Abstract
Serum samples from a total of 71 healthy captive birds belonging to 18 species were collected in July of 2008 in Medellin (Colombia) and tested for flaviviruses. Eighteen of 29 samples from American Flamingoes (Phoenicopterus ruber) were positive for West Nile virus (WNV) by reverse transcription-polymerase chain reaction. Selected positive samples were serially passaged and WNV was confirmed by immunofluorescence. Two isolates (524/08, 9835/08) were characterized in vitro and in vivo. Sequence analysis revealed WNV with 16 nucleotide substitutions resulting in six amino acid changes when compared with the NY99 strain. Colombian (COL) viruses were more closely related to Louisiana isolates from 2001. When compared with attenuated strains isolated from Texas, COL isolates differed in their plaque size and temperature sensitivity phenotype. The COL viruses were pathogenic in embryonated chicken eggs and Balb/c mice.
Introduction
West Nile virus (WNV) is an arboviral zoonotic pathogen, first isolated in 1937, that has been detected throughout Africa, the Middle East, southern Europe, Russia, India, and the Americas.1,2 The virus can affect the nervous system of humans, horses, and birds, causing mild to severe illness and sometimes death. The WNV is a member of the Japanese encephalitis virus antigenic complex in the family Flaviviridae, genus Flavivirus. Flaviviruses are found on every continent except Antarctica, but none is as widely distributed as WNV.3 In its natural cycle, WNV is maintained in birds and Culex spp. mosquitoes. Many wild vertebrates, including wolves, bears, crocodiles, and alligators,4–6 as well as domestic animals such as horses, cats, and dogs can be naturally infected.7,8
In 1999, WNV was introduced into the Americas9 causing an outbreak in New York with subsequent spread throughout the United States. Since the first detection in the United States, WNV has caused over 12,000 cases of meningitis/encephalitis and over 1,100 human fatalities. In the following years, the virus spread through Canada,10 Mexico,11 Central America, the Caribbean,12–14 and South America.2,15
Despite causing disease in more than 12,000 humans, at least 25,000 equids, and hundreds of thousands of birds in the United States and Canada, there have been only a few reports of WNV disease in the Caribbean and Latin America.2 The first report of the virus in Colombia appeared in 2005 following serological surveys of equids from the northern coastal area.16,17 Recently, serologic evidence of WNV activity was found in horses from two Colombian provinces, Antioquia and El Meta, located in the northwestern and central-eastern regions of the country.18 The WNV has been isolated only once in South America, after the death of three horses in Argentina in 2006.19 It is puzzling why WNV has never been isolated or caused severe disease in horses or humans in other South American countries. To increase knowledge about the diversity of arboviruses in Latin America and to confirm the presence of WNV in Colombia, a viral survey was conducted in the summer of 2008 in captive but otherwise healthy wild birds at the Santa Fe zoo collection in Medellin (Colombia).
Materials and Methods
Study sites and collection methods.
Collection of field samples was carried out during the month of July 2008 at the Santa Fe Zoo in Medellin, Colombia (6°13′23.69″N 75°34′48.28″W). Blood samples were collected from captive wild birds belonging mainly to Orders Phoenicopteriformes, Anseriformes, Psittaciformes, and Galliformes. Captive birds were captured within their enclosures by hand. Blood samples were collected from the wing vein by brachial venipuncture, maintained at 4°C overnight and then centrifuged for 15 minutes at 4°C. The sera was separated and then stored at −80°C until tested. All the serological procedures were performed following the guidelines for WNV Surveillance by the Centers for Disease Control and Prevention (CDC).20
Flavivirus detection by reverse transcription-polymerase chain reaction (RT-PCR).
A total of 71 serum samples were pooled into five groups based on the order and species of the birds (Table 1). Total RNA from each pool was extracted using the QIAamp viral RNA mini kit (Qiagen, Inc., Valencia, CA), as per the manufacturer's instructions. The RNA samples were tested for presence of flaviviruses by RT-PCR using a One-Step RT-PCR Kit (Qiagen, Inc.), universal flavivirus FU2/cFD3, and UniF/UniR primers as described elsewhere.21 The reaction conditions are available upon request. The PCR products were visualized by agarose (1%) gel electrophoresis. Individual samples from PCR-positive pools were then analyzed by RT-PCR following the same procedure as described previously.
Table 1.
