Abstract
Secondary heterologous dengue infection is a risk factor for severe disease manifestations because of the immune-enhancement phenomenon. Succeeding clinical infections are seldom reported, and the clinical course of tertiary and quaternary dengue infections is not clear. Cuba represents a unique environment to study tertiary/quaternary dengue infections in a population with known clinical and serologic dengue markers and no dengue endemicity. We took advantage of this exceptional epidemiologic condition to study the effect of primary, secondary, tertiary, and quaternary dengue infection exposure on the expression of pro-inflammatory and regulatory cytokines, critical in dengue infection pathogenesis, by using a dengue infection ex vivo model. Whereas secondary exposure induced a high cytokine response, we found a significantly lower expression of tumor necrosis factor-α, interferon-γ, interleukin-10, and tumor growth factor-β after tertiary and quaternary infectious challenge. Significant differences in expression of the cytokines were seen between the dengue immune profiles, suggesting that the sequence in which the immune system encounters serotypes may be important in determining the nature of the immune response to subsequent infections.
Introduction
Dengue is among the major causes of morbidity and mortality in children in many disease-endemic countries in Asia and the Americas.1 Any of the four dengue virus serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) serotypes, which are transmitted to humans by the urban freshwater-breeding mosquito Aedes aegypti, can produce an infection that ranges from asymptomatic, to dengue fever (DF), characterized by general aches and occasional minor bleeding, to a life-threatening complications known as dengue hemorrhagic fever (DHF) with or without shock, and dengue shock syndrome (DSS).2 Infection induces life-long protective immunity to the infecting serotype, accompanied by short-term cross-protective immunity against the other serotypes. Subsequent infection with a different dengue serotype is more likely to cause DHF/DSS. In 2009, new dengue classification guidelines to streamline dengue diagnosis and treatment were published by the World Health Organization.3 In this article, we will use the prior classification system as the patients from whom the samples were taken were classified by DF, DHF, and DSS.
Epidemiologic data suggest a greater risk of DHF/DSS during secondary infections, and immunopathologic mechanisms, such as immune-enhancement phenomenon, have been proposed to contribute to DHF risk.4 This contribution occurs when non-neutralizing antibodies resulting from the primary infection favor dissemination of the second infecting dengue virus, a phenomenon known as antibody-dependent enhancement,5 and cross-reactive memory T cells from a primary infection recognize antigen from the secondary infection, resulting in increased T cell activation and cytokine production.6
Contrary to most dengue-endemic countries where several dengue serotypes co-circulate, epidemics in Cuba are caused by a single serotype and have been fully controlled, offering the possibility to explore memory T cell response to dengue virus in persons with a well-characterized dengue virus infection history. In 1977–1978 DENV-1 caused a massive DF epidemic that affected 44.5% of the population in Cuba.7 Four years later, a DENV-2 epidemic spread throughout the country with more than 344,203 cases, 10,312 DHF/DSS cases, and 158 deaths.8,9 In 1997, a DENV-2 outbreak was reported in the municipality of Santiago de Cuba with 2,946 cases, 205 DHF/DSS cases, and 12 deaths.10 Finally in 2001, a DENV-3 epidemic was reported in Havana. During this outbreak, there were 12,889 cases of DF, 78 cases of DHF/DSS, and 3 deaths.11
One controversial topic in dengue research is the identification of the factors involved in the infection outcome during the second, third or fourth dengue virus heterotypic re-infection relative to a first or second infection, and which factors are involved in protection or induction of a determined clinical outcome. Taking advantage of the epidemiologic dengue situation in Cuba during 1977–2001, in which sequences of dengue virus infections are well defined, we addressed the role of cross-reactive memory T cells by measuring expression of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), tumor growth factor-β (TGF-β), and interleukin-10 (IL-10) cytokines in peripheral blood mononuclear cells (PBMC) from persons with different dengue immune profiles (dengue naive, primary, secondary, and tertiary dengue infection) after ex vivo re-challenge with DENV-1, DENV-2, and DV-3. Specifically, PBMC collected from healthy persons with primary, secondary, and tertiary dengue infections and dengue-naive persons were challenged with infective DENV-1, DENV-2 and DENV-3. Expression of mRNA for IL-10, TNF-α), IFN-γ, and TGF-β was quantified.
Materials and Methods
Study participants.
The study included 58 residents from Havana (32 women and 26 men 18–60 years of age, mean age = 32.3 years) with IgG titers ≥ 40 against DENV when tested by enzyme-linked immunosorbent assay.12 Because antibodies in serum can neutralize different dengue virus serotypes, serotypes that infected participants were identified by using a plaque-reduction neutralization test.13–15
Twenty-seven persons were classified as having primary dengue immune cases (10 primary DENV-1, 10 primary DENV-2, and 7 primary DENV-3 cases). Thirty-one persons were classified as having secondary dengue immune cases, 10 in the sequence DENV-1/DENV-2, 8 in the sequence DENV-1/DENV-3, and 6 in the sequenceDENV-2/DENV-3. Seven persons were classified as having tertiary cases (DENV-1/DENV-2/DENV-3). The chronologic order of infection is known by the dengue history in Cuba, and particularly in Havana (DENV-1 in 1977–1978,7 DENV-2 in 1981,8,9 and DENV-3 in 200111). Ten persons from Havana (3 women and 7 men 20–60 years of age, mean age = 33.4 years) without antibodies against dengue as tested by enzyme-linked immunosorbent assay,12 were included as negative controls.
This study was conducted according to the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects, and was approved by the institutional ethical review committees of the Institute of Tropical Medicine Pedro Kourí and the Cuban National Academy of Sciences. Written informed consent was obtained from each person at enrollment into the study.
Neutralization assay.
Dengue serotypes that previously infected participants were identified by using a plaque-reduction neutralization test in BHK-2115 cells with some modifications.16 Briefly, BHK-21 cells were grown at 37°C in complete medium E-minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin, 10%l-glutamine, and sodium bicarbonate to adjust the pH to 7.4–7.8. Serum from each person included in the study was diluted from 1:10 to 1:100,000. Hyperimmune ascites fluid to each serotype (Centers for Disease Control and Prevention, Atlanta, GA) and human serum without antibodies to dengue virus were used as positive and negative controls, respectively. Negative and positive controls were used at 1:10 serum dilutions. For antibody titration, 100 μL of each serum dilution was incubated for 1 hour at 37°C with 100 μL of virus working dilution calculated to give 10–20 plaque-forming units/50 μL of the final volume of virus-serum mixture. After incubation, 50 μL of virus-serum mixtures was added to the cell suspension in triplicate. After incubation for 4 hours at 37°C in an atmosphere of CO2, 0.5 mL of overlay medium described above was added to each well. Infected cells were incubated for 5–9 days depending on the dengue virus serotype. After incubation, plates were rinsed with tap water, and cells were stained with a solution of naphthol blue black and acetic acid. The serum dilution that resulted in a 50% reduction in plaque count as determined by probabilistic analysis was considered the end point titer. The neutralizing antibody titer is expressed as the reciprocal of this value.
