Abstract
An important variable in determining the vectorial capacity of malaria mosquito species is the degree of mosquito–human contact. This parameter can be affected by community sleeping behavior and the host-feeding habits of vectors. A cross-sectional study of 775 randomly selected inhabitants, including 385 Baluchi residents and 390 Afghani refugees, was conducted in a malarious area in Sabaz District, Sistan-Baluchestan Province, southeastern Iran. In addition, monitoring of human landing periodicity of main malaria vectors was carried out during an entire transmission season. Afghanis and Baluchis showed diversity in sleeping behavior. Most (79.6%) respondents were familiar with symptoms of malaria and also aware of an association between mosquitoes and malaria. Despite this familiarity, 94.6% of Afghan refugees, 74.8% of Baluch residents, and 87.2% of study participants did not use self-protection preventive measures. Overall, only 8.8% of participants reported using bed nets regularly. Surveyed persons used bed nets mainly during second quarter of night. Three major species of malaria vectors (Anopheles culicifacies, An. fluviatilis, and An. stephensi) started biting by sunset and continued throughout the night. The results of present study indicated that synchronization of encounters between inhabitants and mosquito vectors was caused by poor self-protection and sleeping behavior of inhabitants. In addition, diversity in culture and behavior of the two communities may cause the prevalence of malaria to be different between them. Therefore, promoting awareness of self-protection against mosquito bites could promote community participation in malaria elimination program in this malaria-endemic region.
Introduction
Prevention of mosquito-borne disease through better knowledge and awareness is an appropriate way to keep control diseases. In addition, knowledge of feeding patterns on hosts and resting behavior of mosquito vectors is important to understand the host–vector relationship and the dynamics of disease transmission, and to develop control strategies.1 However, the intensity of transmission of mosquito-borne diseases in an area depends on the degree of human–vector contact.2–4 In addition, variations in the biting rhythm of different species of mosquito vector influence transmission of disease. It has been suggested that mosquito species showed an aggregated feeding pattern, which indicated that most mosquito species shared at least a human host.5 This aggregated pattern could arise from host choice based on host availability as reported.5,6 However, synchronization of mosquito vectors activities and human hosts can promote emergence of mosquito-borne diseases, such as malaria, in any area. Therefore, it is essential to understand the transmission potential of the vector species through time. In addition, information on biting periodicity will be useful to design personal protection measures.
Although great advances in knowledge of blood-feeding patterns have been reported for selected mosquito vector species, little is known about the role of community sleeping behavior in determining such patterns. In addition, in many malarious areas, the socioeconomic condition of the community has a direct impact on the risk of malaria infection.7
Bed nets as mechanical barriers offer limited blood resources to mosquitoes but feeding on human blood still influence by synchronization in the encounter of human host and mosquito-feeding behavior.1 Moreover, recent evidence suggests behavioral changes by malaria mosquito populations to avoid contact with insecticide-treated bed nets, by changing either the feeding places or the time early in the evening. Such changes can drastically compromise the level of personal protection.8
With this background, this study was conducted to compare concurrency of mosquito biting behavior and bed net use among two communities, Baluchi residents and Afghani refugees, in a malarious area in Nickshahr Region, Sarbaz District, Baluchestan Province in southeastern Iran. Most Afghanis moved to Baluchestan because of domestic war and this affected the socioeconomic status of this border region.9
In the past five years, 15,000–25,000 malaria cases have been reported in Iran annually. More than 50% of them occurred in Baluchestan, which constitutes less than 2.5% of the country's population. It is estimated that approximately 10% of the population of this province are Afghani refugees and 25–36% of malaria cases per year are reported among them.10 Generally, the current annual parasite index is 5.5 per 1,000 inhabitants in the malaria-endemic area.11 Plasmodium vivax is responsible for 77.5% of malaria cases in this area.12 The malaria vectors in Baluchestan are Anopheles stephensi, An. culicifacies, and An. fluviatilis.13,14 At the present time, vector control activities in the area are mainly restricted to indoor residual spraying in selected malaria-endemic localities by using pyrethroids, and larviciding with Bacillus thuringiensis.10 Several factors, such as insecticide resistance among vectors,15 parasite drug resistance16 and socioeconomical problems,11 have made malaria elimination difficult in this area.