List of bird species tested and sorting of animal groups
| Number of pool | Number of samples | Common name | Scientific name |
|---|---|---|---|
| 1 | 14 | American Flamingo | Phoenicopterus ruber |
| 2 | 15 | American Flamingo | Phoenicopterus ruber |
| 3 | 2 | Black Swan | Cygnus atratus |
| 5 | Black-Bellied Whistling Duck | Dendrocygna autumnalis | |
| 4 | Blue-and-yellow Macaw | Ara ararauna | |
| 1 | Blue-billed Curassow | Crax alberti | |
| 2 | Blue-headed Parrot | Pionus menstruus | |
| 1 | Cauca Guan | Penelope perspicax | |
| 4 | 2 | Domestic duck | Anas platyrhynchos domesticus |
| 3 | Great Curassow | Crax rubra | |
| 2 | Great Green Macaw | Ara ambiguous | |
| 1 | Scarlet Macaw | Ara macao | |
| 1 | Mealy Amazon | Amazona farinose | |
| 1 | Orange-winged Amazon | Amazona amazonica | |
| 1 | Red-lored Amazon | Amazona autumnalis | |
| 1 | Yellow-crowned Amazon | Amazona ochrocephala | |
| 1 | Orinoco Goose | Neochen jubata | |
| 1 | Bronze-winged Parrot | Pionus chalcopterus | |
| 5 | 13 | Indian Peafowl | Pavo cristatus |
Virus isolation into mosquito cells.
Viral isolation studies were conducted at BSL3 laboratories located at the U.S. Geological Survey-National Wildlife Health Center (USGS-NWHC). Viral isolation of flavivirus RT-PCR positive samples were attempted by three blind passages into C6/36 cells as described elsewhere.22 Briefly, an aliquot of 50 μL from each flavivirus positive serum sample was mixed with 1 mL of Liebovitz L15 medium with L-glutamine (Sigma-Aldrich, Saint Louis, MO) supplemented with 2% fetal bovine serum (FBS), and then inoculated onto subconfluent monolayer of Aedes albopictus C3/36 cells in 25-cm2 flasks. After 1 hour incubation at 28°C, 3 mL of L15 medium containing 10% FBS were added and cell monolayers were incubated at 28°C for 7 days. After two additional blind passages, cell monolayers were harvested and tested for WNV by the indirect fluorescent antibody (IFA) test. The IFA positive cultures were lysed through one freeze-thaw cycle and then supernatants were collected, clarified by centrifugation, and stored in aliquots at −80°C for RT-PCR testing and viral titration.
Flavivirus genome amplification and sequencing.
Total RNA was extracted from WNV-IFA positive supernatants and RT-PCR was performed using universal flavivirus FU2/cFD3 and UniF/UniR mentioned previously. The RT-PCR conditions were similar as previously mentioned. The PCR products were purified using the Gel extraction kit (Qiagen, Inc.) and purified materials were sequenced at the Biotechnology Center at University of Wisconsin (Madison, WI). Sequences were edited and aligned using the BioEdit program (available at: http://www.mbio.ncsu.edu/BioEdit/bioedit.html) and DAMBE software23; after editing, the sequences were compared in GenBank by basic local alignment search tool (BLAST).
WNV antigen expression in mammalian cells.
The WNV antigen expression in mammalian cells was screened by a modified fluorescent assay described elsewhere.24 Briefly, confluent Vero and BHK21 cells in 12-well plates were infected with 100 μL containing 10-fold serial dilutions of each viral isolate. Viral adsorption was allowed to proceed for 1 hour (h) at 37°C in 5% CO2. The media was decanted, and an overlay of Dulbecco's modified eagle medium (DMEM), 2% FBS, and 1% SeaPlaque Agarose (Cambrex Bio Science Rockland, Inc., Charles City, IA) was added and plates were incubated at 37°C in 5% CO2. At 72 h post-infection, the overlay was removed from the wells and replaced with phosphate buffered saline (PBS) (4°C × 5 min). Cells were then fixed for 30 min with cold methanol/acetone (1:1) at −20°C, rinsed twice with 0.1% BSA/PBS 0.05% Tween20 washing buffer (WB), and blocked with 2% FBS/PBS/0.05% Tween20 blocking buffer (BB), at 37°C for 1 h. Antigen was identified using a WNV and Kunjin virus-specific monoclonal antibody (MAB8151 Millipore) diluted 1:500 in BB, incubated for 1 h, and then washed twice with WB. Antibody-labeled cells were detected after 30 min of incubation with a commercial FITC anti-mouse immunoglobulin G (IgG) antibody diluted 1:500, followed by two washes with WB. Finally, a 90% glycerol/PBS solution was applied to cover the monolayer, and cells were observed on a fluorescent microscope.
Viral titration and plaque morphology.