According to previously established criteria,17 serum samples with neutralizing antibody titers ≥ 30 against one of the dengue serotypes were considered positive for a past infection with that serotype. Serum samples with neutralizing antibody titers ≥ 30 to 2 serotypes were considered secondary infections. When a serum sample was monovalent to one of the serotypes, the person was considered to have a primary case. When a serum sample was bivalent and neutralized two serotypes, the person was considered to have a secondary case. When a serum sample had antibodies to three serotypes, the person was considered to have a possible tertiary infection.
Virus strains used in the neutralization assay were DENV-1 Angola 6PC6/36 HT (Angola, 1998), DENV-2 A15 4PR 3PC6/36 HT (Cuba, 1981), and DENV-3 116/00 3PC6/36 HT (Cuba, 2000). The DENV-1 strain was supplied by Robert Shope (University of Texas Medical Branch, Galveston, TX).
Dengue virus preparation.
Dengue virus antigens were prepared as described.18 C636 cell lines from Ae. albopictus mosquitoes were grown to confluence, infected at a multiplicity of infection of 0.1 plaque-forming units/cell with the dengue strains DENV-1-113 Peru 1990, DENV-2 A15 Cuba 1981, and DENV-3 116 Cuba 2000, and cultured in minimum essential medium supplemented with 2% fetal calf serum. When a 50% cytopathic effect was observed, culture supernatant was clarified by centrifugation at 10,000 rpm for 30 minutes at 4°C. Culture supernatant from non-infected C636 cells was used as a negative control antigen (mock control). The BHK-21 clone 15 cell line was used for virus titration as described.15
Dengue virus stimulation of PBMC for reverse transcription–polymerase chain reaction.
Peripheral venous blood (20 mL) was collected from each study participant. PBMC were isolated from 15 mL of citrated venous blood by using standard density gradient centrifugation with Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), and serum samples were obtained from 5 mL of peripheral venous blood without anti-coagulant.19 Cells were adjusted to a concentration of 2 × 106 in 1,000 μL of RPMI 1640 supplemented with 5% autologous serum, 2 mM glutamine, 100 μg of streptomycin/mL, 100 units of penicillin, and cultured for 24 hours at 37°C in the presence of DENV-1, DENV-2, or DENV-3 at a multiplicity of 0.1 or mock control (supernatant of uninfected C636 cells), respectively. Phytohemagglutinin (Sigma, Poole, United Kingdom), 5 μg/mL, served as a positive assay control. After 24 hours, cells were separated from the supernatants and both were frozen at –80°C.
Gene expression analysis.
DNase-treated total RNA was isolated from stimulated PBMC by using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) and evaluated by using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). cDNA was synthesized from mRNA with poly(dT) primers and Superscript II reverse transcriptase (Life Technologies, Rockville, MD) and quantified by real-time PCR analysis by using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA), according to the manufacturer's protocols. Samples were analyzed in triplicate for expression of TNF-α, IFN-γ, TGF-β, IL-10, and the housekeeping gene hypoxanthine phosphoribosyltransferase-1. Specific expression was calculated in relation to that of hypoxanthine phosphoribosyltransferase-1 by using the delta/delta Ct method as recommended by Applied Biosystems.
Statistical analysis.
Means of quantitative variables (TNF-α, IFN-γ, IL-10, and TGF-β) were analyzed between groups according to dengue immunity categories and serotype of dengue virus used for stimulation by using the Mann-Whitney U test. P values < 0.05 were considered statistically significant. SPSS software version 10 (SPSS, Inc., Chicago, IL) was used for all analyses.
Results
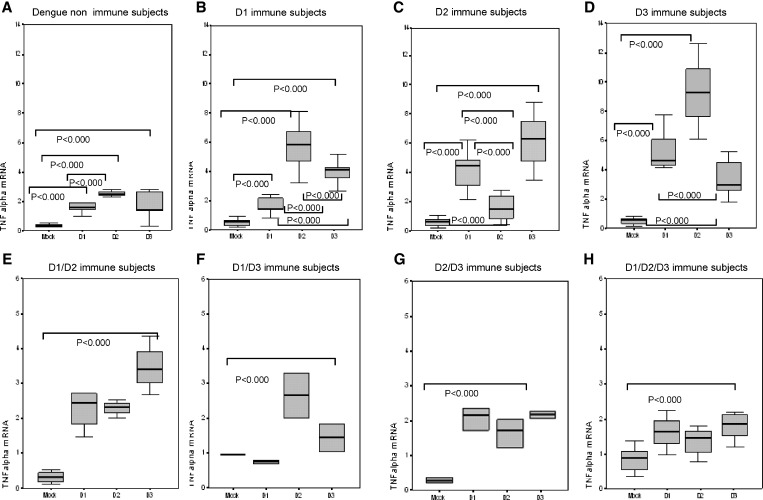
The objective of this study was to explore how prior infection with one or several different dengue serotypes influence expression of TNF-α, IFN-γ, IL-10, and TGF-β. TNF-α mRNA expression is shown in Figure 1. We determined levels of expression of PBMC from dengue-naive persons (control group) and from persons with confirmed primary infection (to DENV-1, DENV-2, and DENV-3) challenged with DV-1, DV-2 and DV-3. The control group showed significantly higher expression of TNFα induced by infective DENV-1, DENV-2, and DENV-3 than uninfected C636 cell culture supernatant used as a mock control (P < 0.001), and primary challenge with DENV-2 induced significantly higher TNFα expression than DENV-1 and DENV-3 (P < 0.001).
Figure 1.
Effect of prior infection with dengue virus on expression of tumor necrosis factor alpha (TNF alpha) in persons with IgG titers ≥ 40 against dengue virus when tested by enzyme-linked immunosorbent assay in Havana, Cuba. Mock, peripheral blood mononuclear cells (PBMC) challenged with uninfected C636 supernatant; D1, PBMC challenged with dengue virus 1; D2, PBMC challenged with dengue virus 2; D3, PBMC challenged with dengue virus 3. Panels show different scales according to the range of values observed.
The three groups of persons with primary dengue infections (Figure 1) also showed significantly higher TNF-α in response to DENV-1, DENV-2, and DENV-3 stimulation compared with the mock control. DENV-1 primary immune persons showed significantly higher TNF-α gene expression in response to DENV-2 and DENV-3 serotype cross-reactive dengue challenge, and the highest response was to DENV-2. DENV-1 and DENV-3 heterotypic challenge also induced significant TNF-α gene expression in dengue 2 primary immune persons, and the highest response was to DENV-3. Dengue 3 primary immune persons showed higher expression of these cytokines in response to secondary DENV-2 heterologous challenge. TNF-α gene expression in cells from persons immune to two and three dengue infections after DENV-1, DENV-2, and DENV-3 challenge is shown in Figure 1.