Recently, Iran has been earmarked for malaria elimination and self-protection by using bed nets. Thus, a cross-sectional study was performed among two ethnic groups, Baluchis and Afghanis, which have different behavior patterns influenced by their distinctive cultures. These groups were interviewed to evaluate their knowledge, attitudes on mosquito bite prevention, sleeping behavior, and self-protection against mosquito biting. In addition, monitoring of human landing periodicity of main malaria vectors was carried out for one malaria transmission season. The information generated by the current study might help in designing and evaluating a malaria elimination program in Baluchestan.
Materials and Methods
Study area.
This study was conducted in the Nickshahr Area during March 2007–February 2008. The area is in Sarbaz District in Sistan-Baluchestan Province. It is a malaria-endemic area with a high risk of transmission and is located at 27°50′–26°45′N and 56°00′–61°60′E. The area is composed of mountainous and hilly regions, and villages are usually located in valleys near rivers. Most agricultural activities are concentrated in these valleys.
The temperature reaches a maximum of 35°C in the highlands and 45°C in the plains during the summer, but it rarely decreases below 5–10°C in both areas during the winter. Annual rainfall ranges from 80 to 100 mm. Rainfall occurs mainly during the summer and fall because of monsoon winds. Features contributing to perennial transmission in the areas are mainly caused by population movement (Baluchis and Afghanis), especially at border crossings, efficient vectors with a high anthropophilic index and frequent human biting habits, and various degrees of resistance to insecticides and drugs. The population of Sarbaz district is estimated to be approximately 132,000 and officially approximately 10,500 Afghani refugees lived in the area during this study.
Sampling technique.
We performed a cross-sectional study. Ten percent of fever cases clinically suspected of having malaria who attended to malaria clinics in Sarbaz Health Centers were selected by systematic random sampling based on number of clinical records. Because children and teenagers in ethnic groups might not be able to respond correctly to the questions, only patients greater than 15 years of age were interviewed.
Selected persons were interviewed by malaria clinic staff member to survey knowledge, attitude, and behavior about malaria, sleeping, and self-protection against mosquito biting and malaria transmission. Structured interview forms were obtained from participants. The structure interview form was explained to local staff in advance. The completed forms and records were then checked and collected by technicians at the Health Research Center of Iranshahr (School of Public Health and Institute of Public Health, Tehran University of Medical Sciences).
Data collection: methods, instruments used, and measurements.
Structure questions consisted of open-ended and closed-ended questions and were grouped into sections, including participants details, sleeping behavior, bed time, mobility, self-protection, history of malaria infection and treatment used, facilities, and access to health services. The questionnaire was written in Farsi, and a few questions were expanded and divided into two questions to make them more understandable by local persons. Interviews were conducted in the native language and dialects by interviewers. The validity of questions was checked in the Statistics Department, School of Public Health, and Tehran University of Medical Sciences. Data were analyzed under supervision of the Statistics Department, School of Public Health. Ethical approval for the study was obtained from the Tehran University of Medical Sciences Research Ethics Committee. Informed consent was obtained from all participants. Anonymity was assured to participants, and they were informed that audio tapes and transcripts would not have identifiable features and would be kept in a secure location. Ethical approval was also provided by Tehran University of Medical Sciences and the Iranian Ministry of Health and Education.
Data management and analysis.
Data were entered and processed by using SPSS version 11.5 for Windows (SPSS, Inc., Chicago, IL) and Microsoft (Redmond, WA) Excel. Dependent variables, including knowledge and practices regarding symptom, cause, and self-protection, and independent variables regarding chosen indoor and outdoor places for sleep were measured by using the chi-square test.