Viral titers were determined by plaque assay in Vero cells and expressed as plaque-forming units (PFU) per mL. Viral titers and plaque phenotypes were determined in Vero cell monolayers in 12-well plates as described elsewhere with some modifications.25 Briefly, viruses were allowed to attach for 1 h at 37°C in 5% CO2 and an overlay media of DMEM containing 1% Methylcellulose and 2% FBS was then added to each well. At 4 days post-infection, neutral red/Dulbecco's phosphate buffered saline (DPBS) solution (0.04%) was added and cells were incubated at 37°C overnight. Plaques were counted and titers were expressed as PFU/mL. In addition, plaque size was measured under a stereomicroscope (10×) and the diameter was calculated by using a linear scale micrometer disc. The small plaque (sp) phenotype was described as a < 1.5 mm diameter whereas large plaque (lp) was > 1.5 mm in diameter.25
Temperature-sensitivity (TS) assays.
The COL WNV isolates were further characterized by their TS phenotypes. Vero and BHK21 cells were adapted to grow at 39.5°C. The TS assays were conducted at 37 and 39.5°C. Cells were seeded in 6-well plates and infected in duplicates at MOI of 0.01. At 24–72 h intervals post-infection, cells and media from each well were harvested and centrifuged at 3,000 rpm for 5 min. The TS phenotype was defined as a > 2.5 log10 reduction in infectivity titer determined by PFU/mL for both cell lines. The WNV titers in both cells lines at 39.5 and 37.0°C were determined. In addition, cell pellets were spotted onto glass slides, air dried for 5 min, fixed with cold methanol/acetone (1:1) at −20°C for 30 min, and then tested by IFA as described previously to confirm viral replication.
Sequencing of WNV isolates and phylogenetic analysis.
The entire virus genome (11,029 bp) from four viral isolates was PCR amplified into 12 overlapping segments. The PCR products were purified using a gel extraction kit (Qiagen, Inc.) and sequenced at UW Biotechnology Center (primers sequences and RT-PCR conditions are available upon request). Sequences obtained in this study were deposited in GenBank (accession nos.: JN716371 and JN716372). The resulting sequences were edited and assembled into complete genome sequences using BioEdit program and ContigExpress (VectorNTI software, Invitrogen, Grand Island, NY).
For the Phylogenetic and BEAST analysis, 52 WNV full-genome sequences, from North and Latin America were downloaded from GenBank. Nucleotide sequences were aligned using the ClustalW component of DAMBE,23 and then manually edited using the BioEdit program. The phylogenetic relationship between COL WNV isolates and published sequences was estimated using the Bayesian MCMC approach available in the BEAST package.26 Briefly, the XML file was produced by BEAUTI in the BEAST package, using a model with the following parameters: nucleotide substitution (TN93), estimated base frequencies, homogeneous sites, and a strict molecular clock. The MCMC analysis was run for 10 million chains. A maximum clade credibility (MCC) tree was produced and annotated by Tree Annotator in the BEAST package. The tree was visualized and edited with FigTree software (available from http://tree.bio.ed.ac.uk/software/figtree). Nucleotide alignments from COL isolates were translated into amino acid sequences using the BioEdit program and substitutions in comparison with reference viruses were identified using DNAsp software.27 Amino acid substitutions were compared for closely related isolates.
Comparative virulence and pathogenicity studies.
Animal procedures were carried out in Biosafety Level 3 (BSL3) containment facilities and approved by the Institutional Animal Care and Use Committee (IACUC) of the School of Veterinary Medicine of the University of Wisconsin-Madison.
Inoculation in embryonated chicken eggs.
The WNV is known to cause mortality of chicken embryos. Thus, COL WNV isolates were tested for their virulence by inoculation into the allantoic cavity of 9 to 10-day-old specific pathogen free embryonated chicken eggs (N = 3 per treatment; Sunnyside Hatchery, Beaver Dam, WI) as described elsewhere.28 Briefly, eggs were incubated at 37°C following allantoic cavity inoculation with 100 μL of 10-fold dilutions of infected supernatants yielding a starting infectious dose of 1.8 × 105 PFU/mL and 1.5 × 107 PFU/mL for COL524/08 and COL9835/08 isolates, respectively. A control group was inoculated with 100 μL of PBS. Eggs were candled daily for up to 7 days. At that time, samples of allantoic fluid were aseptically harvested and were inoculated into cultures of Vero cells, which were subsequently examined by IFA to confirm the presence of WNV. The minimum lethal dose was determined as the highest virus dilution that caused 50% mortality.
Virulence in newborn and 4-week-old mice.
