Abstract
Four terpenoid derivatives were examined for their activity against Trypanosoma cruzi. Our results show that two compounds were very active in vitro against both extra- and intracellular forms. These compounds, non-toxic for the host cells, are more effective than the reference drug benznidazole. The capacity to infect cells was negatively affected and the number of amastigotes and trypomastigotes was reduced. A wide range of ultrastructural alterations was found in the epimastigote forms treated with these compounds. Some metabolic changes occurred presumably at the level of succinate and acetate production, perhaps caused by the disturbance of the enzymes involved in sugar metabolism inside the mitochondria. In vivo results were consistent with those observed in vitro. The parasitic load was significantly lower than in the control assay with benznidazole. The effects of these products showed the reduction of the anti-T. cruzi antibodies level during the chronic stage.
Introduction
Tropical and subtropical diseases caused by protozoal parasites remain a major public health problem in many of the less developed countries of the world, because of the lack of effective drugs or increasing resistance against the few affordable drugs available.1 American trypanosomiasis, also known as Chagas disease, is one of the most devastating parasitic diseases. It is caused by the kinetoplastid protozoan Trypanosoma cruzi, which is vectorially transmitted by a hemiptera depositing feces on the skin surface, containing metacyclic forms, after the blood meal. Other pathways of infection include contaminated blood transfusions, organ transplants, oral contamination caused by food/beverage,2,3 and even transmission from mother to child during pregnancy or breastfeeding.4
Chagas disease manifests itself in the form of an acute infection, during which most patients do not know that they are infected. Actually, the acute phase can be asymptomatic but also the Romana's sign can be present in around 15% of the cases. Only a 30–40% of acute individuals undergo further development and it becomes chronic and systemic, severely affecting the heart, esophagus, and colon.5 Endemic throughout Latin America, it is the third most widespread tropical disease after malaria and schistosomiasis, according to the World Health Organization (WHO).5
An estimated 10 million people are infected worldwide, mostly in Latin America where Chagas disease is endemic. More than 25 million people are at risk of the disease. It is estimated that in 2008 Chagas disease killed more than 10,000 people6; unfortunately, current treatment of this disease is very limited, and no successful vaccine has been developed.7
Available drugs are mainly nitroheterocyclic compounds such as nitrofuranenifurtimox or the nitroimidazole derivative benznidazole (BZN), but they have proved effective only against the acute phase, exhibiting very limited efficacy in the chronic stage.8 Furthermore, they are quite toxic, causing severe side effects such as pancreatitis and cardiac toxicity.2 The search for more effective drugs focuses mainly on their potential action over essential and exclusive components of the trypanosomatids.
The use of natural products as new chemotherapeutic agents has been investigated for some time. Among these, terpenoids, an important group of secondary metabolites, must be emphasized, mainly because of their structural diversity and natural abundance. During the last few years trypanocidal activities for some triterpenes9 and diterpenes, with ent-kaurene,10 pimarane,11 and spongiane skeleton,12 have been reported. Recently, the trypanocidal activity of several oxygenated abietane diterpenoids has been described.13 Diterpene resin acids are important defense compounds from conifers against potential herbivores and pathogens.14 The biological activity of natural abietane acids has been reviewed.15 In recent years, interest in these types of terpenoids has increased as a result of the isolation of compounds, mainly phenols and related derivatives, showing remarkable biological activities.16–18 Other significant oxidized abietane diterpenes have shown strong inhibition of various human tumors and oncogen-transformed cells.19 The widespread use of these agents has not yet been established, however, and chemotherapeutic armament against kinetoplastic parasites remains limited. New drug options are clearly needed to fight these pathogens.
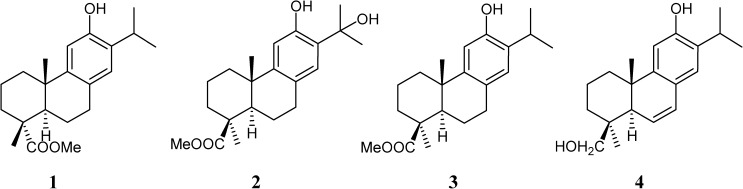
Recently, our group synthesized abietane phenols 1–420,21 (Figure 1), and their antiproliferative in vitro and in vivo activities against T. cruzi (epimastigote, amastigote, and trypomastigote forms) have been investigated in this work. Unspecific mammal cytotoxicity of the most active compounds was evaluated in vitro, and less toxic derivatives have been submitted to in vivo experimentation in a more thorough study. Furthermore, we also included a nuclear magnetic resonance (1H NMR) study concerning the nature and percentage of the excretion metabolites to gain information concerning the inhibitory effect of our compounds over the glycolytic pathway, because it represents the prime source of energy for the parasite. Finally, the effect of compounds on the ultrastructure of T. cruzi is considered the basis of transmission electronic microscopy (TEM) experiments.
Figure 1.
Terpenoid derivatives structure.
Materials and Methods
Chemical compounds.
Compound 1, the methyl ester of 12-hydroxydehydroabietic acid, recently described as a new natural product,22 has been synthesized from commercial abietic acid.20
Compounds 2–4 were prepared from trans-communic acid,21 a labdane diterpene very abundant in some species of Juniperus and Cupressus. Compound 3 is the methyl ester of lambertic acid, isolated from Podocarpus lambertius and compound 4, 6,7-dehydroabieta-8,11,13-trien-12,19-diol, named sugikurojin A, is a new diterpene recently isolated from Cryptomeria japonica. Compound 2, methyl 12,15-dihydroxyabieta-8,11,13-trien-19-oate, has not yet been found in nature.
Parasite strain, culture.
Trypanosoma cruzi SN3 strain of (IRHOD/CO/2008/SN3) was isolated from domestic Rhodnius prolixus and the biological origin is Guajira (Colombia).23
Epimastigote forms were obtained in biphasic blood-agar NNN medium (Novy-Nicolle-McNeal) supplemented with minimal essential medium and 20% inactivated fetal bovine serum and afterwards reseeded in a monophasic culture (MTL), following the method of Luque and others.24
Cell culture and cytotoxicity tests.