Mosquito collection (mosquito landing catches).
Blood feeding activity of malaria vectors was estimated by using human landing catches on two baits in a fixed catching station in a rice irrigation area in the Nikshahr Region in the Sarbaz District. The area had no interventions for mosquito control during time of the study. The survey was carried out twice a month during seasonal activity of mosquitoes (March 10–July 20). Human landing catch was carried out continuously during the entire night from sunset to sunrise (7:00pm–6:00am) by trained staff using mouth aspirators. Two human volunteers of acted as baits while wearing usual clothing. Exposed body surfaces were searched and mosquitoes that were attempting to bite were collected by insect collectors using mouth aspirators. During human landing collection, collector workers for two-hour intervals. Collected mosquitoes were brought to the field laboratory alive. Mosquitoes were anesthetized by using chloroform and identified based on species identification keys.17 The human biting rate was calculated directly from human landing catches as the average number of bites per person per night.
Results
Knowledge of symptoms and causes of malaria.
Information for 775 respondents was considered (385 Baluchi residents and 390 Afghani refugees) The mean ± SEM age of participants was 28.5 ± 15.5 years (age range = 15.4–80 years). Most Baluchi and Afghan participants were familiar with different symptoms of malaria, including fever, chills, and muscle aches, and 76.6% of them were also aware of an association between mosquitoes and malaria (Table 1). Despite this knowledge, 94.6% of Afghan refugees, 74.8% of Baluchi residents, and 87.2% of participants did not use self-protection preventive measures, and 8.8% of participants reported using bed nets regularly.
Table 1.
Participants knowledge about signs, symptoms, and causes of malaria in Sarbaz District, Nickshahr Region, Iran
| Variable | Baluchi residents, no. (%) | Afghani refugees, no. (%) |
|---|---|---|
| Symptom | ||
| Fever | 320 (83.1) | 297 (76.1) |
| Chills | 279 (72.5) | 245 (62.8) |
| Muscle ache | 163 (42.3) | 63 (16.2) |
| Vomiting | 68 (17.7) | 26 (6.7) |
| Cough | 43 (11.2) | 7 (1.8) |
| Other | 58 (15.1) | 59 (15.1) |
| Cause | ||
| Mosquito bites | 336 (87.3) | 258 (66.2) |
| Dirty water | 104 (27.0) | 39 (10.0) |
| Polluted food | 10 (2.6) | 30 (7.7) |
| Other | 5 (1.3) | 87(22.3) |
Generally, 78.9% of Afghan and 52.6% of Baluchi participants (65.84% of all participants) stated that they had never been infected with malaria. Moreover, 77.4% of Afghanis and 52.0% of Baluchis said that they had not been treated with any antimalaria drugs; 31.2% of Afghans and 95.1% of Baluchis who had fever and sought treatment went to health houses or local health centers for medical attention. Although all respondents affirmed that they had access to health care, only 60.8% them stated that “the nearest health services is less than half an hour walk away.” However, results indicated that Afghanis (45.4%) have less access to health services (one hour or less walk) compared with Baluchis (75.4%).
Significant diversity of knowledge about cause of malaria by mosquito bites was observed between two communities (χ2 = 25.2, degrees of freedom [df] = 8, P < 0.001). Mosquito bites were mentioned as the main cause of malaria by 87.3% of Baluchis and 66.2% of Afghanis. In addition, more Baluchis (27.0%) than Afghanis (10.0%) believed that malaria could also be caused by dirty domestic water (χ2 = 11.2, df = 8, P < 0.001) (Table 1).
Sleeping and biting protection behavior.
