opossums in the same area as canine transmission is a predisposing factor for canine infection, although the role of these animals as reservoirs and their impact on the transmission of L. infantum chagasi in urban areas are not completely established. Nonetheless, opossums are usually found in urban areas.4,6,7 The aim of the present study was to investigate the occurrence of Leishmania spp. infection in specimens of the white-eared opossum (Didelphis albiventris) captured in an urban area of the city of Campo Grande, Brazil.
Campo Grande is located in the central region of the state of Mato Grosso do Sul, Brazil (Figure 1). For cases of human VL, the Campo Grande Secretary of Health classifies different regions of the city as sporadic transmission zones, moderate transmission zones, intense transmission zones, and zones without transmission.
Figure 1.
Location of the study area in Campo Grande Municipality, Mato Grosso do Sul (MS), Brazil.
Didelphis albiventris were captured in different areas of the city, kept at a wild animal rehabilitation center, and screened for L. i. chagasi infection. Animals were defined as being in good health on the basis of physical examination at arrival. A microchip was implanted in all animals after species identification and clinical screening. Samples from opossums were typically collected within 1–2 days of arrival at the center; the maximum interval between arrival and collection procedures was three days.
After intramuscular anesthetic procedures with xylazine chlorhydrate (2 mg/kg) and ketamine chlorhydrate (30 mg/kg), bone marrow was collected by aspiration of the tibiofibular joint, and peripheral blood was collected by cardiac puncture. Samples were microscopically examined for Leishmania amastigotes and were inoculated in Neal, Novy and Nicolle medium and Schneider's medium. All procedures were carried out after approval from the local ethics committee (CEUA/UFMS protocol no. 179/2008) and the government wildlife authority (SISBIO-IBAMA protocol no. 13838-1).
DNA was extracted from samples by using the Wizard DNA Purification Kit (Promega, Madison, WI) according to the manufacturer's instructions. A polymerase chain reaction was designed to amplify a 300-basepair fragment of the internal transcribed spacer 1 of ribosomal RNA of Leishmania spp. Restriction fragment length polymorphism analyses were conducted as described.8
A total of 54 white-eared opossums were screened for L. i. chagasi infection. All animals exhibited good health and no symptoms of VL at the time of clinical screening.
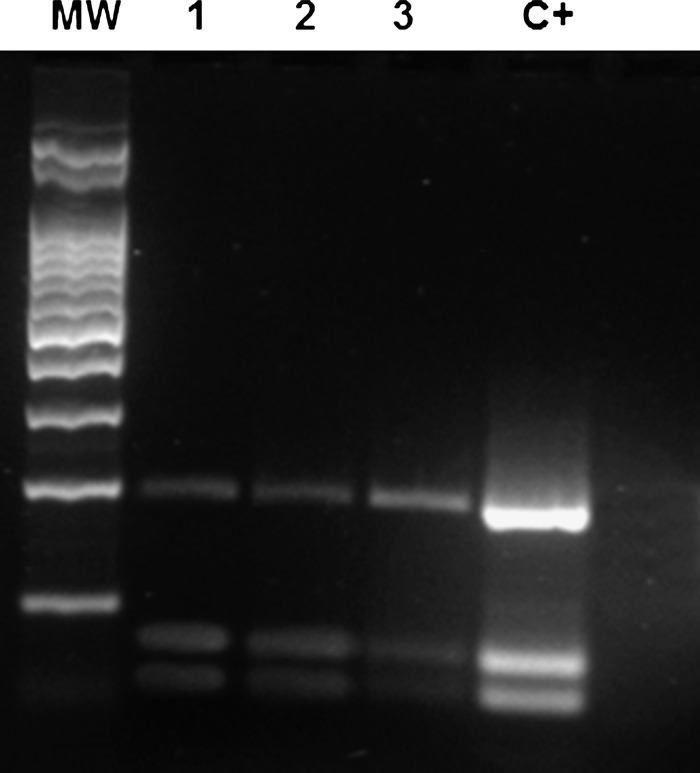
Leishmania DNA was detected by PCR in 11 (20.37%) animals. Seven of 21 blood marrow samples and 4 of 33 peripheral blood samples were positive for Leishmania spp. Amastigote forms were found in peripheral blood samples from two animals, but isolation in culture media was not possible. Restriction fragment length polymorphism analysis identified the parasites from bone marrow and peripheral blood samples as Leishmania i. chagasi (Figure 2).