Vero cells (Flow) were grown in RPMI (Gibco, Madrid, Spain) supplemented with l0% inactivated fetal bovine serum and adjusted to pH 7.2, in a humidified 95% air-5% CO2 atmosphere at 37°C for 2 days. For the cytotoxicity test, cells were placed in 30 mL sterile polystyrene container (Deltalab, Barcelona, Spain), and centrifuged at 100 g for 5 min. The culture medium was removed, and fresh medium was added to a final concentration of 1×105 cells/mL. This cell suspension was distributed in a culture tray (with 24 wells) at a rate of 100 μL/well and incubated for 2 days at 37°C in humid atmosphere enriched with 5% CO2.
The medium was removed, and the fresh medium was added together with the product to be studied (at concentrations of 100, 50, 25, l0 and 1 μM). After 72 h of treatment, the cell viability was determined by flow cytometry. Thus, 100 μL/well of propidium iodide (PI) solution (100 μg/mL) was added and incubated for 10 min at 28°C in darkness. Afterward, 100 μL/well of fluorescein diacetate (FDA) (100 ng/mL) was added and incubated under the same conditions as above. Finally, the cells were recovered by centrifugation at 400 g for l0 min and the precipitate washed with phosphate buffer solution (PBS). Flow cytometric analysis was performed on a FACS Vantageflow cytometer (Becton Dickinson, Madrid, Spain). The live cells with their plasma membrane intact were associated with the green fluorescence, because of the effect of sterases on FDA. On the other hand, cells that had lost the membrane integrity and were dead allowed the penetration of the IP by passive diffusion and specifically bound to their DNA and then, fluoresce in the range of 580 nm. The percentage of viability was calculated in comparison to that of the control culture (infected but untreated cultures), and the IC50 (the concentration required to give 50% of inhibition) was calculated by linear-regression analysis from the Kc values at the concentrations used.
In vitro trypanocidal activity assay.
Epimastigote assay.
The parasite suspension was obtained for the trypanocidal assay by concentrating the epimastigote culture in the exponential growth phase by centrifugation at 1,000 g for 10 min, whereupon the number of flagellates were counted in a hemocytometric chamber and distributed into aliquots of 5×105 parasites/mL. The compounds were dissolved in dimethyl sulfoxide at a concentration of 0.01%, after being assayed as non-toxic and without inhibitory effects on the parasite growth. The compounds were dissolved in the culture medium, and the dosages used were 100, 50, 25, 10, and 1 μM. After 72 h of incubation, the effect of each compound was evaluated by light microscopy, through the quantification of viable parasite using a Neubauer chamber.
Metacyclic trypomastigotes assay.
Metacyclogenesis was induced by culturing parasites at 28°C in modified Grace's medium (Gibco) for 12 days as described previously.25 Twelve days after cultivation at 28°C, metacyclic forms were counted to infect Vero cells. The proportion of metacyclic forms was around 40% at this stage.
After the metacyclic forms were obtained, Vero cells were cultured in RPMI medium in a humidified 95% air-5% CO2 atmosphere at 37°C. Cells were seeded at a density of 1× 104 cells/well in 24-well microplates (Nunc, Roskilde, Denmark) with rounded coverslips on the bottom and cultivated for 2 days. Afterward, the cells were infected in vitro with metacyclic forms of T. cruzi at a ratio of 10:1. The compounds (IC25 concentrations, concentration required to give 25% of inhibition) were added immediately after infection and were incubated for 12 h at 37°C in a 5% CO2. The non-phagocytosed parasites and the drugs were removed by washing, and the infected cultures were then grown for 10 days in fresh medium without drugs. Fresh culture medium was added every 48 h.
The activity of the compounds was determined from the percentage of infected cells and the number of amastigotes founded per cells infected in treated and untreated cultures in methanol-fixed and Giemsa-stained preparations. The percentage of infected cells and the mean number of amastigotes per infected cell were determined by analyzing more than 100 host cells distributed in randomly chosen microscopic fields. Values are the means of three separate determinations. The number of trypomastigotes in the medium was determined as described previously.24
Axenic amastigotes assay.
Axenic amastigote forms of T. cruzi were cultured following the methodology described previously by Moreno.26 Thus, epimastigotes transformed into amastigotes after 3 days of culture in M199 medium (Invitrogen, Leiden, The Netherlands) supplemented with 10% heat-inactivated FCS, 1 g/L β-alanine, 100 mg/L L- asparagine, 200 mg/L sucrose, 50 mg/L sodium pyruvate, 320 mg/L malic acid, 40 mg/L fumaric acid, 70 mg/L succinic acid, 200 mg/L α-ketoglutaric acid, 300 mg/L citric acid, 1.1 g/L sodium bicarbonate, 5 g/L MES, 0.4 mg/L hemin, and 10 mg/L gentamicin pH 5.4 at 37°C. The effect of each compound against 1× 106 axenic amastigote forms/mL, was tested at 48 h using a Neubauer hemocytometric chamber. The inhibitory effect is expressed as IC50 values.
In vivo trypanocidal activity assay.
This experiment was performed with the permission of the ethical committee of the University of Granada, Spain. Groups of three BALB/c female mice (6 to 8 weeks of age; 25 g) maintained under standard conditions were infected with 1×105 T. cruzi metacyclic forms by the intraperitoneal route. The animals were divided into the following groups: i) group 1: uninfected (not infected and not treated); ii) group 2: untreated (infected with T. cruzi but not treated); iii) group 3: uninfected (not infected and treated: with 1 mg/kg of body weight/day, for five consecutive days (7–11 post-infection) by the intraperitoneal route27; and iv) group 4: treated (infected and treated with 1 mg/kg of body weight/day for five consecutive days (7 to 11 post-infection) by the intraperitoneal route with the compounds and BZN).
Treatments were started 7 days after infection with the parasites. Compounds were administered in a similar way to that explained previously and at the same concentrations.