Overall, inhabitants in Baluchestan traditionally do not use bed nets when they sleep in indoor places. Therefore, all respondents stated that they did not sleep under a bed net in indoor places. More Afghans (91.8%) than Baluchis (63.4%) preferred to sleep inside (χ2 = 13.3, df = 3, P < 0.001). In addition, 14.3% of Baluchis and 1.2% of Afghans reported that they occasionally slept indoors or outdoors (Table 2). Differences in sleeping behavior between Afghans and Baluchis were significantly different (χ2 = 8.6, df = 3, P < 0.001).
Table 2.
Sleeping behavior and self-protection action of residents against mosquito biting in Sarbaz District, Nickshahr Region, Iran
| Participants | Sleeping behavior, no. (%) | Having bed net, no. (%) | Bed net use, no. (%) | Screen on windows, no. (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Indoors | Outdoors | Both | Yes | No | Yes | No | Yes | No | |
| Baluchi | 244 (63.4) | 86 (22.3) | 55 (14.3) | 146 (37.9) | 239 (62.1) | 97 (66.4) | 49 (33.6) | 135 (35.1) | 250 (64.9) |
| Afghani | 358 (91.8) | 27 (7.0) | 5 (1.2) | 40 (10.3) | 350 (89.7) | 21 (52.5) | 19 (47.5) | 33 (8.5) | 357 (91.5) |
| Total | 602 | 113 | 60 | 186 | 589 | 118 | 68 | 168 | 607 |
Twenty-four percent of participants, 37.9% of Baluchis, and 10.3% of Afghanis reported to own at least one bed net. Generally, all respondents with bed nets stated that they used bed nets more often from the beginning of April to the end of September. Furthermore, 25.2% (n = 97) of Baluchis and 5.4% (n = 21) of Afghanis with nets stated that they slept under bed nets regularly (Table 2). Nevertheless, most participants stated that they slept indoors, and 78.3% stated they did not have any screens on windows or doors. Use of screens against mosquitoes was reported by 35.1% (n = 135) Baluchi respondents and only 8.5% (n = 33) of Afghan respondents.
Mosquito biting periodicity.
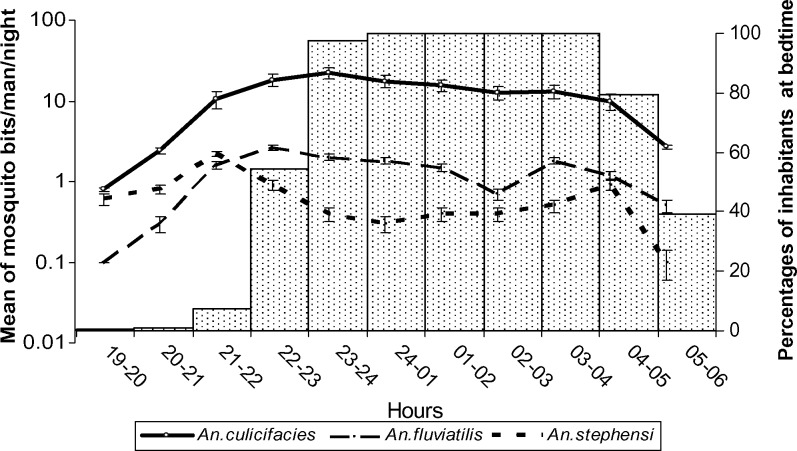
Biting periodicity and bedtimes of inhabitants are shown in Figure 1. Results showed that the three mosquito vectors started biting continuously from sunset to sunrise. Anopheles culicifacies was predominantly captured on human baits, and the number of An. fluviatilis and An. stephensi captured was lower (Table 3). The average number of An. culicifacies caught was 126.3 females/human bait/night. In comparison, the average number of An. fluviatilis and An. stephensi collected were 14 and 7.5 females/human bait/night, respectively. Maximum biting activity for three mosquito species occurred during the second quarter of night until midnight (Table 3).
Figure 1.