Figure 2.
Restriction fragment length polymorphism pattern of internal transcribed spacer 1 region polymerase chain reaction products in bone marrow (lanes 1 and 2) and peripheral blood (lane 3) samples positive for Leishmaniai. chagasi after digestion with Hae III. Lane MW = molecular weight standard; lane C+ = positive control.
The distribution of the animals among the VL transmission zones and number of opossums positive or negative for L. i. chagasi is shown in Table 1. The distribution of animals positive for L. i. chagasi among the VL transmission zones according to sex and age is shown in Table 2.
Table 1.
Distribution of Didelphis albiventris among VL transmission zones and number of animals positive or negative for Leishmania infantum chagasi infection in Campo Grande, Mato Grosso do Sul, 2008 and 2009*
| VL transmission zones | Results | |
|---|---|---|
| No. positive | No. negative | |
| Sporadic | 5 | 22 |
| Moderate | 2 | 07 |
| Intense | 2 | 04 |
| No transmission | 0 | 10 |
| Not informed | 2 | 0 |
| Total | 11 | 43 |
VL = visceral leishmaniasis.
Table 2.
Distribution of specimens of Didelphis albiventris positive for Leishmania infantum chagasi among VL transmission zones according to sex and age, Campo Grande, Mato Grosso do Sul, 2008 and 2009*
| VL transmission zones | No. positive | Sex | Age | ||
|---|---|---|---|---|---|
| Male | Female | Adult | Juvenile | ||
| Sporadic | 5 | 2 | 3 | 4 | 1 |
| Moderate | 2 | 1 | 1 | 2 | 0 |
| Intense | 2 | 1 | 1 | 2 | 0 |
| Not informed | 2 | 2 | 0 | 2 | 0 |
| Total | 11 | 6 | 5 | 10 | 1 |
VL = visceral leishmaniasis.
Opossums (Didelphis sp.) have been incriminated as major reservoirs of Trypanosomatidae, such as Trypanosoma cruzi and various viscerotropic and dermatotropic species of Leishmania.4,6,7,9,10 During the clinical examination, no animal exhibited any symptoms of VL infection, which corroborates data from studies carried out in Colombia by Travi and others.5
Most wild animals considered to be reservoir species for Leishmania spp. are asymptomatic for leishmaniasis infection.2 In cases where clinical signs are present, the expected symptoms include skin lesions with or without ulceration, enlarged lymph nodes, corneal ulceration, splenomegaly, and hepatomegaly.4 None of these were observed in the opossums tested.
The larger number of infected adult opossums (10 adults versus 1 juvenile) suggests that these animals likely become infected over time because of living in close contact with human populations and, consequently, infected dogs. Moreover, opossums expand their area of dispersion as they get older.11
The city of Campo Grande has a large number of wooded areas, which accounts for the presence of opossums near human households. In addition, forest degradation caused by urban development may expel opossums from their natural habitat and force these animals to seek shelter in domestic environments. Another peculiarity that supports this synanthropic behavior is the habit among the local human population of turning their backyards into rural areas by breeding animals, such as chickens and pigs, and maintaining a variety of fruit trees. These conditions also contribute to proliferation and maintenance of vectors. Lutzomyia longipalpis is known to be distributed throughout the city of Campo Grande.12 and this insect can feed on D. albiventris.13
Currently, data on opossum population size in the Campo Grande area is insufficient to enable accurate estimation of population size and, subsequently, ideal sample size. The sample size used here was based on the number of opossums that were brought to the wild animal rehabilitation center during the course of the study.