DENV-1/DENV-2 secondary immune persons showed significantly higher TNF-α expression after DENV-1, DENV-2, and DENV-3 challenge than with the mock control, and higher expression was observed after heterotypic tertiary challenge with DENV-3. We also measured levels of TNFα expression induced by DENV-1, DENV-2, and DENV-3 tertiary challenge in cells from DV-1/DV-3 secondary immune persons. There was a significant induction of TNF-α by DENV-1, DENV-2, and DENV than with the mock control, but there were no differences in TNFα expression induced by the three DENV serotypes. Induction of TNFα expression in DENV-2/DENV-3 immune persons was significantly higher than with the mock control, but lower than that observed in DENV-1/DENV-2 and DENV-1/DENV-3 secondary immune groups. Quaternary challenge in DENV-1/DENV-2/DENV-3 immune persons showed low levels of expression of TNF-α, which were comparable with those observed in dengue-naive persons, and there were no differences among serotypes.
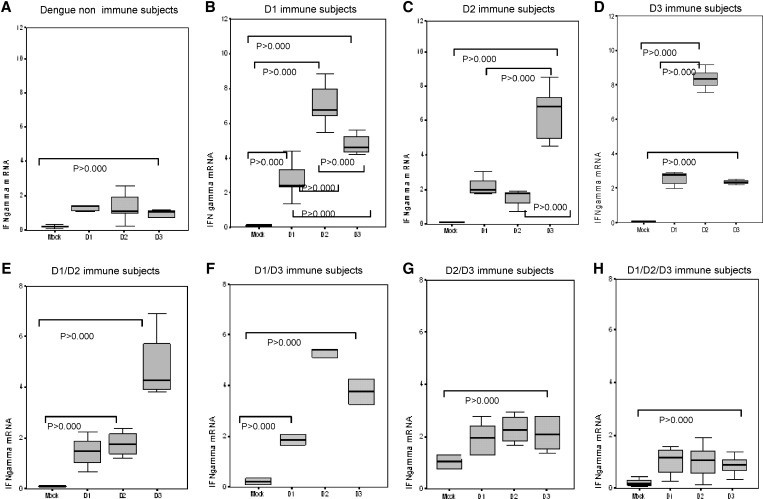
IFN-γ expression is shown in Figure 2. We measured the response of dengue-naive persons and dengue primary immune persons. Significantly higher expression of IFN-γ induced by infective DENV-1, DENV-2, and DENV-3 was observed in cells collected from dengue-naive persons than that induced by uninfected C636 cell culture supernatant, indicating that induction of IFN-γ resulted from dengue infection.
Figure 2.
Effect of prior infection with dengue virus on expression of interferon-gamma (IFN gamma) in persons with IgG titers ≥ 40 against dengue virus when tested by enzyme-linked immunosorbent assay in Havana, Cuba. Mock, peripheral blood mononuclear cells (PBMC) challenged with uninfected C636 supernatant; D1, PBMC challenged with dengue virus 1; D2, PBMC challenged with dengue virus 2; D3, PBMC challenged with dengue virus 3. Panels show different scales according to the range of values observed.
IFN-γ expression in DENV-1 primary immune persons was higher after exposure to DENV-1, DENV-2 and DENV-3 than with the mock control. The highest expression was obtained after DENV-2 challenge. Dengue 2 and dengue 3 immune persons also showed significantly higher expression to the three infective dengue serotypes than to the mock control. The highest IFN-γ expression in the dengue 2 primary infected group was in response to heterologous DENV-3 re-exposure, and dengue 3 immune persons showed highest expression after DENV-3 challenge. IFN-γ expression of DENV-1/DENV-2, DENV-1/DENV-3, and DENV-2/DENV-3 secondary immune cases and IFN-γ expression of DENV-1/DENV-2/DENV-3 immune (tertiary) cases is shown in Figure 2.
The DENV-1/DENV-2 immune persons showed significantly higher IFN-γ expression to heterotypic tertiary challenge with DENV-3. The DENV-1/DENV-3 immune persons showed significantly higher IFN-γ mRNA expression after dengue infective challenges than with the mock control, and the highest response was to DENV-2, followed by DENV-3 and DENV-1. Lower levels of IFN-γ expression were observed in cells from the DENV-2/DENV-3 secondary immune group, and there were no differences among the three DENV serotypes. Induction of IFN-γ expression in DENV-1/DENV-2/DENV-3 tertiary persons after quaternary challenge showed lower levels.
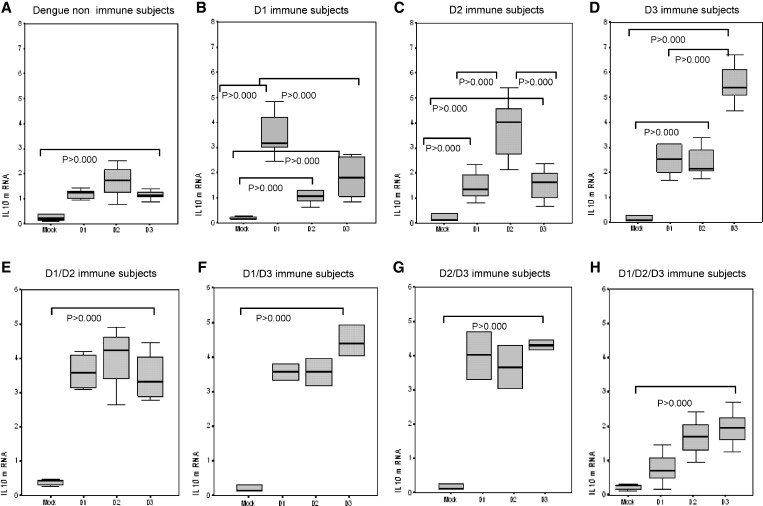
Levels of IL-10 expression are shown in Figure 3. Infective DENV-1, DENV-2, and DENV-3 induced significantly higher mRNA expression of IL-10 in dengue-naive persons and in dengue 1, dengue 2, and dengue 3 primary immune persons than with the mock control. Homologous DENV-1 re-challenge induced a higher up-regulation of IL-10 gene expression for dengue 1, Dengue-2, and dengue-3 primary immune persons. There were no statistically significant differences in mRNA expression of IL-10 in DENV-1/DENV-2, DENV-1/DENV-3, and DENV-2/DENV-3 secondary immune persons after DENV-1, DENV-2, and DENV-3 challenge, but the levels of expression were similar to those observed in primary immune persons in response to homotypic challenge. The IL-10 expression described in response to quaternary challenge was notably low for all three dengue challenging serotypes.