Histogram of inhabitant bed time and biting activities of main vectors, Anopheles culicifacies, An. fluviatilis, and An. stephensi in Nikshahr, Sarbaz District, Baluchestan Province. Iran. Most inhabitants stated that they went to bed during second quarter of the night. Results of mosquito landing collections indicated that biting activities of vectors started by sunset. Inhabitants may be exposed to mosquito bites at least for three hours if they do not use bed nets during this time. Error bars indicate mean ± SEM.
Table 3.
Mean and accumulative bites of Anopheles spp. malaria vectors during the entire night in Sarbaz District, Nickshahr Region, Iran*
| Time | An. culicifacies | An. stephensi | An. fluviatilis | |||
|---|---|---|---|---|---|---|
| Bites/hour (%) | Accumulated bites | Bites/hour (%) | Accumulated bites | Bites/hour (%) | Accumulated bites | |
| 7:00–7:59 pm | 0.8 (0.6) | 0.8 | 0.6 (8.0) | 0.6 | 0.0 (0.0) | 0.0 |
| 8:00–8:59 pm | 2.4 (1.9) | 3.2 | 0.8 (10.7) | 1.4 | 0.3 (2.1) | 0.3 |
| 9:00–9:59 pm | 10.6 (8.4) | 13.8 | 2.2 (29.3) | 3.6 | 1.6 (11.4) | 1.9 |
| 10:00–10:59 pm | 18.3 (14.5) | 32.1 | 0.9 (12.0) | 4.5 | 2.6 (18.6) | 4.5 |
| 11:00–11:59 pm | 22.5 (17.8) | 54.6 | 0.4 (5.3) | 4.9 | 2.0 (14.3) | 6.5 |
| Midnight–12:59 am | 17.7 (14.0) | 72.3 | 0.3 (4.0) | 5.2 | 1.8 (12.9) | 8.3 |
| 1:00–1:59 am | 15.5 (12.3) | 87.8 | 0.4 (5.3) | 5.6 | 1.5 (10.7) | 9.8 |
| 2:00–2:59 am | 12.6 (10.0) | 100.4 | 0.4 (5.3) | 6.0 | 0.7 (5.0) | 10.5 |
| 3:00–3:59 am | 13.2 (10.5) | 113.6 | 0.5 (6.7) | 6.5 | 1.8 (12.9) | 12.3 |
| 4:00–4:59 am | 10.0 (7.9) | 123.6 | 0.9 (12.0) | 7.4 | 1.2 (8.6) | 13.5 |
| 5:00–5:59 am | 2.7 (2.1) | 126.3 | 0.1 (1.3) | 7.5 | 0.5 (3.6) | 14.0 |
| Total bites | 126.3 (100) | 7.5 (100) | 14 (100) | |||
Biting collections were performed biweekly during March–July.
Inhabitants sleeping behavior.
Questions about personal sleeping habits of inhabitants showed that adults in both groups wake up at approximately 5:00–6:00am and most are in bed by 10:00–11:00pm (Figure 1). Children usually wake up between 6:00am and 7:00am and go to bed between 9:00pm and 10:00pm, depending on age and time of sunset. All informants who had bed nets reported that they used the nets after 9:00pm and 11:00pm, depending on time of sunset.
Discussion
This study showed that two ethnic communities live in a malarious area had different protective behaviors against mosquito bites. Although awareness of both communities about malaria disease and the role of mosquitoes in transmission was relatively high (Table 1), their knowledge about mosquito biting and protection measures against malaria transmission did not affect their malaria prevention behavior. It is possible that malaria is not perceived as a major health problem in both communities.
In addition, most respondents were familiar with signs and symptoms associated with malaria but a diversity of knowledge about malaria causes was observed between the two communities. Some factors such as language barriers, mistrust of health services, and a fear of asking questions may increase the knowledge gap for malaria causes between Afghans and Baluchis.