A blood sample (5 μL) drawn from the mandibular vein of each treated mouse was taken and diluted 1:15 (50 μL of citrate buffer and 20 μL of lysis buffer at pH 7.2). The parasites were counted by fields with the immersion objective. The number of bloodstream T. cruzi metacyclic forms were recorded every 2 days from 7 to 40 days post-infection. The number of metacyclic forms was expressed per 100 microscopic fields.
Circulating anti-T. cruzi antibodies were quantitatively evaluated at Days 40 and 120 post-infection by the use of an enzyme-linked immunoassay. The blood, diluted to 1:50 in PBS, was reacted with an antigen constituted by a soluble Fe-SODe from T. cruzi epimastigotes. The results are expressed as the ratio of the absorbance (Abs) of each serum sample at 490 nm to the cutoff value. The cutoff for each reaction was the mean of the values determined for the negative controls plus three times the standard deviation.28
Metabolite excretion.
Cultures of T. cruzi epimastigotes (initial concentration 5×105 cells/mL) received IC25 of the compounds (except for control cultures). After incubation for 96 h at 28°C, the cells were centrifuged at 400 g for l0 min. The supernatants were collected to determine excreted metabolites by 1H-NMR spectroscopy as previously described.29 The chemical displacements were expressed in parts per million (ppm), using sodium 2,2 dimethyl-2-silapentane-5-sulfonate as the reference signal. The chemical displacements used to identify the respective metabolites were consistent with that described.29
Ultrastructural alterations.
The parasites, at a density of 5×105 cells/mL, were cultured in their corresponding medium, containing the drugs at the IC25 concentration. After 96 h, the cultures were centrifuged at 400 g for 10 min, and the pellets washed in PBS and then fixed with 2% (v/v) p-formaldehyde-glutaraldehyde in 0.05M cacodylate buffer (pH 7.4) for 5 h at 4°C. Pellets were prepared for TEM (Zeiss model, Barcelona, Spain) following the technique of Luque.24
Results
In vitro anti-T. cruzi and cytotoxicity evaluation.
The inhibitory effect of the new terpenoid Compounds 1–4 was measured at concentrations ranging from 1 to 100 μM on the in vitro growth of. T. cruzi epimastigotes.30 The IC50 values registered after 72 h of exposure are shown in Table 1, including BZN as the reference drug. The trypanocidal activity of the derivatives was slightly higher (Compounds 1 and 2, IC50 6.10 and 7.98 μM, respectively) or even slightly less (Compounds 3 and 4, IC50 50.03 and 69.01 μM) with respect to that found for BZN (15.89 μM).
Table 1.
In vitro activity, toxicity, and selectivity index found for the terpenoids derivatives on epimastigote and axenic-amastigote forms of Trypanosoma cruzi*
| Compound | Activity IC50 (mM)† | Toxicity on Vero cell IC50 (mM)‡ | SI§ | ||
|---|---|---|---|---|---|
| Epimastigote forms | Axenic amastigote forms | Epimastigote forms | Axenic amastigote forms | ||
| Benznidazole | 15.89 ± 1.1 | 18.92 ± 1.1 | 13.60 ± 0.9 | 0.8 | 0.7 |
| Compound 1 | 6.10 ± 0.2 | 6.03 ± 0.3 | 98.95 ± 6.2 | 16.2 (20) | 16.4 (23) |
| Compound 2 | 7.98 ± 0.8 | 6.81 ± 0.1 | 154.66 ± 17.3 | 19.4 (24) | 22.7 (32) |
| Compound 3 | 50.03 ± 6.6 | 30.44 ± 2.3 | 117.67 ± 21.8 | 2.4 (3) | 3.9 (6) |
| Compound 4 | 69.01 ± 7.7 | 38.72 ± 3.6 | 163.14 ± 14.5 | 2.4 (3) | 4.2 (6) |
Results are averages of three separate determinations.
IC50 = the concentration required to give 50% inhibition, calculated by linear regression analysis from the Kc values at concentrations used (1, 10, 25, 50, and 100 μM).
On Vero cells after 72 h of culture. IC50 = the concentration required to give 50% inhibition, calculated by linear regression analysis from the Kc values at concentrations used (1, 10, 25, 50 and 100 μM).
Selectivity index = IC50 Vero cells/IC50 epimastigote and axenic amastigote forms of the parasite. In brackets: number of times that compound SI exceeds the reference drug SI.
The cytotoxicity evaluation against mammalian cells by using Vero cells as the mammal-cell model (Table 1) showed that the four derivatives were much less toxic than BZN. On the other hand, the selectivity index calculated for the derivatives was about 20-fold (Compound 1) and 24-fold (Compound 2) higher than that of BZN. The SI values found for Compounds 3 and 4 were significantly lower than those found for Analogues 1 and 2.
Axenic amastigotes obtained following the technique described in the Materials and Methods section, were assayed to determine the IC50 against the four terpenoids, using BZN as the reference drug (Table 1). Compounds 1 and 2 proved the most effective with IC50 of 6.03 and 6.81 μM, respectively. When the SI was determined for Compound 2, it was 32-fold better than the reference drug, whereas Compound 1 was 23-fold better that the BZN again.