Figure 3.
Effect of prior infection with dengue virus on expression of interleukin-10 (IL-10) in persons with IgG titers ≥ 40 against dengue virus when tested by enzyme-linked immunosorbent assay in Havana, Cuba. Mock, peripheral blood mononuclear cells (PBMC) challenged with uninfected C636 supernatant; D1, PBMC challenged with dengue virus 1; D2, PBMC challenged with dengue virus 2; D3, PBMC challenged with dengue virus 3. Panels show different scales according to the range of values observed.
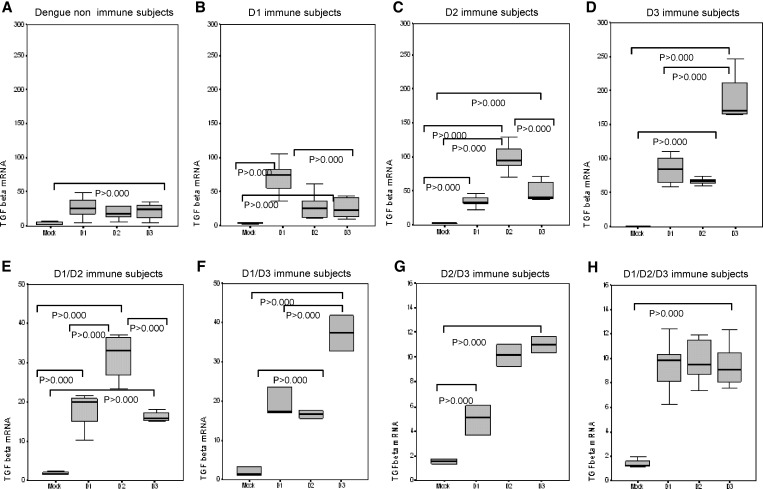
TGF-β expression is shown in Figure 4. Significantly higher TGF-β expression was observed in dengue-naive persons after challenge with DENV-1, DENV-2, and DENV-3 than with the mock control. Dengue 1 primary immune persons showed higher TGFβ gene expression in response to homologous DENV-1 re-challenge, dengue 2 immune persons showed the highest TGF-β expression to DENV-2 challenge, and dengue 3 immune persons showed the highest TGF-β expression to DENV-3 challenge.
Figure 4.
Effect of prior infection with dengue virus on expression of tumor growth factor β (TGFβ) in persons with IgG titers ≥ 40 against dengue virus when tested by enzyme-linked immunosorbent assay in Havana, Cuba. Mock, peripheral blood mononuclear cells (PBMC) challenged with uninfected C636 supernatant; D1, PBMC challenged with dengue virus 1; D2, PBMC challenged with dengue virus 2; D3, PBMC challenged with dengue virus 3. Panels show different scales according to the range of values observed.
TGF-β expression in response to tertiary challenge with DENV-1, DENV-2, and DENV-3 (Figure 4) showed differences according to the dengue immune profile of the person. DENV-1/DENV-2 persons had significantly higher expression to DENV-2 challenge than to DENV-1 and DENV-3 challenge. TGF-β expression in DENV-1/DENV-3 persons was significantly higher to DENV-3. DENV-2/DENV-3 persons showed significantly higher expression in response to DENV-2 and DENV-3 than to DENV-1, but significantly lower than that observed in DENV-1/DENV-2 and DENV-1/DENV-3 persons. Quaternary challenge of persons with DENV-1/DENV-2/DENV-3 tertiary cases also showed low levels of expression to DENV-1, DENV-2, and DENV-3, but significantly higher than with the mock control.
Discussion
Immunity to a single dengue virus infection does not provide heterologous protective immunity to subsequent infections. Exposure to a second heterologous dengue infection is considered the main risk factor for DHF/DSS.20 However, there are few studies on the incidence of dengue disease after a third or fourth dengue infection.21–23
Data presented compare the inflammatory/anti-inflammatory response after primary, secondary, tertiary, and quaternary challenge. Our results confirm that primary dengue challenge induces TNF-α, IFN-γ, IL-10, and TGF-β expression at early stages after infection, but notably lower than the secondary exposure. Our results also corroborate that heterologous secondary re-challenge induced a dominant inflammatory (IFN-γ, TNF-α) response, and homologous re-challenge triggered a pattern dominated by regulatory cytokines.24
We also show that tertiary and quaternary exposure results in a milder inflammatory response with a high regulatory immune response compared with secondary exposure. It is believed that after a secondary dengue infection, a heterologous protective immunity is raised and the threat of dengue is reduced, although there are reports of possible tertiary infection resulting in DHF.21,22
Havana has a unique epidemiologic dengue situation in which residents could have had immunity to two of the four dengue viruses before the DENV-3 epidemic in 2001–2002. DENV-1 circulated in 1977–19797 and DENV-2 infections occurred in 1981.25,26 This pattern resulted in a number of possible combinations of dengue immune profiles for secondary and tertiary heterotypic infections after the 2001–2002 DENV-3 epidemic (DENV-1/DENV-3, DENV-2/DENV-3, and DENV-1/DENV-2/DENV-3).
The immunopathologic mechanisms of DHF include a series of altered immune response mechanisms. Among them are a rapid and uncontrolled increase in the levels of cytokines, especially TNF-α and IFN-γ, which in turn induce the clinical manifestations of DHF such as plasma leakage, shock, and hemorrhagic manifestations.27–29
In the present study, we examined the memory cellular immune response by measuring the expression of two critical mediators of pro-inflammatory response (IFN-γ and TNF-α) and two central mediators of regulatory response (TGF-β and IL-10).24 Our results show that TNF-α and IFN-γ mRNA expression was significantly lower in the dengue-naive control group compared with all dengue-immune persons (P < 0.001). However, when we compared the pro-inflammatory response induced by primary dengue challenge of PBMC collected from dengue-naive persons to DENV-1, DENV-2, and DENV-3, the response was significantly higher to DENV-2.