Moreover, 65.84% of participants stated that they had never been infected with malaria. Therefore, past malaria infection should be considered a reason that may change the prevention behavior of inhabitants in malarious area. In addition, 77.4% of Afghanis and 52.0% of Baluchis had never had antimalaria treatment. In contrast, 31.2% of Afghanis and 95.1% of Baluchis went to health houses or local health centers for malaria treatment. Most Afghanis preferred private health services instead of public health services because they worried about being deported. In addition, the reason that Afghani residents (45.4%) had less access to health services than Baluchi residents (75.4%) could be explained by the fact Afghan refugees primarily live at the margin of cities and villages.
Although Afghani refugees are a minority population in Baluchestan, one-third of malaria cases occur among them (Department of Communicable Disease Control, unpublished data). Afghan culture and language are different from Baluchi culture and language. Therefore, differences in life style, lack of communication, and preventive behavior among Afghanis should be addressed to reduce the number of malaria cases among the entire population in Baluchestan.
The three main malaria vectors in Baluchestan (An. culicifaces, An. fluviatilis, and An. stephensi) have developed resistance to some major groups of insecticides.18 Drug resistance of P. falciparum has also been reported in the study area.16,19 Therefore, bed net use is a potential tool for malaria prevention in this area, given the confluence of drug and insecticide resistance and a deficient health infrastructure. In the present study, most respondents, both Afghanis and Baluchis (62%), stated that they did not have any bed nets and were not using any malaria household protection because malaria is not perceived as a priority health problem.
The Ministry of Public Health in Iran has a plan to use an integrated vector management program. This program includes insecticide residual spraying, larval control, and insecticide-impregnated bet nets to protect humans as part of the malaria elimination program in Baluchestan Province.11
Some Baluchi residents use bed nets mainly for personal protection against nuisance biting mosquitoes and other nuisance domestic insects. Although this approach can be used as a promotion tool for bed net use, it may have limitations because bed nets are used only when the mosquito density is high. For example, the biting of the three vectors in Baluchestan, especially An. culicifacies, occurred mainly in the early evening and may put the community at more risk of malaria infection because the relatively perceived low mosquito density may be large enough for malaria transmission.
The average number of vector bites was 147.8/human/night during this study (Table 3). This finding indicates that human–vector contact was relatively high in Baluchestan. As shown in Figure 1, biting activities of vectors started by sunset, and most inhabitants stated that they used bed nets during the second quarter of the night. Therefore, mosquitoes have a greater chance to feed on humans for at least three hours if persons do not use bed nets after sunset.
The degree of mosquito vector and human contact is recognized as an important variable in determining the vectorial capacity of mosquito species.2 Accordingly, An.culicifacies showed a large vectorial potential capacity in Baluchestan. This species is predominantly a zoophilic mosquito in India,20 Pakistan,21 and southern Iran.13 Similarly, An. stephensi is predominantly a domestic vector and primarily a zoophilic species.22–24 In contrast, An. fluviatilis has been reported to be highly anthropophilic and exophilic in Iran and India.25–27 However, environmental change may influence biting behavior of mosquitoes in Baluchestan. These changes may include biting behavior expressed by place and/or time of biting. Generally, mosquitoes may change their host preference because of unavailability of favorite hosts.20,26,28 These issues may explain why An. culicifacies showed high tendency to feed on humans in Baluchestan than in neighbor countries.
Moreover, most Afghanis (91.8%) and Baluchis (63.4%) slept in indoors shelters without using window screens or bed nets. Because An. culicifacies and An. stephensi are mostly endophilic and endophagous species,29,30 both communities are exposed to mosquito bites.
In conclusion, although most participants were familiar with malaria and the role of mosquito bites on malaria transmission, ownership and use of bed nets was low among Afghanis and Baluchis communities. However, a bet net distribution plan by health authorities may fail to achieve desirable effect in the area because bet net users may receive infectious bites when they are outside the net during the early evening. Therefore, incorporating social, cultural, and economic aspects of both communities during design of elimination projects and their implementation is essential. This policy would enhance and ensure sustainability of malaria control interventions in southern Iran and neighboring countries. Delivery of health education to Afghan refugees and Baluchi residents has to be amended to achieve the required changes in current practice. In addition, information on biting periodicity will be useful in designing personal protection measures.