Compounds 3 and 4 did not reach values of SI ≥ 20-fold and therefore these compounds were not included in the subsequent studies.31
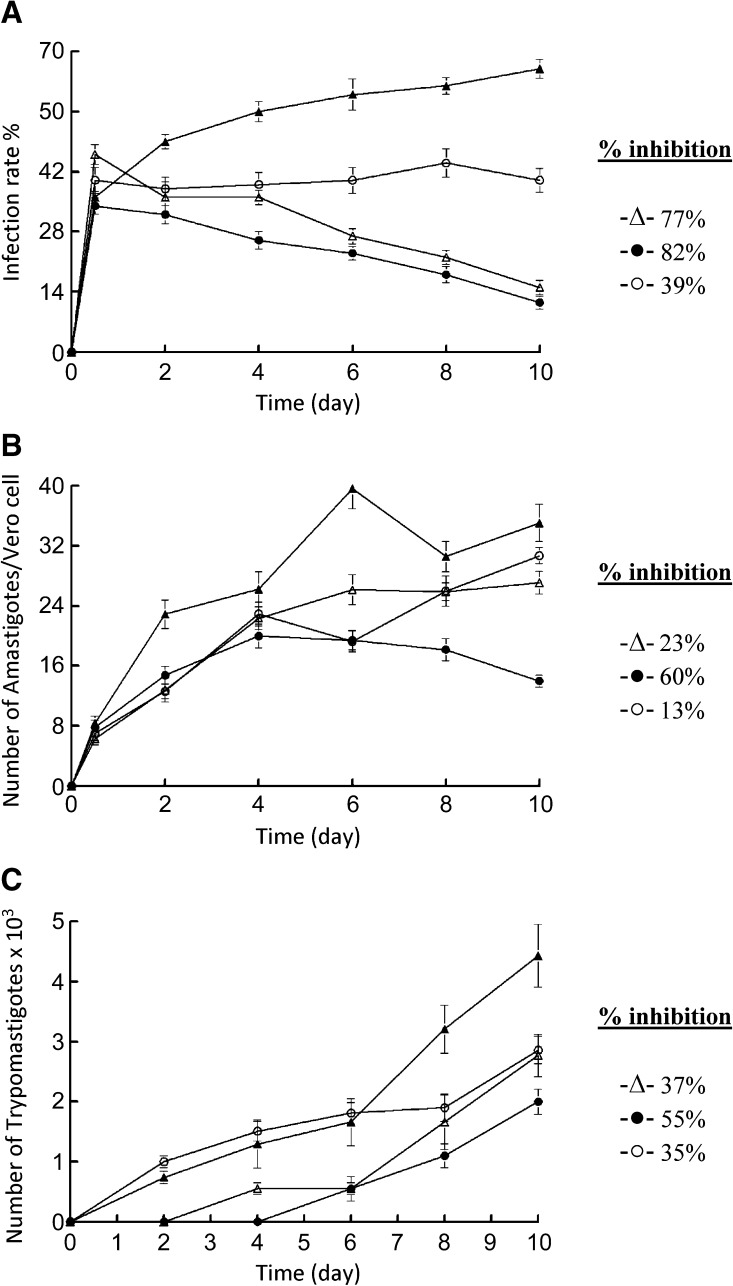
Because the metacyclic trypomastigote forms are responsible for the infection of the mammalian host, Compounds 1 and 2 were assayed against the metacyclic forms to evaluate the infection rate. When 1×104 Vero cells were incubated for 2 days and then infected with 1×105 metacyclic forms, obtained in the way described in the Materials and Methods section (control experiment; Figure 2A), the parasites invaded the cells and underwent the morphological transformation to amastigotes within 1 day after infection, following the parasite's normal life cycle. On Day 10, the rate of host-cell infection peaked. When Compounds 1 or 2 were added to the infected Vero cells with T. cruzi metacyclic forms (IC25 concentration), the infection rate significantly decreased with respect to the control, reaching 77% (Compound 1) and 82% (Compound 2) on Day 10 of the experiment. The infection-rate decline found using the compounds tested was substantially more pronounced than that measured for BZN (39%). Furthermore, another indication of the effectiveness of the infection-rate decrease was the average number of amastigotes per infected cell (Figure 2B) increased to 39.6 amastigotes/cell in the control experiment on Day 6, decreased to a value of 30.6 on Day 8, and increased again to 35.0 on Day 10. The break-up of Vero cells implies the transformation of amastigotes into trypomastigotes and release to spread the infection. Therefore, the variation of the trypomastigote number in the culture medium was also measured (Figure 2C) as a third way to test whether the infection rate was initially affected as a consequence of the exposure to the compounds. The control experiment afforded a trypomastigote number of 4.4×103 on Day 10, and reductions of 37% and 55% are found for Compounds 1 and 2, respectively. The reduction was higher for Compound 2 than that found for BZN (35%).
Figure 2.
Effect of activity of terpenoid compounds on the infection rate and T. cruzi growth. (A) Rate of infection. (B) Mean number of amastigotes per infected Vero cell. (C) Number of trypomastigotes in the culture medium. Control (-▴-); benznidazole (BZN) (-○-); Compound 1 (-▵-) and Compound 2 (-●-) (at IC25 conc.). The values are means of three separate experiments.
In vivo anti-T. cruzi evaluation.
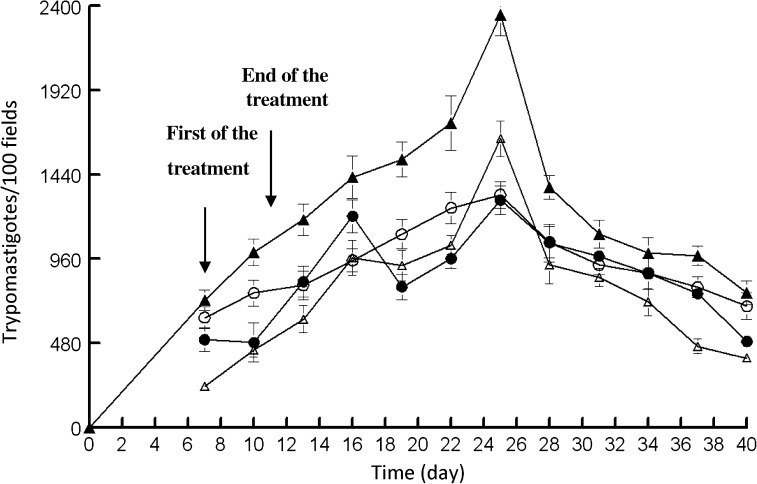
The good results obtained in vitro with Compound 1 and 2 prompted us to study their in vivo activity in mice. Their impact on the two significant stages of Chagas disease was evaluated: the acute phase, considered until 60 days post-infection, and the chronic phase, from 60 days post-infection. It has been published that the intravenous doping route results in high mortality rates,27 and therefore we used the intraperitoneal route, using a concentration of 5 mg/kg,32 which did not result in any animal mortality. Female Swiss mice were inoculated intraperitoneally with 1×105 metacyclic trypomastigotes, and treatment began 7 days post-infection with the intraperitoneally route of 1 mg/kg/day of each compound for 5 days. Administration was performed using a saline solution. A group treated in the same manner with the vehicle (control) was included. During the study of the acute-phase activity, the level of parasitemia was determined every 2 days (Figure 3).