There are several reports on mild or asymptomatic DENV-2 primary infection.29–35 As our results suggest, DENV-2 seems to be more immunogenic than the other serotypes, and generates a stronger pro-inflammatory immunologic response, which is necessary to control viral infection. Much of the antiviral potential of effector cells of the innate and adaptive immune system (natural killer cells and cytotoxic T cells) is caused by production of antiviral cytokines such as IFN-γ and TNF-α at the site of the infection. Importantly, the same cytokines also control viral infections indirectly by modulating the induction, amplification, recruitment, and effector functions of the immune response and by up-regulating antigen processing and display of viral epitopes at the surface of infected cells.30 Recently, a human challenge model for dengue infection showed a possible protective role for sustained IFN-γ levels during the acute phase of illness.31
Significantly higher gene expression of TNF-α and IFN-γ in response to DENV-2 and DENV-3 heterologous challenge in DENV-1 primary immune cases was found, and the highest response was to DENV-2 Although DENV-1/DENV-2 and DENV-1/DENV-3 sequences of dengue infection were associated with severe illness during the 1981 (DENV-2) and 2001 (DENV-3) epidemics, respectively, greater severity was observed during the 1981 epidemic. Previous studies demonstrated that in 1981, 98% of DHF cases occurred during a secondary DENV-1/DENV-2 infection while in 2001, 73% of DHF cases occurred during a secondary DENV-1/DENV-3 infection.9,32
Population-based epidemiologic studies have shown that infection with DENV-2 in persons previously infected with a different dengue serotype is a major risk factor for DHF and DSS.33–37 However, despite co-circulation of several dengue serotypes in the Americas, it was not until the epidemic of 1981 in Cuba that the first DHF cases occurred; this coincided with the introduction of a new genetic type of DENV-2, the Southeast Asian genotype.38,39 Since that time, other countries in the Americas have reported DHF associated with secondary dengue serotype 2 viruses of the Southeast Asian genotype and not the indigenous American genotype.40,41 Thus, it has been suggested that a specific genotype of DENV-2 may have the propensity to cause DHF, whereas other genotypes are associated with the less severe DF.42–48
Several reports examined differences between DENV-2 American versus Asian strains, and attempted to explain why the American DENV-2 genotype strains lack the properties necessary to cause severe disease in a secondary heterotypic infection. Results of these studies support the hypothesis that specific structural differences between the Southeast Asian and American genotypes may be involved in disease pathogenesis.49–53
In this study, we compared only the ability of induction of the studied cytokines in secondary heterotypic infection between dengue serotypes and we used only the DV-2 A15 4PR 3PC6/36 HT Cuban strain (A15/81 strain) a Southeast Asian genotype. It would be interesting in future studies to address differences in the cellular immune response to the different strains of the four serotypes, in particular the American and Southeast Asian genotypes of DENV-2. Differences in the pattern of cellular immune response induced by the different strains could also be associated with differences in disease severity.
TNF-α and IFN-γ gene expression in DENV-2 primary immune cases was significantly higher in response to challenge with DENV-1, DENV-2, and DENV-3 than with the mock control, and significantly higher with heterologous DENV-1 and DENV-3 stimulation than with homologous stimulation. In addition, expression in response to DENV-3 was significantly higher than that seen with DENV-1 Because TNF-α and IFN-γ have been reported as critical cytokines in DHF pathogenesis,27 our ex vivo model results may support that association.
Heterotypic challenge also induced significant TNF-α and IFN-γ gene expression in dengue 3 persons with primary infections, and the highest response was seen with DENV-2 challenge. DENV-2 has been the serotype most associated with disease severity in the course of a secondary infection,33–37 and high levels of TNF-α and IFN-γ have been frequently associated with severe dengue.6,28,54–60 This finding is not consistent with higher IFNγ and TNF-α responses observed in PBMC from dengue-naive persons in response to DENV-2 challenge, especially if one considers that DENV-2 primary infection is frequently mild or asymptomatic. However, IFN-γ and TNF-α are essential for effective control of viral infection. IFN-γ and TNF-α are not per se the causes of human dengue infection pathogenesis; it is the complex interplay of self-reinforcing pathologic events that ultimately manifests in DHF/DSS and increased vascular permeability and circulatory failure involved in a secondary dengue heterotypic infection.
DENV-1/DENV-2 and DENV-1/DENV-3 secondary immune persons showed the highest expression of pro-inflammatory mediators in response to the serotype to which persons have not exposed, after tertiary challenge with DENV-1, DENV-2, and DENV-3. This finding is consistent with that observed in dengue 1 or dengue 3 immune persons, and can be explained for similar reasons: higher proinflammatory cytokine secretion and lower avidity cross-reactive T cells during the course of heterotypic dengue virus infection. Cross-reactive T cells with higher avidity for an infecting serotype are preferentially activated, and cells specific to the current infecting serotype are killed by activation-induced cell death.61 The consequences may be inefficient viral clearance and enhanced immunopathologic changes caused by massive and uncontrolled production of proinflammatory mediators targeting vascular endothelial cells, which leads to fluid and protein leakage that ultimately results in DHF/DSS.62
Although tertiary challenge generally showed a weaker inflammatory response to heterologous dengue virus challenge than the secondary challenge, DENV-1/DENV-2 immune persons showed higher TNF-α and IFN-γ expression after heterotypic tertiary challenge with DENV-3. We previously reported six confirmed DHF/DSS patients with tertiary DENV-3 infections (DENV-1/DENV-2/DENV-3) during the 2001 outbreak.21,22 Others reports on dengue hospital admissions found no differences in the risk between a first dengue infection and a third/fourth dengue infection.21,22 The significantly higher expression of TNFα and IFNγ found in our ex vivo model after a tertiary challenge with DENV-3 could be related to reports of a possible tertiary infection by this serotype, resulting in dengue disease and even in the severe clinical outcome observed in some persons.21,22
DENV-2/DENV-3 immune persons showed significantly lower TNF-α and IFN-γ gene expression than DENV-1/DENV-2 and DENV-1/DENV-3 immune persons after tertiary challenge (P < 0.0001). A stronger immunologic response induced by DENV-2 could also imply a wider and specific memory immune response that confers not only a life-long protective immunity to the primary infecting serotype, but a longer cross-protective immunity against other serotypes. This suggestion might also explain why dengue 2 immune persons that had a heterotypic secondary infection show development of mild or asymptomatic disease, as was observed in the DENV-3 epidemic in Cuba in 2000–2001.11
In general, tertiary challenge (simulation of a tertiary infection), showed a milder Th1 response to heterologous dengue virus challenge than secondary challenge. This finding could be the result of the heterotypic neutralizing capacity of antibodies against DENV-1 and DENV-2 in persons with secondary dengue infections. The weaker inflammatory Th1 response to a tertiary heterologous dengue virus challenge observed is also consistent with an increase in protective immunity after two heterotypic dengue infections. There are few reports of tertiary dengue infections, and those have been associated mostly with milder dengue.21,22
Homologous dengue virus re-challenge induced higher up-regulation of TGF-β and IL-10 gene expression in persons with dengue 1, dengue 2, and dengue 3 primary infections. The highest expression of TGF-β and IL-10 in PBMC from dengue 1, dengue 2 and dengue 3 primary immune persons was in response to the homotypic challenge, which is associated with an effective, balanced, and protective serotype-specific memory immune response.