ACKNOWLEDGMENTS
We thank the personnel of the Iranshahr Training and Research Center for kind assistances and maintenance of the laboratory mosquito strain and Dr. Mahmoud Iranpour (University of Manitoba, Winnipeg, Manitoba, Canada) for critically reviewing the manuscript and invaluable comments.
Footnotes
Financial support: This study was support by the Regional Office for the Eastern Mediterranean of the World Health Organization and the School of Public Health and Institute of Public Health, Tehran University of Medical Sciences.
Authors' addresses: Hamid R. Basseri and Mohammad R. Abai, Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, PO Box 6446-14155, Tehran, Iran, E-mails: basserih@tums.ac.ir and abaimr@tums.ac.ir. Ahmad Raeisi, Centers for Disease Control, Malaria Control Section, Ministry of Health and Education, Tehran, Iran, E-mail: raeisia@tums.ac.ir. Khandan Shahandeh, Center for Community Based Participatory Research, Tehran University of Medical Sciences, Tehran, Iran, E-mail: shahandeh@razi.tums.ac.ir.
References
- 1.Chaves LF, Harrington LC, Keogh CL, Nguyen AM, Kitron UD. Blood feeding patterns of mosquitoes: random or structured? Front Zool. 2010;7:3. doi: 10.1186/1742-9994-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shriram AN, Ramaiah KD, Krishnamoorthy K, Sehgal SC. Diurnal pattern of human-biting activity and transmission of subperiodic Wuchereria bancrofti (Filariidea: Dipetalonematidae) by Ochlerotatus niveus (Diptera: Culicidae) on the Andaman and Nicobar islands of India. Am J Trop Med Hyg. 2005;72:273–277. [PubMed] [Google Scholar]
- 4.Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukherjee SR, Dunn JR, Timothy TF, Wilson R, Moncayo AC. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am J Trop Med Hyg. 2009;81:452–456. [PubMed] [Google Scholar]
- 5.Edman JD, Spielman A. Blood-feeding by vectors: physiology, ecology, behavior, and vertebrate defense. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 153–189. [Google Scholar]
- 6.Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- 7.Killeen GF, McKenzie FE, Foy BD, Bogh C, Beier JC. The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by African mosquito populations. Trans R Soc Trop Med Hyg. 2001;95:469–476. doi: 10.1016/s0035-9203(01)90005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 9.Basseri HR, Raeisi A, Holakoui K, Shanadeh K. Malaria prevention among Afghan refugees in a malarious area, southeastern Iran. Bull Soc Pathol Exot. 2010;103:340–345. doi: 10.1007/s13149-010-0050-3. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control . Annual Report for Malaria Control Program [in Persian] 23rd edition Tehran: Education IMoHaM; 2008. [Google Scholar]
- 11.Raeisi A. Malaria Elimination in Iran, Progress Achievements and Challenges. Sixth Meeting of National Malaria Programme Managers; Cairo, Egypt: 2006. [Google Scholar]
- 12.Edrissian GH. Malaria in Iran: past and present situation. Iran J Parasitol. 2006;1:1–14. [Google Scholar]
- 13.Zaim M, Subbarao SK, Manouchehri AV, Cochrane AH. Role of Anopheles culicifacies s.l. and An. pulcherrimus in malaria transmission in Ghassreghand (Baluchistan), Iran. J Am Mosq Control Assoc. 1993;9:23–26. [PubMed] [Google Scholar]