The virulence of COL WNV isolates was evaluated in both 1 to 3-day-old and 4-week-old Balb/c mice (Harlan Sprague Dawley, Inc., Mapleton, IN). Groups of three newborn mice were inoculated intracranially (i.c.) with 20 μL of 10-fold dilutions of COL WNV isolates. Viral doses ranged between 1.5 × 104 PFU and 1.0 × 102 PFU for COL9835/08 and 1.8 × 103 and 1.8 PFU × 10° for COL524/08. Groups of four 4-week-old mice were injected with either 20 μL i.c. or 100 μL intraperitoneally (i.p.). Similar 10-fold dilution doses as in newborns were used for both inoculation routes. Control groups for both ages were inoculated with PBS. Mice were monitored daily for 14 days to determine clinical signs of disease such as lethargy, anorexia, and paralysis. Severely ill animals were euthanized following IACUC guidelines. Brain homogenates from selected dead animals were subsequently examined by IFA and RT-PCR to confirm the presence of WNV.
Results
A total of 71 birds belonging to 18 species were sampled in July of 2008 at the Santa Fe Zoological Park in the Medellin city, province of Antioquia, Colombia (see Table 1 for a complete list of species sampled). All birds appeared to be in good health at the sampling time.
Flavivirus detection by RT-PCR.
Universal FU2/cFD3 and UniF/UniR primers detected flavivirus cDNAs only in two serum pools (29 samples) that originated from American Flamingos (Phoenicopterus ruber). These primers amplified the NS5 and E genes, yielding detectable products of ∼845 and 872 bp for each primer pair, respectively (data no shown). Individual testing of the specimens revealed that 18 out of 29 samples (62%) were positive for flavivirus by RT-PCR.
Virus isolation into C6/36 cells.
Ten of the flavivirus PCR-positive samples were randomly selected for virus isolation attempts in C3/36 cells. After three serially blind passages, spotted cells from four out of the 10 samples were confirmed for WNV by IFA (data not shown). The WNV was also confirmed in these samples by RT-PCR (data not shown). Viral titers in infected cell supernatants ranged between 1.8 × 105 PFU/mL and 1.5 × 107 PFU/mL for the COL524/08 and COL9835/08 isolates, respectively.
Sequencing and phylogenetic analysis.
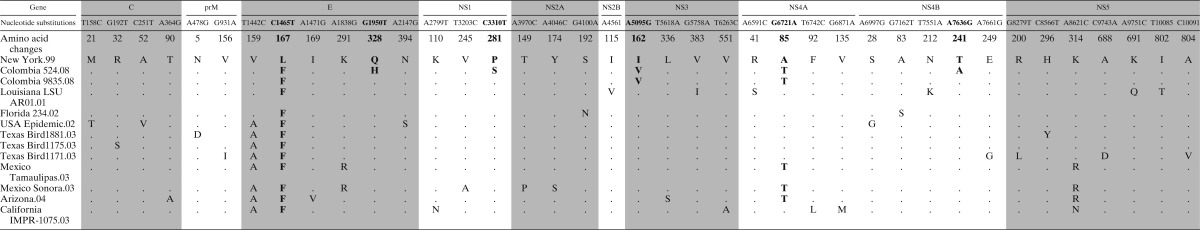
Four isolates (COL524/08, COL739/08, COL928/08, and COL9835/08) were selected for full sequence analysis and two distinct variants (524/08 and 9835/08) were further characterized in vitro and in vivo. Four contiguous sequence fragments (10,754 bp – 97.5% genome) comprising the entire coding region and partial UTRs (76–96 and 10,399–10,829) were assembled and aligned. All RT-PCR products revealed WNV sequence similarity by BLAST comparison. The WNV isolates COL9835/08, COL928/08, and COL739/08 were 100% identical, but differed from COL524/08 in three amino acid substitutions in the E (Q328H), NS1 (P281S), and NS4B (T241A) genes, indicating that at least two WNV genotypes are currently circulating in Colombia (Table 2). Sequence comparisons between NY99 and COL isolates revealed a total 16 nucleotide changes; all of them were located in the viral coding region and resulted in six amino acid substitutions: two in the E (L167F, Q328H) and single ones in the NS1, NS3, NS4A, and NS4B genes (Table 2).
Table 2.
Amino acid changes and nucleotide substitutions among COL WNV isolates*
Amino acid sequences for COL524/08 and COL9835/08 isolates were compared with NY99 (GenBank accession no. DQ211652) and other isolates from the region (LA, FL, TX, MEX, CA, and AZ).