In a study carried out in the city of Jacobina (Bahia State, Brazil), it was found that 2.3% of specimens of D. albiventris were naturally infected with L. donovani. This mammal was also found naturally infected with other species of Leishmania (L.) amazonensis (1.1%), L. (Viannia) braziliensis (1.1%), and Trypanosoma cruzi (3.5%), thereby corroborating the hypothesis that D. albiventris is one of the links of domestic and sylvatic transmission.4
Brandão-Filho and others14 captured D. albiventris in different ecotopes in the coastal zone of the state of Pernambuco and reported Leishmania (Viannia) infection in 13.5% of specimens. Other studies report Didelphis marsupialis infected with L. i. chagasi.9 The results of the present study demonstrate an association between cases of human VL and the presence of infected opossums in same area. Likewise, the absence of infected opossums coincided with areas without human cases of the disease. According to Santiago and others,6 high infection rates in opossums in an urban area suggest that this species may act as a natural link in the domestic and sylvatic parasite life cycle because opossums and dogs can inhabit the same environment.
According to Zoonoses Control Center of Campo Grande city, there are VL-seropositive dogs throughout Campo Grande, and the prevalence of disease in dogs is 25.30%.15 Nevertheless, more data on the size of the opossum population level are needed to determine how large a representative sample of opossums is required for definitive association between human and opossum cases of leishmaniasis. The sampling method we used was convenience sampling of all opossums brought to the wild animal rehabilitation center during the study. However, the prevalence within our sample of 11 opossums (20.37%) suggests an association between the presence of opossums and cases of leishmaniasis in Campo Grande. Thus, the presence of L. i. chagasi in D. albiventris captured in all disease transmission areas demands further research to elucidate the role of this animal in the epidemiology of VL.
ACKNOWLEDGMENTS
We thank the Wild Animals Rehabilitation Center and the Instituto de Meio Ambiente de Mato Grosso do Sul for their contributions to this study.
Footnotes
Financial support: This study was supported by DECIT/FUNDECT-23/200.041/2008.
Authors' addresses: Roberta M. P. Humberg, Instituto de Meio Ambiente de Mato Grosso do Sul, GCF/Fiscalizaçao, Avenida Desembargador Leão Neto do Carmo, s/n, Quadra 03, Lote 03, Parque dos Poderes, Campo Grande, Mato Grosso do Sul CEP 79031-902, Brazil. Elisa T. Oshiro, Alda M. T. Ferreira, and Alessandra Gutierrez de Oliveira, Centro de Ciência Biológicas e da Saúde, Universidade Federal de Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil, E-mails: elisa.teruya.oshiro@gmail.com, alda.ferreira@ufms.br, and alessandra.oliveira@ufms.br. Maria do Socorro Pires e Cruz, Departamento de Morfofisiologia Veterinária, Universidade Federal do Piauí, Teresina, Piauí, Brazil, E-mail: mspcruz@gmail.com. Paulo E. M. Ribolla and Diego P. Alonso, Instituto de Biociências de Botucatu, Universidade Estadual Júlio de Mesquita Botucatu, São Paulo, Brazil, E-mails: pribolla@ibb.unesp.br and alonso@ibb.unesp.br. Raquel A. Bonamigo and Norton Tasso, Jr., Faculdade de Medicina, Universidade Federal de Mato Grosso do Sul Campo Grande, Mato Grosso do Sul, Brazil, E-mails: raquelbonamigo@hotmail.com and noortonjr@hotmail.com.