Figure 3.
Parasitemia in the murine model of acute Chagas disease. Control (-▴-) and dose receiving 5 mg/kg of: Benznidazole (BZN) (-○-); Compound 1 (-▵-) and Compound 2 (-●-). The values are means of three separate experiments.
None of the animals treated with Compounds 1 and 2 died during the treatment, whereas the surviving percentage of the mice treated with BZN was 80%.
The data represented in Figure 3 show that the two compounds tested diminished the trypomastigote number on the day of maximum parasitic load, which was Day 25 post-infection, with respect to the control with untreated mice. On Day 40 post-infection a reduction of the parasitemia was found for the two compounds. From these data, the following order for in vivo activity could be established: Compound 1 ≈ Compound 2 > BZN.
Concerning the activity in the chronic phase, serological tests were performed 40 and 120 days post-infection (Table 2). Compounds 1 and 2 decreased antibody levels between Days 40 and 120, showing higher performance than did BNZ in this assay.
Table 2.
Differences in the level of anti-Trypanosoma cruzi antibodies between Days 40 and 120 post-infection for Compounds 1 and 2 and benznidazole (BZN), expressed in absorbance units (abs)
| Compounds* | ΔA† |
|---|---|
| Control (untreated) | 0,206 ± 0.07 |
| BZN | 0,116 ± 0.03 |
| Compound 1 | −0.237 ± 0.01 |
| Compound 2 | −0.005 ± 0.00 |
1 mg/kg/day, intraperitoneal route administered during 5 days (see Material and Methods).
▵A = absorbance at 490 nm, Day 120 p.i. absorbance at 490 nm, day 40 p.i.
Studies on the action mechanism.
To gain information on the possible mechanism of action of Compounds 1 and 2 on the parasite, we performed the following experiments:
Metabolite-excretion effect.
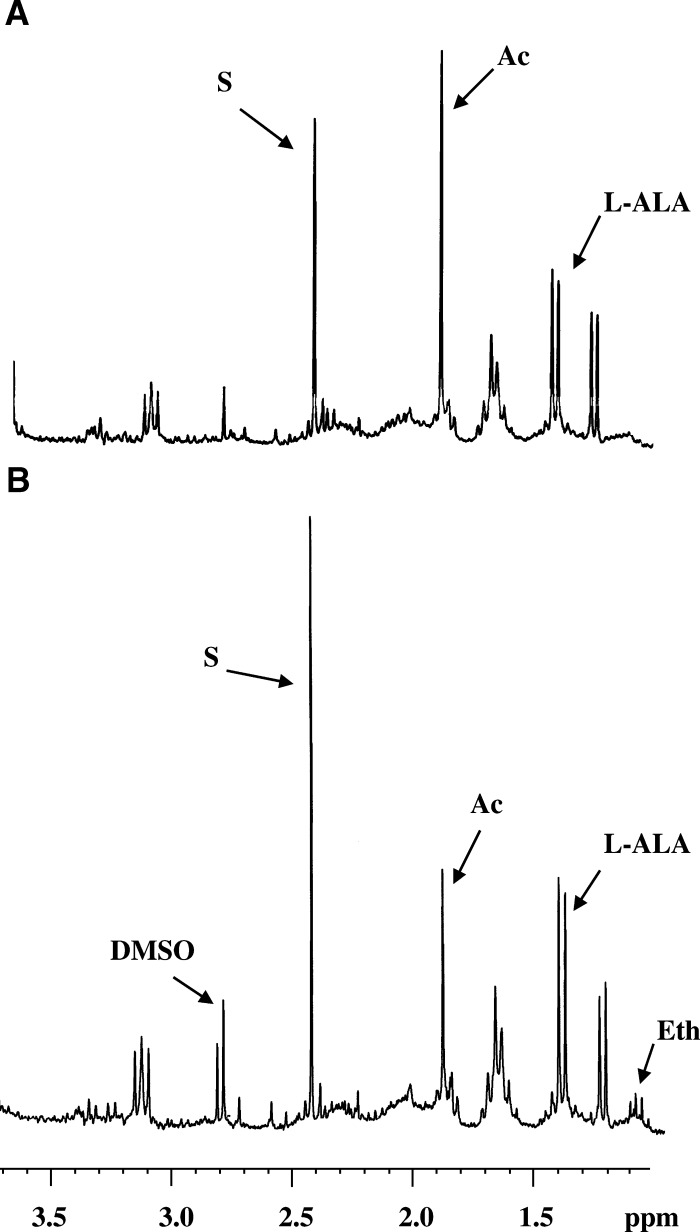
For information concerning the effect of Compounds 1 and 2 on metabolite excretion, the 1H-NMR spectra were registered. As a control, a culture of parasites untreated was used (Figure 4A). In this control experiment, T. cruzi excreted acetate and succinate as the major metabolites and, in a lower percentage, L-alanine. These data agree with those reported previously.33 When the trypanosomatids were treated with Compound 2, the excretion of some of these catabolites (mainly acetate) was clearly disturbed (Figure 4B) at the dosages assayed (IC25). Similarly, succinate, L-alanine and ethanol levels rose in comparison with the control experiment.
Figure 4.
Nuclear magnetic resonance (1H-NMR) spectra epimastigote forms of Trypanosoma cruzi treated against terpenoids compounds (at a concentration of IC25): (A) Control (untreated) and (B) Compound 2. Ala = L-alanine; Ac = acetate; S = succinate; Eth = ethanol, and DMSO = Dimethyl sulfoxide.
Compound 1 provoked similar effects, although at a lesser magnitude (spectra not shown). The BZN did not trigger any alteration in the energy metabolism of the parasites (spectra not shown).
Ultrastructural alterations.