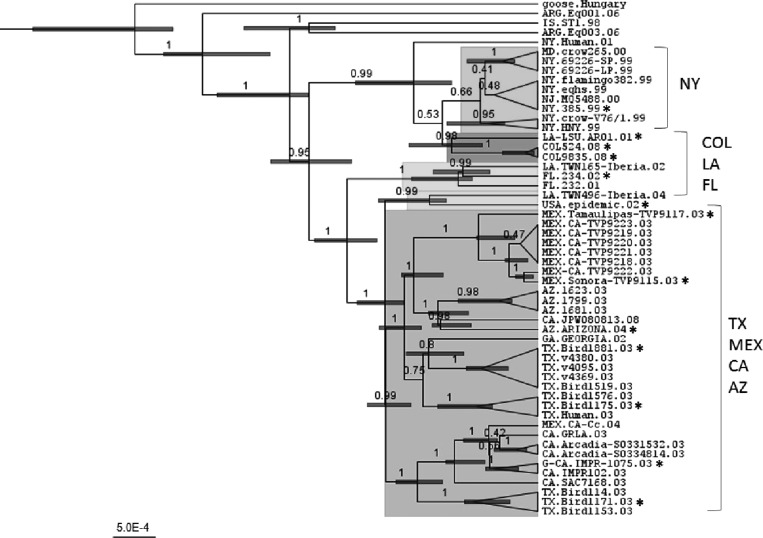
When compared with more recent United States isolates such as WNV LA01, the COL isolates show single amino acid changes in E (Q328H), NS1 (P281S), NS3 (I162V), NS4A (A85T), and NS4B (T241A) genes, indicating the existence of genetic differences between COL WNV isolates and other isolates from other countries in the Americas (Table 2). A phylogenetic analysis using the sequences of COL WNV isolates and 52 complete coding region sequences of GenBank from the Americas, clearly shows that COL WNV isolates are different than the NY99 strain and that these viruses are more closely related to Louisiana (LA) isolates from 2001 (Figure 1).
Figure 1.
Phylogenetic distribution of West Nile virus (WNV) isolates from Colombia and other American isolates. Maximum clade credibility (MCC) tree of the whole encoded region of WNV genomes constructed using BEAST. Colored boxes correspond to the principal branches predicted. The number at the nodes corresponds to the level of posterior probability. Asterisks mark the sequences used to create amino acid comparisons in Table 2.
Tissue culture characterization.
The WNV isolates COL524/08 and COL9835/08 were characterized in Vero and BHK21 cell cultures. Both cell lines showed initial cytopathic effect (CPE) at 24 h post-infection for both isolates. However, CPE was observed in greater scale in BHK21 cells than in Vero cells. The COL524/08 isolate caused more CPE than COL9835/08 in both cell lines, but both isolates produced complete CPE by 72 h post-infection. All isolates had similar growth characteristics and permissive infections were confirmed on both cell lines (Table 3). Additionally, both isolates exhibited large plaque phenotype (> 1.5 mm) that were highly homogeneous at 4 days post-infection in Vero cells (data not shown). The plaque size was slightly larger for the COL524/08 (N = 12, 3.5 ± 0.41 mm) than for COL9835/08 (N = 30, 3.1 ± 0.42 mm), although no statistically significant difference was found.
Table 3.
Viral titers of Colombian West Nile virus (COL WNV) isolates at 37 and 39.5°C in BHK21 and Vero cells*
| WNV isolate | Cell line | Viral Titers Times Post-Infection, PFU (Log10) | |||||
|---|---|---|---|---|---|---|---|
| 37°C | 39.5°C | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| COL524/08 | Vero | 4.8 | 6.8 | 7.8 | 4.8 | 6.8 | 6.8 |
| BHK21 | 5.8 | 8.8 | 6.8 | 4.8 | 5.8 | 6.8 | |
| COL9835/08 | Vero | 6.8 | 6.8 | 7.8 | 6.8 | 7.8 | 7.8 |
| BHK21 | 6.8 | 7.8 | 7.8 | 6.8 | 8.8 | 6.8 | |
WNV isolates COL524/08 and COL9835/08 were characterized by their temperature sensitivity (TS) phenotypes in Vero and BHK21 cells. Temperature sensitivity assays were conducted at 37 and 39.5°C. Cells were seeded in 6-well plates and infected in duplicates at MOI of 0.01. At 24–72 h intervals post-infection, cells, and media from each well were harvested and centrifuged at 3,000 rpm for 5 min.
Temperature-sensitivity assays.
The TS assays in Vero and BHK21 cells showed that virus replication was supported in both cell lines at permissive and non-permissive temperatures (Table 3). Both COL9835/08 and COL524/08 isolates caused CPE that reached 100% cell mortality at 72 h post-infection. The WNV antigen expression for each time point in both cell lines was confirmed by IFA. At 39.5°C, no marked differences were observed in the growth and viral yields of both isolates.
In vivo studies: inoculation in embryonated chicken eggs.