Because over-expression of pro-inflammatory cytokines and over-activation of the immune system during heterologous secondary infection has been associated with severe dengue,63–66 the secondary homologous protective response could be associated with a balanced inflammatory/regulatory response.67–69 Regulatory activities of the cytokines are probably crucial in controlling the inflammatory response during dengue disease, which is necessary for control of viral infection, and the balance among inflammatory/regulatory mediators could be related to recovery of most patients with a dengue secondary infection.9,31,70,71
The IL-10 response of persons with a bivalent infection history showed higher expression in response to the third challenge with the three serotypes. This finding could be explained by the bivalent immunity, which confers wider memory protective immunity associated with IL-10, which in addition to its regulatory role of anti-inflammatory response, can perform other functions, such as leukocyte recruitment, particularly cytotoxic T-cells.72–74
The IL-10 response in persons with a DENV-1/DENV-2/DENV-3 history is notably lower when compared with that of other groups of study participants. A previous trivalent immunity, which increases serotype cross-reactive immune protectiveness, enables rapid and effective control of the infection. This control avoids an excess of pro-inflammatory cytokines (levels of which are also low in tertiary-infected persons). As a result, there is low induction of regulatory cytokines such as IL-10.
DENV-1/DENV-2 immune persons showed significantly higher expression of TGF-β to DENV-2 tertiary challenge. In DENV-1/DENV-3 persons, TGF-β expression was significantly higher to DENV-3 challenge. In DENV-2/DENV-3 persons, there was significantly higher TGFβ expression in response to challenge with DENV-2 and DENV-3. These findings might indicate that DENV-2 and DENV-3 are more immunogenic than DENV-1 in terms of regulatory cytokines induction, thereby inducing a stronger regulatory DENV-2/DENV-3–specific memory immune response.
DENV-2/DENV-3 challenge showed significantly lower levels of TGF-β expression than DENV-1/DENV-2 and DENV-1/DENV-3 challenges (P < 0.0001), which is similar to tertiary DENV-1/DENV-2/DENV-3 challenge. Interestingly, these secondary infection sequence groups also showed significantly lower expression of TNF-α and IFN-γ. Effective and rapid control of infection by heterovalent antibodies, which are products of the memory humoral immune response produced after DENV-2 and DENV-3 infections, could prevent activation of the TH antiviral and pro-inflammatory TNF-α and IFN-γ response. Low levels of TGF-β expression could be caused by absence of the stimulus of inflammatory cytokines.75,76
It is also interesting that persons with DENV-2/DENV-3 secondary infections showed levels of TNF-α and IFN-γ expression as low as those seen in persons with tertiary DENV-1/DENV-2/DENV-3 infections, and tentatively, lower TGF-β levels of expression. This finding could indicate that DENV-2/DENV-3 secondary infection may induce a cross-reactive cellular protective immunity that confers protection against the four dengue virus serotypes. These results could be useful for vaccine strategies.
Quaternary challenge in DENV-1/DENV-2/DENV-3 persons showed low expression levels of the four cytokines. This finding could be the result of a previous trivalent immunity, which increases serotype cross-reactive immune protectiveness, enabling rapid and effective control of infection. There are recent reports that confirm the importance of cell-mediated response in conferring protective response against dengue virus.77,78 This is the first report of cytokine expression after a quaternary dengue challenge, and supports the importance of heterovalent immunity to achieve a successful dengue vaccine.
In contrast to other countries characterized by co-circulation of several dengue virus serotypes, Cuba represents a unique environment to study tertiary and quaternary dengue challenges in a population with a well-defined history of dengue virus infections. We took advantage of this situation to investigate variations in expression of four cytokines with critical roles in DHF immunopathogenesis after secondary, tertiary, and quaternary ex vivo challenge in a sequence-specific manner. We confirmed that previous immunity to a dengue virus serotype determines the cellular immune response pattern in subsequent infections.
ACKNOWLEDGMENTS
We thank Dr. Linda Lloyd for excellent revision of the manuscript.
Footnotes
Authors' addresses: Beatriz Sierra, Ana B. Pérez, Mayling Alvarez, Gissel García, Eglys Aguirre, and María G. Guzmán, Tropical Medicine Institute Pedro Kourí, Autopista Novia del Mediodía, Km 6 ½, Entre Autopista Nacional y Carretera Central, La Lisa, Apartado 601, Marianao 13 C, Havana, Cuba, E-mails: siebet@ipk.sld.cu, anab@ipk.sld.cu, mayling@ipk.sld.cu, gmd@ipk.sld.cu, eglys@ipk.sld.cu, and lupe@ipk.sld.cu. Katrin Vogt, Kathrin Schmolke, and Hans-Dieter Volk, Institute for Medical Immunology, Charité-Universitätsmedizin Berlin, CCM, Charitéplatz 1,D-10117 Berlin, Germany, E-mails: katrin.vogt@charite.de, kathrin.schmolke@charite.de, and hans-dieter.volk@charite.de.