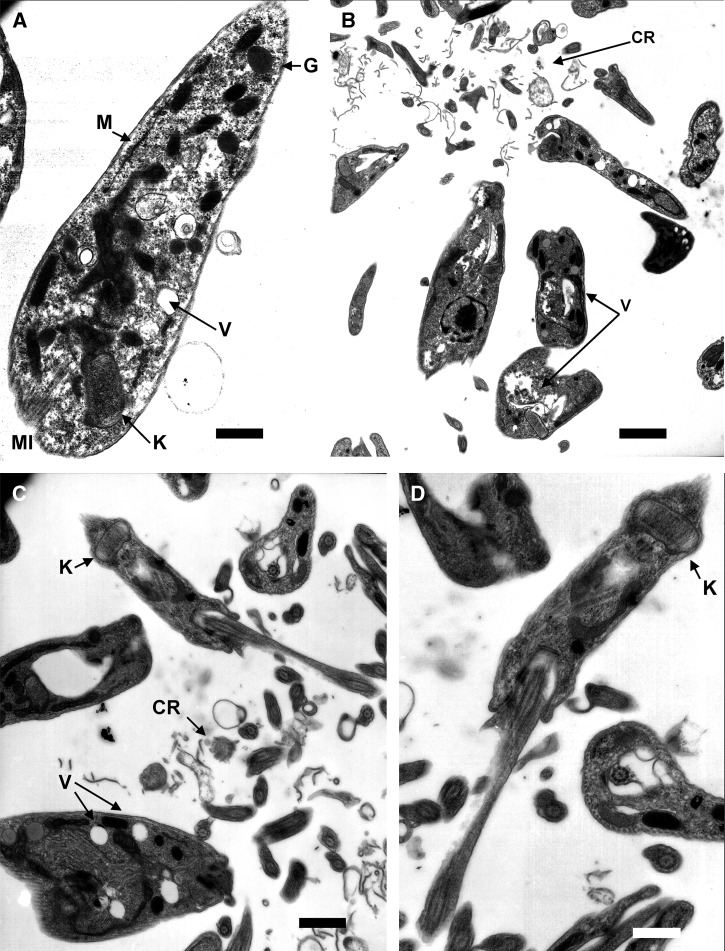
The study of the ultrastructural alterations caused by Compounds 1 and 2 in the T. cruzi epimastigotes reflected notable changes in parasites, as reflected in (Figure 5B, C, and D), with respect to the control cultures (Figure 5A). Both compounds induced ultrastructural alterations. The most evident changes were the destruction of the treated parasites. Furthermore, there was evident intense vacuolization in a high number of parasites. Compound 2 triggered the dilatation of the kinetoplast of some parasites (Figure 5D).
Figure 5.
Ultrastructural alterations by transmission electron microscopy (TEM) in Trypanosoma cruzi treated with terpenoids compounds. (A) Control parasite of T. cruzi with structures as vacuoles (V) and mitochondrion (M), kinetoplast (K), glycosomes (G), and microtubules (MI) (Bar: 583 μm). (B) Epimastigotes of T. cruzi treated with Compound 1 with cellular debris from dead parasites (CR) and vacuoles (V) (Bar: 1.59 μm). (C and D) Epimastigotes of T. cruzi treated with Compound 2 with cellular rest (CR), vacuoles (V), and swelling kinetoplast (K) (Bar: 1.00 μm and Bar: 583 μm, respectively).
Discussion
In most studies on activity assays of new compounds against parasites, forms that develop in invertebrate host are used because they are easier to handle in vitro, but a preliminary test using extracellular epimastigote forms should always be complemented by a subsequent evaluation using intracellular forms (amastigotes in vertebrate host cells) for a better understanding of the activity results found. For this reason, we studied the activity of axenic amastigote forms.
The data on the axenic amastigotes match those found for the extracellular forms, where Compounds 1 and 2 were the most effective.
The trypanocidal effect of terpenoid compounds against epimastigote and amastigote forms, has been previously shown. Cassane diterpenes isolated from leaves of Myrospermum frutescens, have anti-T. cruzi activity against these forms.34
According to the activity criteria for the development of new drugs,31 only the compounds that SI ≥≥ 20-fold were included in the following studies.
The assays showed that Compounds 1 and 2 were effective enough to undergo the next stage. To study the drug's effect on the infective parasite forms, we performed the metacyclic assay. The IC25 of each product was used as the test dosage. This concentration was chosen for being harmful but not totally lethal,35 revealing that Compound 2 was the most effective.
Diterpenes isolated from Aristolochia cymbifera have been shown to be selective against trypomastigotes.36
The in vivo assays showed that the differences in the level of anti-T. cruzi antibodies are consistent with the parasitemia findings.
As far as is known, no trypanosomatid studied to date is capable of completely degrading glucose to CO2 under aerobic conditions, and thus these parasites excrete into the medium a great part of their carbon skeleton as fermented metabolites, which differ depending on the species considered.37,38 Trypanosoma cruzi consumes glucose at a high rate, thereby acidifying the culture medium caused by incomplete oxidation to acids. The 1H-NMR spectra enables us to determine the fermented metabolites excreted by the trypanosomatid during in vitro culture. One of the major metabolites excreted by T. cruzi is succinate, the main role of which is probably to maintain the glycosomal redox balance, by providing two glycosomal oxidoreductase enzymes that allow reoxidation of NADH, produced by glyceraldehydes-3-phosphate dehydrogenase in the glycolytic pathway. Succinic fermentation offers the significant advantage of requiring only half of the phosphoenolpyruvate produced to maintain the NAD+/NADH balance. The remaining phosphoenolpyruvate is converted into acetate, L-lactate, L-alanine, and/or ethanol, depending on the species considered. The role of the acetate is probably to maintain the glycosomal redox balance. In the case of Compound 2 the inhibition of acetate excretion explains the observed increase in succinate, L-alanine, and ethanol production, probably because these compounds act over any level in the energy metabolism, still unknown.