The avian virulence and pathogenicity of COL9835/08 and COL524/08WNV isolates was confirmed by inoculation into the allantoic cavity of 10-day-old chicken embryos. Both viruses produced 100% mortality at high concentrations with a mean death time ranging between 152 and 162 h post-infection. The embryo 50% lethal dose was 4.7 × 103 and 1 × 102 PFU/mL for COL9835/08 and COL524/08, respectively.
Virulence in newborn and 4-week-old mice
The inoculation of COL9835/08 and COL524/08 WNV isolates in newborn Balb/c mice resulted in significant neurovirulence and mortality. All viral isolates induced similar clinical signs including anorexia and lethargy and were lethal at all doses tested (data not shown). The WNV antigen expression was confirmed in the brain of infected mice by IFA (data not shown).
Inoculation of WNV isolates into 4-week-old Balb/c mice by the i.c. or i.p. routes induced clinical sings that included hind limb paralysis, ruffled fur, hunched posture, anorexia, and lethargy. For both isolates the clinical signs were first observed between Days 3 and 4 and all animals died by Day 6 post-inoculation. Neurovirulence and neuroinvasiveness of COL isolates was shown in mice (data not shown). The WNV antigen expression was also confirmed in the brain of infected mice by IFA.
Discussion
This is the first study in which WNV has been isolated and characterized from Colombia. These viruses were found in captive but otherwise healthy birds at a zoological collection of Medellin, suggesting the possibility of circulation of less virulent strains in Colombia. Sequence analysis showed nucleotide differences between COL viruses, indicating that at least two WNV genotypes are circulating in the area. Further analysis revealed that COL viruses are distinct from the WNV NY99 strain and more closely related to LA isolates from 2001. Phenotypic characterization in vitro showed differences between COL and attenuated WNV isolated from Texas (TX) on their growth in cell culture. Although these were isolated from healthy birds, in vivo studies show that COL viruses were pathogenic in embryonated chicken eggs and highly virulent in newborn and 4-week-old Balb/c mice.
For several years, there has been some evidence of natural circulation of WNV in Colombia and other surrounding countries. Separate serological studies in 2005 and 2006 have suggested the presence of the virus in the northern coastal areas.16,17 We recently also documented serologic evidence of WNV activity in horses from two central Colombian provinces.18 One of these sites is located in the province of Antioquia and ∼30 miles southwest from the zoo collection surveyed in this study. With evidence of WNV in central and northern regions, it is likely that the virus is circulating in other regions as well and possibly spreading to new regions.
It is interesting that all WNV sequences were detected and isolated from American Flamingoes, but not from other captive birds that were sampled in this study. Eighteen of 29 flamingoes sampled were positive by RT-PCR. It is possible that mutations detected in COL isolates have restricted the virus host range to only flamingoes or viremia levels in other captive birds are below the threshold of detection for the RT-PCR used in the current study. An alternative explanation is that there was local transmission of WNV between flamingoes in the aquatic environment in which they are group housed. Other birds sampled in this study were housed in separate enclosures. The high prevalence among this particular group of flamingoes may support this hypothesis. We did not test mosquito populations to determine if local WNV transmission is occurring. A third possible explanation is that the flamingoes were infected before they were housed in the zoo and maintained a persistent viremia. This explanation seems less likely because experimental studies of WNV infection in birds and mammals indicate that most species have a transient viremia (3–6 days),29 however the length of viremia is unknown in American flamingoes. A review of the history and source records of these animals showed that they arrived to the zoo between 1 and 5 years ago from different areas of Colombia.
A second peculiarity of these results in that these viruses were isolated from apparently healthy birds, although in vivo studies indicate that they are pathogenic in mammalian and avian models. One possibility is that that these flamingoes possess antibodies to another flavivirus that altered the progression of subsequent WNV infection. Diaz and others30 showed that infection with St. Louis encephalitis virus was modified in house sparrows previously infected with other flaviruses. Many other flaviruses are known to circulate in Colombia and neighboring countries, such as yellow fever, dengue, and others including Bussuquara, Cacipacore, Ilheus, Iguape, and Rocio viruses.31 We cannot explore this possibility further, because the antibody status of the birds in this study is unknown. Additionally, clinical signs of WNV vary greatly between species, with some experiencing severe disease, whereas others can be viremic with no apparent signs.29 A second possibility is that the flamingoes were experiencing mild clinical signs, such as lethargy and inappetence, but that these were not easily detected by zoo staff. It is a well-known occurrence for wild animals to hide illness until disease is very severe or fatal. Since the time of sampling, no clinical illnesses have been reported in any of the WNV-positive Flamingoes.