References
- 1.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 4.Halstead SB. Immune enhancement of viral infection. Prog Allergy. 1982;31:301–364. [PubMed] [Google Scholar]
- 5.Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11((Suppl 4)):S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 6.Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- 7.Cantelar de Francisco N, Fernandez A, Albert Molina L, Perez Balbis E. Survey of dengue in Cuba, 1978–1979 [in Spanish] Rev Cubana Med Trop. 1981;33:72–78. [PubMed] [Google Scholar]
- 8.Guzman MG, Kouri GP, Bravo J, Calunga M, Soler M, Vazquez S, Venereo C. Dengue haemorrhagic fever in Cuba. I. Serological confirmation of clinical diagnosis. Trans R Soc Trop Med Hyg. 1984;78:235–238. doi: 10.1016/0035-9203(84)90285-2. [DOI] [PubMed] [Google Scholar]
- 9.Guzman MG, Kouri G, Morier L, Soler M, Fernandez A. A study of fatal hemorrhagic dengue cases in Cuba, 1981. Bull Pan Am Health Organ. 1984;18:213–220. [PubMed] [Google Scholar]
- 10.Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, Halstead SB. Epidemiological studies on dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–799. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez D, Castro OE, Kouri G, Perez J, Martinez E, Vazquez S, Rosario D, Cancio R, Guzman MG. Classical dengue hemorrhagic fever resulting from two dengue infections spaced 20 or more years apart: Havana, dengue 3 epidemic, 2001–2002. Int J Infect Dis. 2005;9:280–285. doi: 10.1016/j.ijid.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez S, Bravo JR, Perez AB, Guzman MG. Inhibition ELISA: its utility for classifying a case of dengue [in Spanish] Rev Cubana Med Trop. 1997;49:108–112. [PubMed] [Google Scholar]
- 13.Alvarez Vera M, Valdes Palacios D, Vazquez Ramudo S, Delgado Hernandez I, Garcia Infante S, Morier Diaz L, Guzman Tirado MG. The standardization of the plaque reduction technique for differentiating a dengue infection from a yellow fever infection [in Spanish] Rev Cubana Med Trop. 1998;50:177–181. [PubMed] [Google Scholar]
- 14.Alvarez M, Rodriguez-Roche R, Bernardo L, Morier L, Guzman G. Improved dengue virus plaque formation on BHK21 and LLCMK2 cells: evaluation of some factors. Dengue Bull. 2005;29:49–57. [Google Scholar]
- 15.Morens DM, Halstead SB, Repik PM, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol. 1985;22:250–254. doi: 10.1128/jcm.22.2.250-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez Vera M, Valdes Palacios D, Vazquez Ramudo S, Delgado Hernandez I, Garcia Infante S, Morier Diaz L, Guzman Tirado MG. The standardization of the plaque reduction technic for differentiating a dengue infection from a yellow fever infection. Rev Cubana Med Trop. 1998;50:177–181. [PubMed] [Google Scholar]
- 17.Guzman MG, Kouri G, Bravo J, Soler M, Martinez E. Sequential infection as risk factor for dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) during the 1981 dengue hemorrhagic Cuban epidemic. Mem Inst Oswaldo Cruz. 1991;86:367. doi: 10.1590/s0074-02761991000300011. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc C, Deriaud E, Megret F, Briand JP, Van Regenmortel MH, Deubel V. Identification of helper T cell epitopes of dengue virus E-protein. Mol Immunol. 1993;30:613–625. doi: 10.1016/0161-5890(93)90072-j. [DOI] [PubMed] [Google Scholar]
- 19.Böyusn A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;21((Suppl 9)):77–89. [PubMed] [Google Scholar]
- 20.Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81:816–820. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez M, Rodriguez-Roche R, Bernardo L, Vazquez S, Morier L, Gonzalez D, Castro O, Kouri G, Halstead SB, Guzman MG. Dengue hemorrhagic fever caused by sequential dengue 1-3 virus infections over a long time interval: Havana epidemic, 2001–2002. Am J Trop Med Hyg. 2006;75:1113–1117. [PubMed] [Google Scholar]
- 22.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP, Jr, Srikiatkhachorn A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–913. [PubMed] [Google Scholar]
- 23.Guzman MG, Alvarez A, Vazquez S, Alvarez M, Rosario D, Pelaez O, Cruz G, Rodriguez-Roche R, Pavon A, Gonzalez A, Morier L, Ruiz D, Kourí G, Halstead SB. Epidemiologic studies on dengue 3 In Playa Municipality, Havana, Cuba, 2001–2002. Int J Infect Dis. 2012;16:e198–e203. doi: 10.1016/j.ijid.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Sierra B, Perez AB, Vogt K, Garcia G, Schmolke K, Aguirre E, Alvarez M, Kern F, Kourí G, Volk HD, Guzman MG. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol. 2010;262:134–140. doi: 10.1016/j.cellimm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Guzman MG. Global voices of science. Deciphering dengue: the Cuban experience. Science. 2005;309:1495–1497. doi: 10.1126/science.1115177. [DOI] [PubMed] [Google Scholar]
- 26.Guzman MG, Kouri G, Bravo J, Soler M, Morier L, Vazquez S, Diaz A, Fernandez R, Ruiz A, Ramos A. Dengue in Cuba: history of an epidemic [in Spanish] Rev Cubana Med Trop. 1988;40:29–49. [PubMed] [Google Scholar]
- 27.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis. 2007;30:329–340. doi: 10.1016/j.cimid.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Cardier JE, Marino E, Romano E, Taylor P, Liprandi F, Bosch N, Rothman AL. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine. 2005;30:359–365. doi: 10.1016/j.cyto.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 30.Guidotti LG, Chisari FV. Noncytolitic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Gunther VJ, Putnak R, Eckels KH, Mammen MP, Schererd JM, Lyonsa A, Szteine MB, Sunf W. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine. 2011;29:3895–3904. doi: 10.1016/j.vaccine.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Guzman MG, Pelaez O, Kouri G, Quintana I, Vazquez S, Penton M, Avila LC. Final characterization of and lessons learned from the dengue 3 epidemic in Cuba, 2001–2002 [in Spanish] Rev Panam Salud Publica. 2006;19:282–289. doi: 10.1590/s1020-49892006000400014. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 34.Ocazionez RE, Gomez SY, Cortes FM. Dengue hemorrhagic fever serotype and infection pattern in a Colombian endemic area [in Spanish] Rev Salud Publica (Bogota) 2007;9:262–274. doi: 10.1590/s0124-00642007000200010. [DOI] [PubMed] [Google Scholar]
- 35.Thomas L, Verlaeten O, Cabié A, Kaidomar S, Moravie V, Martial J, Najioullah F, Plumelle Y, Fonteau C, Dussart P, Césaire R. Influence of the dengue serotype, previous dengue infection, and plasma viral load on clinical presentation and outcome during a dengue-2 and dengue-4 co-epidemic. Am J Trop Med Hyg. 2008;78:990–998. [PubMed] [Google Scholar]
- 36.Tsai JJ, Chan KS, Chang JS, Chang K, Lin CC, Huang JH, Lin WR, Chen TC, Hsieh HC, Lin SH, Lin JC, Lu PL, Chen YH, Lin CY. Effect of serotypes on clinical manifestations of dengue fever in adults. J Microbiol Immunol Infect. 2009;42:471–478. [PubMed] [Google Scholar]
- 37.Kumaria R. Correlation of disease spectrum among four dengue serotypes: a five years hospital based study from India. Braz J Infect Dis. 2010;14:141–146. [PubMed] [Google Scholar]
- 38.Guzman MG, Deubel V, Pelegrino JL, Rosario D, Marrero M, Sariol C, Kouri G. Partial nucleotide and amino acid sequences of the envelope and the envelope/nonstructural protein-1 gene junction of four dengue-2 virus strains isolated during the 1981 Cuban epidemic. Am J Trop Med Hyg. 1995;52:241–246. doi: 10.4269/ajtmh.1995.52.241. [DOI] [PubMed] [Google Scholar]