In conclusion, our results show that four new terpenoid derivative compounds were active in vitro against both extra- and intracellular forms of T. cruzi (in the order 2 > 1 > 3 > 4 > BZN). These compounds are not toxic for the host cells and are effective at concentrations lower than the reference drug used in this study. The in vitro growth rate of T. cruzi was reduced, its capacity to infect cells was negatively affected, and the multiplication of the amastigotes and subsequent transformation into trypomastigotes was greatly lowered. Moreover, a wide range of ultrastructural alterations in epimastigote forms of T. cruzi treated with these new terpenoid compounds were found. These alterations mainly at the mitochondria level could explain the metabolic changes in the productions of succinate and acetate, which may be caused by the disturbance of the enzymes involved in sugar metabolism within the mitochondria. The in vivo studies revealed results that were consistent with those observed in vitro. On the one hand, during the treatment of mice with the compounds (Compounds 1 and 2), no signs of toxicity were observed. On the other hand, the parasitic load was significantly decreased in comparison with the reference drug. The effects of these products were also demonstrated with the anti-T. cruzi antibody level modification during the chronic stage. So far nothing is known about the structure-activity relationship for these substances, and the results described here do not allow it. Additional research is needed for the activity of other structurally related molecules. These results support further research of terpenoid compounds as potential agents against Chagas disease. The synthesis of new derivatives and preclinical studies, such as doses, schedule, strains, and toxicological studies, is currently in progress.
ACKNOWLEDGMENTS
We thank the Proyecto de Excelencia de la Junta de Andalucía Project (P07-FQM-03101) and MEC (Spain) (CGL2008-03687-E/BOS), for financial support. We are also grateful to the services of transmission-electron microscopy, flow cytometry, and nuclear magnetic resonance spectroscopy of the CIC-University of Granada.
Footnotes
Authors' addresses: Inmaculada Ramírez-Macías, Clotilde Marín, Francisco Olmo, María José Rosales, and Manuel Sánchez-Moreno, Department of Parasitology, University of Granada, Granada, Spain, E-mails: iramirezm@ugr.es, cmaris@ugr.es, folmoarevalo@ugr.es, mjrosale@ugr.es, and msanchem@ugr.es. Rachid Chahboun, Ibtissam Messouri, and Enrique Alvarez-Manzaneda, Department of Organic Chemistry, Institute of Biothenology, University of Granada, Granada, Spain. E-mails: spr@ugr.es, imessouri@yahoo.fr, and eamr@ugr.es. Ramòn Gutierrez-Sánchez, Department of Statistics, University of Granada, Granada, Spain, E-mail: ramongs@ugr.es.
References
- 1.Souza W. Chagas' disease: facts and reality. Microbes Infect. 2007;9:544–545. doi: 10.1016/j.micinf.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104:41–45. doi: 10.1590/s0074-02762009000900007. [DOI] [PubMed] [Google Scholar]
- 3.Kribs-Zaleta CM. Alternative transmission modes for Trypanosoma cruzi. Math Biosci Eng. 2010;7:657–673. doi: 10.3934/mbe.2010.7.657. [DOI] [PubMed] [Google Scholar]
- 4.Brener Z, Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Appl Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 5.Strosberg AM, Barrio K, Stinger VH, Tashker J, Wilbur JC, Wilson L, Woo K. Chagas Disease: A Latin Nemesis. San Francisco, CA: Institute for OneWorld Health; 2007. [Google Scholar]
- 6.World Health Organization Chagas disease (American trypanosomiasis) 2010. http://www.who.int/mediacentre/factsheets/fs340/en/index.html#. Available at. Accessed January 2010. [PMC free article] [PubMed]
- 7.Croft S, Barrett M, Urbina J. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 2005;21:508–512. doi: 10.1016/j.pt.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Hinojosa-Valdez R, Düsman-Tonin LT, Ueda-Nakamura T, Dias-Filho BP, Morgado-Diaz A, Sarragiotto MH, Vataru-Nakamura C. Biological activity of 1,2,3,4-tetrahydro-β-carboline-3-carboxamides against Trypanosoma cruzi. Acta Trop. 2009;110:7–14. doi: 10.1016/j.actatropica.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Leite JP, Oliveira AB, Lombardi JA, Filho JD, Chair E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol Pharm Bull. 2006;29:2307–2309. doi: 10.1248/bpb.29.2307. [DOI] [PubMed] [Google Scholar]
- 10.do Nascimento AM, Chaves JS, Albuquerque S, de Oliveira DC. Trypanocidal properties of Mikania stipulacea and Mikania hoehnei isolated terpenoids. Fitoterapia. 2004;75:381–384. doi: 10.1016/j.fitote.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Ambrósio SR, Arakawa NS, Esperandim VR, de Albuquerque S, Da Costa FB. Trypanocidal activity of pimarane diterpenes from Viguiera arenaria (Asteraceae) Phytother Res. 2008;22:1413–1415. doi: 10.1002/ptr.2512. [DOI] [PubMed] [Google Scholar]
- 12.Orhan I, Şener B, Kaiser M, Brun R, Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera JC, Troncone G, Henriquez D, Urdaneta N. Trypanocidal activity of abietane diterpenoids from the roots of Craniolaria annua. Z Naturforsch C. 2008;63:821–829. doi: 10.1515/znc-2008-11-1207. [DOI] [PubMed] [Google Scholar]
- 14.Martin D, Tholl D, Gershenzon J, Bohlmann J. Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol. 2002;129:1003–1018. doi: 10.1104/pp.011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San Feliciano A, Gordaliza M, Salinero MA, Miguel del Corral JM. Abietane acids: sources, biological activities, and therapeutic uses. Planta Med. 1993;59:485–490. doi: 10.1055/s-2006-959744. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Yang L, Jia ZJ. A new eremophilane sesquiterpenoid and a new iridoid from Pedicularis striata subsp arachnoides. Planta Med. 1997;63:248–250. doi: 10.1055/s-2006-957664. [DOI] [PubMed] [Google Scholar]