In addition to genetic similarities between WNV isolates from LA and COL, there are also phenotypic similarities. In vitro characterization shows that COL isolates induce CPE in Vero cells, similar to LA AR01. Both COL524/08 and COL9835/08 isolates were highly lethal in 4-week-old mice by the i.c. and i.p. routes. The low LD50 value (< 1.8 PFU) and clinical signs clearly indicate that, like LA viruses, COL viruses are highly neurovirulent and neuroinvasive and can induce severe disease in the mouse animal model.32
The LA AR01 strain was isolated from a dead blue jay, whereas our isolates are from healthy birds. This fact raises the question if these strains may be attenuated. However, in comparison to attenuated strains from TX, COL isolates are more pathogenic in vitro. The COL isolates grew similarly at 37 and 39.5°C, whereas attenuated TX viruses grew only at permissive temperatures and had reduced replication in Vero cells. Both COL viruses had large and homogenous plaque size, whereas attenuated TX viruses had small plaques.25 Additionally, some TX strains exhibit neurovirulence but not neuroinvasiveness.25 The COL isolates were lethal in chicken embryos, but only at high concentrations. This could be evidence of virulence in avian species. However, we do not know if COL viruses are pathogenic in adult chickens or wild birds. Future studies should include experimental infection of COL WNV strains in chickens, flamingoes and other wild birds.
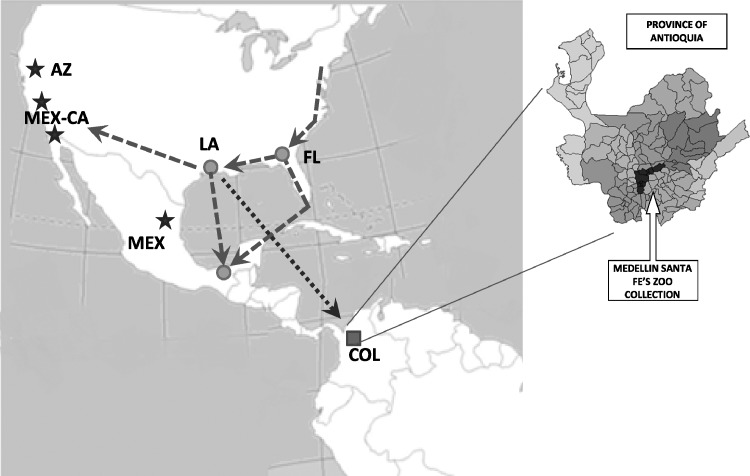
The closeness between COL and LA isolates leads to speculations that these viruses were perhaps introduced into Colombia by migratory birds from either the Atlantic coast or the Mississippi valley flyways. The Atlantic coast or eastern flyway overlaps both New York and Florida, while the Mississippi Valley flyway overlaps Louisiana, the Yucatan Peninsula of Mexico and Central American and Caribbean countries. Thus, migratory birds could have carried WNV from the southeastern United States into Colombia, either directly or through the Caribbean. Figure 2, shows the hypothetical routes of West Nile virus spread in America and introduction into Colombia. Serosurveys have suggested WNV circulation among birds in various Caribbean islands since 2002.13 It is also possible for the virus to have entered Colombia by other means such as the importation of infected mosquitoes by air travel, sick horses, or infected people.
Figure 2.
Map showing the hypothetical route of West Nile virus introduction into Colombia. Circles indicate locations of isolates in the Florida-Louisiana-Tabasco 2001–2003 clade. Stars indicate locations of isolates in the California-Arizona-northern Mexico clade. The square indicates location of Colombian study.
The genetic similarity of COL isolates with LA strains could support the hypothesis of early WNV expansion to Central and South America. Furthermore, amino acid comparisons show one ancestral mutation E-V159 shared only by NY, LA, FL, and COL isolates, which confirms the early introduction of WNV to South America (Table 2). The constant rate of evolution observed in the phylogenetic tree and the fixed and unique amino acid changes found in COL isolates provide evidence for the fast emergence of WNV through all America after its introduction in 1999.33,34 Although there are fewer amino acid differences between COL 9835 and NY99, than between LA AR01 and NY99, analysis of the nucleotide sequences showed a considerable number of silent mutations which are shared between Colombian isolates and the LA isolate (data not shown). This supports the phylogenetic grouping between LA and COL isolates.
The isolation of WNV in Colombia could have important implications in human, veterinary, and wildlife medicine. It is possible that these WNV strains are causing mild to severe human disease in some parts of the country. In terms of wildlife, Colombia has a great variety of ecosystems, providing habitat to a large number of migratory and resident birds. An outbreak of WNV in this area could have devastating effects on susceptible species. The findings of the current study supports the need for larger ecological and field surveillance studies in wild birds, domestic animals, and humans to better understand the impact of WNV and native flavivirus circulation in Colombia. It is also important to determine the mosquito vectors involved in the transmission of these WNV strains in Colombia. We are currently conducting field investigations that can provide some insight into the natural history of WNV and related flaviviruses in Colombia.