- 39.Sariol CA, Pelegrino JL, Martinez A, Arteaga E, Kouri G, Guzman MG. Detection and genetic relationship of dengue virus sequences in seventeen-year-old paraffin-embedded samples from Cuba. Am J Trop Med Hyg. 1999;61:994–1000. doi: 10.4269/ajtmh.1999.61.994. [DOI] [PubMed] [Google Scholar]
- 40.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 41.Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, Halstead SB. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 42.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 43.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 44.Halstead SB. Immunological parameters of togavirus disease syndromes. In: Schlesinger RW, editor. The Togaviruses: Biology, Structure, Replication. New York: Academic Press; 1980. pp. 107–173. [Google Scholar]
- 45.Russell PK, Yuill TM, Nisalak A, Udomsakdi S, Gould DJ, Winter PE. An insular outbreak of dengue hemorrhagic fever. II. Virologic and serologic studies. Am J Trop Med Hyg. 1968;17:600–608. doi: 10.4269/ajtmh.1968.17.600. [DOI] [PubMed] [Google Scholar]
- 46.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, Cummings DAT. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4:e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen RF, Yang KD, Wang L, Liu JW, Chiu CC, Cheng JT. Different clinical and laboratory manifestations between dengue haemorrhagic fever and dengue fever with bleeding tendency. Trans R Soc Trop Med Hyg. 2007;101:1106–1113. doi: 10.1016/j.trstmh.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.White NJ. Variation in virulence of dengue virus. Lancet. 1999;354:1401–1402. doi: 10.1016/S0140-6736(99)00236-6. [DOI] [PubMed] [Google Scholar]
- 49.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de Chacon, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong PM, Rico-Hesse R. Differential susceptibility of Aedes aegypti to infection by the American and Southeast Asian genotypes of dengue type 2 virus. Vector Borne Zoonotic Dis. 2001;1:159–168. doi: 10.1089/153036601316977769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey BD, Morita K, Hasebe F, Parquet MC, Igarashi A. Molecular evolution, distribution and genetic relationship among the dengue 2 viruses isolated from different clinical severity. Southeast Asian J Trop Med Public Health. 2000;31:266–272. [PubMed] [Google Scholar]
- 52.Pryor MJ, Carr JM, Hocking H, Davidson AD, Li P, Wright PJ. Replication of dengue virus type 2 in human monocyte-derived macrophages: comparisons of isolates and recombinant viruses with substitutions at amino acid 390 in the envelope glycoprotein. Am J Trop Med Hyg. 2001;65:427–434. doi: 10.4269/ajtmh.2001.65.427. [DOI] [PubMed] [Google Scholar]
- 53.Cologna R, Rico-Hesse R. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J Virol. 2003;77:3929–3938. doi: 10.1128/JVI.77.7.3929-3938.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hober D, Delannoy AS, Benyoucef S, De Groote D, Wattre P. High levels of sTNFR p75 and TNF alpha in dengue-infected patients. Microbiol Immunol. 1996;40:569–573. doi: 10.1111/j.1348-0421.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 55.Hober D, Nguyen TL, Shen L, Ha DQ, Huong VT, Benyoucef S, Nguyen TH, Bui TM, Loan HK, Le BL, Bouzidi A, De Groote D, Drouet MT, Deubel V, Wattre P. Tumor necrosis factor alpha levels in plasma and whole-blood culture in dengue-infected patients: relationship between virus detection and pre-existing specific antibodies. J Med Virol. 1998;54:210–218. doi: 10.1002/(sici)1096-9071(199803)54:3<210::aid-jmv12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere JL, Vergez-Pascal R, Wattre P. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 57.Suharti C, Van Gorp EC, Setiati TE, Dolmans WM, Djokomoeljanto RJ, Hack CE, Ten CH, Van der Meer JW. The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb Haemost. 2002;87:42–46. [PubMed] [Google Scholar]
- 58.Gagnon SJ, Mori M, Kurane I, Green S, Vaughn DW, Kalayanarooj S, Suntayakorn S, Ennis FA, Rothman AL. Cytokine gene expression and protein production in peripheral blood mononuclear cells of children with acute dengue virus infections. J Med Virol. 2002;67:41–46. doi: 10.1002/jmv.2190. [DOI] [PubMed] [Google Scholar]
- 59.Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Ann N Y Acad Sci. 2009;1171:E36–E41. doi: 10.1111/j.1749-6632.2009.05055.x. [DOI] [PubMed] [Google Scholar]
- 60.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunological Rew. 2008;225:300–313. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 61.Dong T, Moran E, Vinh Chau N, Simmons C, Luhn K, Peng Y, Wills B, Phuong Dung N, Thi Thu Thao L, Hien TT, McMichael A, Farrar J, Rowland-Jones S. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS ONE. 2007;2:e1192. doi: 10.1371/journal.pone.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv Virus Res. 2003;60:397–419. doi: 10.1016/s0065-3527(03)60010-2. [DOI] [PubMed] [Google Scholar]
- 63.Kurane I, Innis BL, Nimmannitya S, Nisalak A, Rothman AL, Livingston PG, Janus J, Ennis FA. Human immune responses to dengue viruses. Southeast Asian J Trop Med Public Health. 1990;21:658–662. [PubMed] [Google Scholar]
- 64.Kurane I, Innis BL, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis FA. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Invest. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green S, Pichyangkul S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Nisalak A, Kurane I, Rothman AL, Ennis FA. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis. 1999;180:1429–1435. doi: 10.1086/315072. [DOI] [PubMed] [Google Scholar]
- 66.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 67.Guzman MG, Kouri G, Valdes L, Bravo J, Vazquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11:223–227. doi: 10.1590/s1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 68.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 69.Halstead SB. Dengue. Curr Opin Infect Dis. 2002;15:471–476. doi: 10.1097/00001432-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Santos M, Villaescusa R, Basanta P, Indan G, Ballester JM. Dengue haemorrhagic fever in Cuba. II. Clinical investigations. Trans R Soc Trop Med Hyg. 1984;78:239–241. doi: 10.1016/0035-9203(84)90286-4. [DOI] [PubMed] [Google Scholar]
- 71.Kouri G, Guzman MG, Bravo J. Hemorrhagic dengue in Cuba: history of an epidemic. Bull Pan Am Health Organ. 1986;20:24–30. [PubMed] [Google Scholar]
- 72.Jinquan T, Larsen CG, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. 1993;151:4545–4551. [PubMed] [Google Scholar]
- 73.Vora M, Romero LI, Karasek MA. Interleukin-10 induces E-selectin on small and large blood vessel endothelial cells. J Exp Med. 1996;184:821–829. doi: 10.1084/jem.184.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santin AD, Hermonat PL, Ravaggi A, Bellone S, Pecorelli S, Roman JJ, Parham GP, Cannon MJ. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2000;74:4729–4737. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 76.Taylor AW. Review of the activation of TGF-beta in immunity. J Leukoc Biol, 2009 Jan. 2009;85((1)):29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gil L, López C, Lazo L, Valdés I, Marcos E, Alonso R, Gambe A, Martin J, Romero Y, Guzmán M, Guillén G, Hermida L. Recombinant nucleocapsid-like particles from dengue-2 virus induce protective CD4+ and CD8+ cells against viral encephalitis in mice. Int Immunol. 2009;21:1175–1183. doi: 10.1093/intimm/dxp082. [DOI] [PubMed] [Google Scholar]
- 78.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]