- 17.Marrero JG, Andres LS, Luis JG. Semisynthesis of rosmanol and its derivatives. Easy access to abietatriene diterpenes isolated from the genus Salvia with biological activities. J Nat Prod. 2002;65:986–989. doi: 10.1021/np010565o. [DOI] [PubMed] [Google Scholar]
- 18.Tan N, Kaloga M, Radtke OA, Kiderlen AF, Oksuz S, Ulubelen A, Kolodziej H. Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry. 2002;6:881–884. doi: 10.1016/s0031-9422(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 19.Son KH, Oh HM, Choi SK, Han DC, Kwon BM. Anti-tumor abietane diterpenes from the cones of Sequoia sempervirens. Bioorg Med Chem Lett. 2005;15:2019–2021. doi: 10.1016/j.bmcl.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Manzaneda E, Chahboun R, Cabrera E, Alvarez E, Alvarez-Manzaneda R, Lachkar M, Messouri I. First synthesis of picealactone C. A new route toward taxodione-related terpenoids from abietic acid. Tetrahedron Lett. 2007;48:989–992. [Google Scholar]
- 21.Alvarez-Manzaneda E, Chahboun R, Cabrera E, Alvarez E, Alvarez-Manzaneda R, Lachkar M, Messouri I. Synthesis of phenol abietane diterpenes based on the oxidative radical cyclization utilizing the Mn(OAc)3/Ac2O System. Synlett. 2007;15:2425–2429. [Google Scholar]
- 22.Kinouchi Y, Ohtsu H, Tokuda H, Nishino H, Matsunaga S, Tanaka R. Potential antitumor-promoting diterpenoids from the stem bark of Piceaglehni. J Nat Prod. 2000;63:817–820. doi: 10.1021/np0000217. [DOI] [PubMed] [Google Scholar]
- 23.Téllez-Meneses J, Mejía-Jaramillo AM, Triana-Chávez O. Biological characterization of Trypanosoma cruzi stocks from domestic and sylvatic vectors in Sierra Nevada of Santa Marta, Colombia. Acta Trop. 2008;108:26–34. doi: 10.1016/j.actatropica.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Luque F, Fernández-Ramos C, Entrala E, Rosales MJ, Navarro JA, Romero MA, Salas-Peregrín JM, Sánchez-Moreno M. In vitro evaluation of newly synthesized [1,2,4]triazolo[1,5-a]pyrimidine derivatives against Trypanosoma cruzi, Leishmania donovani and Phytomonas staheli. Comp Biochem Physiol. 2000;126:39–44. doi: 10.1016/s0742-8413(00)00093-1. [DOI] [PubMed] [Google Scholar]
- 25.Osuna A, Adroher FJ, Lupiañez JA. Influence of electrolytes and non-electrolytes on growth and differentiation of Trypanosoma cruzi. Cell Differ Dev. 1990;30:89–95. doi: 10.1016/0922-3371(90)90077-a. [DOI] [PubMed] [Google Scholar]
- 26.Moreno D, Plano D, Baquedano Y, Jiménez-Ruiz A, Palop JA, Sanmartín C. Antileishmanial activity of imidothiocarbamates and imidoselenocarbamates. Parasitol Res. 2011;108:233–239. doi: 10.1007/s00436-010-2073-x. [DOI] [PubMed] [Google Scholar]
- 27.da Silva CF, Batista MM, Batista DG, de Souza EM, da Silva PB, de Oliveira GM, Meuser AS, Shareef AR, Boykin DW, Soeiro MN. In vitro and in vivo studies of the trypanocidal activity of a diarylthiophenediamidine against Trypanosoma cruzi. Antimicrob Agents Chemother. 2008;9:3307–3314. doi: 10.1128/AAC.00038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado C, Marin C, Olmo F, Huertas O, Quiros M, Sánchez-Moreno M, Rosales MJ, Salas JM. In vitro and in vivo trypanocidal evaluation of nickel complexes with an azapurine derivative against Trypanosoma cruzi. J Med Chem. 2010;53:6964–6972. doi: 10.1021/jm100581z. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Becerra C, Sánchez-Moreno M, Osuna A, Opperdoes FR. Comparative aspects of energy metabolism in plant trypanosomatids. J Eukaryot Microbiol. 1997;44:523–529. [Google Scholar]
- 30.Porcal W, Hernandez P, Aguirre G, Boiani L, Boiani M, Merlino A, Ferreira A, Di Maio R, Castro A, Gonzalez M, Cerecetto H. Second generation of 5-ethenylbenzofuroxan derivatives as inhibitors of Trypanosoma cruzi growth: synthesis, biological evaluation, and structure-activity relationships. Bioorg Med Chem. 2007;15:2768–2781. doi: 10.1016/j.bmc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Natl Rev. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 32.Sülsen VP, Frank FM, Cazorla SI, Anesini CA, Malchiodi EL, Freixa B, Vila R, Muschietti LV, Martino VS. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) Antimicrob Agents Chemother. 2008;52:2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turrens J. More differences in energy metabolism between Trypanosomatidae. Parasitol Today. 1999;15:346–348. doi: 10.1016/s0169-4758(99)01479-9. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza DT, Ureña González LD, Ortega-Barría E, Capson TL, Rios LC. Five new cassane diterpenes from Myrospermum frutescens with activity against Trypanosoma cruzi. J Nat Prod. 2003;66:928–932. doi: 10.1021/np030010o. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Moreno M, Sanz AM, Gomez-Contreras F, Navarro P, Marín C, Ramírez-Macias I, Rosales MJ, Olmo F, Garcia-Aranda I, Campayo L, Cano C, Arrebola F, Yunta MJ. In vivo trypanosomicidal activity of imidazole- or pyrazole-based benzo[g]phthalazine derivatives against acute and chronic phases of Chagas disease. J Med Chem. 2011;54:970–979. doi: 10.1021/jm101198k. [DOI] [PubMed] [Google Scholar]
- 36.Sartorelli P, Carvalho CS, Reimão JQ, Lorenzi H, Tempone AG. Antitrypanosomal activity of a diterpene and lignans isolated from Aristolochia cymbifera. Planta Med. 2010;76:1454–1456. doi: 10.1055/s-0029-1240952. [DOI] [PubMed] [Google Scholar]
- 37.Bringaud F, Riviere L, Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. 2006;149:1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Ginger M. Trypanosomatid biology and euglenozoan evolution: new insights and shifting paradigms revealed through genome sequencing. Protist. 2005;156:377–392. doi: 10.1016/j.protis.2005.10.001. [DOI] [PubMed] [Google Scholar]