- 14.Basseri HR, Doosti S, Akbarzadeh K, Nateghpour M, Whitten MM, Ladoni H. Competency of Anopheles stephensi mysorensis strain for Plasmodium vivax and the role of inhibitory carbohydrates to block its sporogonic cycle. Malar J. 2008;7:131. doi: 10.1186/1475-2875-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djadid ND, Barjesteh H, Raeisi A, Hassanzahi A, Zakeri S. Identification, sequence analysis, and comparative study on GSTe2 insecticide resistance gene in three main world malaria vectors: Anopheles stephensi, Anopheles culicifacies, and Anopheles fluviatilis. J Med Entomol. 2006;43:1171–1177. doi: 10.1603/0022-2585(2006)43[1171:isaacs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Edrissian GH, Afshar A, Sayedzadeh A, Mohsseni G, Satvat MT. Assessment of the response in vivo and in vitro of Plasmodium falciparum to sulphadoxine-pyrimethamine in the malarious areas of Iran. J Trop Med Hyg. 1993;96:237–240. [PubMed] [Google Scholar]
- 17.Smart J. A Handbook for the Identification of Insects of Medical Importance. New Delhi, India: Vedams eBooks; 2003. [Google Scholar]
- 18.Djadid ND, Forouzesh F, Karimi M, Raeisi A, Hassan-Zehi A, Zakeri S. Monitoring pyrethroid insecticide resistance in major malaria vector Anopheles culicifacies: comparison of molecular tools and conventional susceptibility test. Iran Biomed J. 2007;11:169–176. [PubMed] [Google Scholar]
- 19.Zakeri S, Afsharpad M, Raeisi A, Djadid ND. Prevalence of mutations associated with antimalarial drugs in Plasmodium falciparum isolates prior to the introduction of sulphadoxine-pyrimethamine as first-line treatment in Iran. Malar J. 2007;6:148. doi: 10.1186/1475-2875-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Curr Sci. 2007;92:1571–1578. [Google Scholar]
- 21.Herrel N, Amerasinghe FP, Ensink J, Mukhtar M, van der Hoek W, Konradsen F. Adult anopheline ecology and malaria transmission in irrigated areas of South Punjab, Pakistan. Med Vet Entomol. 2004;18:141–152. doi: 10.1111/j.0269-283X.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 22.Manouchehri AV, Zaim M, Emadi AM. A review of malaria in Iran, 1975–90. J Am Mosq Control Assoc. 1992;8:381–385. [PubMed] [Google Scholar]
- 23.Hati AK. Urban malaria vector biology. Indian J Med Res. 1997;106:149–163. [PubMed] [Google Scholar]
- 24.Dash AP, Raghavendra K, Pillai MK. Resurrection of DDT: a critical appraisal. Indian J Med Res. 2007;126:1–3. [PubMed] [Google Scholar]
- 25.Basseri H, Moosakazemi S, Yosafi M, Mohebali M, Hajaran H. Anthropophily of malaria vectors in Kahnouj District, south of Kerman, Iran. Iran J Public Health. 2005;34:27–37. [Google Scholar]
- 26.Nanda N, Yadav RS, Subbarao SK, Joshi H, Sharma VP. Studies on Anopheles fluviatilis and Anopheles culicifacies sibling species in relation to malaria in forested hilly and deforested riverine ecosystems in northern Orissa, India. J Am Mosq Control Assoc. 2000;16:199–205. [PubMed] [Google Scholar]
- 27.Parida SK, Hazra RK, Marai N, Tripathy HK, Mahapatra N. Host feeding patterns of malaria vectors of Orissa, India. J Am Mosq Control Assoc. 2006;22:629–634. doi: 10.2987/8756-971X(2006)22[629:HFPOMV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Pemola Devi NJ. Relationship between Anopheles fluviatilis and A. stephensi (Diptera: Culicidae) catches and the prevalence of malaria cases at Kalsi area in Dehradun district (Uttaranchal) Indian J Med Res. 2006;123:151–158. [PubMed] [Google Scholar]
- 29.Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran, 2002. Acta Trop. 2006;97:196–203. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan province, south-east Islamic Republic of Iran. East Mediterr Health J. 2011;17:439–445. [PubMed] [Google Scholar]