References
- 1.Lainson R, Killick-Kendrick R, Flisser A. Ecological interactions in the transmission of the leishmaniases. Philos Trans R Soc Lond B Biol Sci. 1988;321:389–404. doi: 10.1098/rstb.1988.0099. [DOI] [PubMed] [Google Scholar]
- 2.Lainson R, Shaw JJ, Silveira FT, Braga RR. American visceral leishmaniasis: on the origin of Leishmania (Leishmania) chagasi. Trans R Soc Trop Med Hyg. 1987;81:517. doi: 10.1016/0035-9203(87)90187-8. [DOI] [PubMed] [Google Scholar]
- 3.Nunes VL, Galati EA, Nunes DB, Zinezzi RO, Savani ES, Ishikawa E, Camargo MC, D'áuria SR, Cristaldo G, Rocha HC. Ocorrência de leishmaniose visceral canina em assentamento agrícola no Estado de Mato Grosso do Sul, Brasil. Rev Soc Bras Med Trop. 2001;34:301–302. doi: 10.1590/s0037-86822001000300014. [DOI] [PubMed] [Google Scholar]
- 4.Sherlock A, Miranda JC, Sadigurski M, Grimaldi G., Jr Observações sobre o calazar em Jacobina, Bahia: IV-Investigações sobre reservatórios silvestres e comensais. Rev Soc Bras Med Trop. 1988;21:23–27. doi: 10.1590/s0037-86821988000100005. [DOI] [PubMed] [Google Scholar]
- 5.Travi BL, Osorio Y, Guarin N, Cadena H. Leishmania (Leishmania) chagasi: clinical and parasitological observations in experimentally Didelphis marsupialis, reservoir of New World visceral leishmaniasis. Exp Parasitol. 1998;88:73–75. doi: 10.1006/expr.1998.4214. [DOI] [PubMed] [Google Scholar]
- 6.Santiago ME, Vasconcelos RO, Fattori KR, Munari DP, Michelin AF, Lima VM. An investigation of Leishmania spp. from urban and peri-urban areas in Bauru (São Paulo, Brazil) Vet Parasitol. 2007;150:283–290. doi: 10.1016/j.vetpar.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Schallig HD, Da Silva ES, Van Der Meide WF, Schoone GJ, Gontijo CM. Didelphis marsupialis (common opossum): a potential reservoir host for zoonotic leishmaniasis in the metropolitan of Belo Horizonte (Minas Gerais, Brazil) Vector Borne Zoonotic Dis. 2007;7:387–393. doi: 10.1089/vbz.2006.0651. [DOI] [PubMed] [Google Scholar]
- 8.Schonian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, Jaffe CL. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–358. doi: 10.1016/s0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 9.Corredor A, Gallego JF, Tesh RB, Peláez D, Dias A, Montilla M, Palau MT. Didelphis marsupialis, an apparent wild reservoir of Leishmania donovani chagasi in Colombia, South America. Trans R Soc Trop Med Hyg. 1989;83:195. doi: 10.1016/0035-9203(89)90640-8. [DOI] [PubMed] [Google Scholar]
- 10.Travi BL, Jaramillo CD, Montoya J, Segura I, Zea A, Gonçalves A, Vellez ID. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am J Trop Med Hyg. 1994;50:557–565. doi: 10.4269/ajtmh.1994.50.557. [DOI] [PubMed] [Google Scholar]
- 11.Cáceres NC, Monteiro-Filho ELA. Os Marsupiais do Brasil: Biologia, Ecologia e Evolução. Second edition. Campo Grande, Brazil: UFMS; 2006. [Google Scholar]
- 12.Oliveira AG, Galati EA, De Oliveira O, De Oliveira GR, Espindola CE, Dorval ME, Brazil RP. Abundance of Lutzomyia longipalpis (Diptera, Psychodidae, Phlebotominae) and urban transmission of visceral leishmaniasis in Campo Grande, State of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 2006;101:869–874. doi: 10.1590/s0074-02762006000800008. [DOI] [PubMed] [Google Scholar]
- 13.Sherlock IA, Miranda JC, Sadigurski M, Grimaldi G., Jr Natural infection of the opossum Didelphis albiventris (Marsupialia, Didelphidae) with Leishmania donovani, in Brazil. Mem Inst Oswaldo Cruz. 1984;79:511. doi: 10.1590/s0074-02761984000400020. [DOI] [PubMed] [Google Scholar]
- 14.Brandão-Filho SP, Brito ME, Carvalho FG, Ishikawa EA, Cupolillo E, Floeter-Winter L, Shaw JJ. Wild and synanthropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco State, Brazil. Trans R Soc Trop Med Hyg. 2003;97:291–296. doi: 10.1016/s0035-9203(03)90146-5. [DOI] [PubMed] [Google Scholar]
- 15.Furlan MB. Epidemia de leishmaniose visceral no município de Campo Grande-MS, 2002 a 2006. Epidemiol Serv Saúde. 2010;19:15–24. [Google Scholar]