ACKNOWLEDGMENTS
We thank the veterinary and animal care staff of Zoologico Santa Fe (Medellin, Colombia), and Melissa Lund and Kathryn Griffin (USGS- National Wildlife Health Center) for technical support. We also thank Thomas M. Yuill, Tonie E. Rocke, Charalambos D. Partidos, and Tony Goldberg for the critical review of this manuscript. Ivan Dario Velez and members of the Programa para Estudio y Control de Enfermedades Tropicales (PECET) from the Universidad de Antioquia (Medellin, Colombia) for their contribution to this manuscript. Use of trade or product names does not imply endorsement by the U.S. Government.
Footnotes
Authors' addresses: Jorge E. Osorio, Karl A. Ciuoderis, Juan G. Lopera, Leidy D. Piedrahita, Darby Murphy, and Jim LeVasseur, Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI, E-mails: Osorio@svm.vetmed.wisc.edu, adolfmvz@gmail.com, juanguilopera@gmail.com, piedritas@gmail.com, darbymurphy@wisc.edu, and jalevasseur@wisc.edu. Lina Carrillo, Universidad de Antioquia - Facultad de Ciencias Agrarias, Medellin, Colombia, E-mail: linacarrillo@gmail.com. Martha C. Ocampo, Zoológico Santa Fe de Antioquia – Zoologico, Medellin, Colombia, E-mail: martha.ocampo@zoologicosantafe.com. Erik Hofmeister, USGS - National Wildlife Health Center, Madison, WI, E-mail: ehofmeister@usgs.gov.
References
- 1.Hayes CG. West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci. 2001;951:25–37. doi: 10.1111/j.1749-6632.2001.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 2.Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- 3.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 4.Lichtensteiger CA, Heinz-Taheny K, Osborne TS, Novak RJ, Lewis BA, Firth ML. West Nile virus encephalitis and myocarditis in wolf and dog. Emerg Infect Dis. 2003;9:1303–1306. doi: 10.3201/eid0910.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farajollahi A, Panella NA, Carr P, Crans W, Burguess K, Komar N. Serologic evidence of West Nile virus infection in black bears (Ursus americanus) from New Jersey. J Wildl Dis. 2003;39:894–896. doi: 10.7589/0090-3558-39.4.894. [DOI] [PubMed] [Google Scholar]
- 6.Klenk K, Snow J, Morgan K, Bowen R, Stephens M, Foster F, Gordy P, Beckett S, Komar N, Gubler D, Bunning M. Alligators as West Nile virus amplifiers. Emerg Infect Dis. 2004;10:2150–2155. doi: 10.3201/eid1012.040264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- 8.Read RW, Rodriguez DB, Summers BA. West Nile virus encephalitis in a dog. Vet Pathol. 2005;42:219–222. doi: 10.1354/vp.42-2-219. [DOI] [PubMed] [Google Scholar]
- 9.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 10.Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B, Simor A, Low DE, McGeer A, Mazzulli T, Burton J, Jaigobin C, Fearon M, Artsob H, Drebot MA, Halliday W, Brunton J. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ. 2003;168:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Salas I, Contreras-Cordero JF, Blitvich BJ, Gonzalez-Rojas JI, Cavazos-Alvarez A, Marlenee NL, Elizondo-Quiroga A, Lorono-Pino MA, Gubler DJ, Cropp BC, Calisher CH, Beaty BJ. Serologic evidence of West Nile Virus infection in birds, Tamaulipas State, Mexico. Vector Borne Zoonotic Dis. 2003;3:209–213. doi: 10.1089/153036603322662192. [DOI] [PubMed] [Google Scholar]
- 12.Cruz L, Cardenas VM, Abarca M, Rodriguez T, Reyna RF, Serpas MV, Fontaine RE, Beasley DW, Da Rosa AP, Weaver SC, Tesh RB, Powers AM, Suarez-Rangel G. Short report: serological evidence of West Nile virus activity in El Salvador. Am J Trop Med Hyg. 2005;72:612–615. [PubMed] [Google Scholar]
- 13.Komar N, Clark GG. West Nile virus activity in Latin America and the Caribbean. Rev Panam Salud Publica. 2006;19:112–117. doi: 10.1590/s1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- 14.Barrera R, Hunsperger E, Munoz-Jordan JL, Amador M, Diaz A, Smith J, Bessoff K, Beltran M, Vergne E, Verduin M, Lambert A, Sun W. First isolation of West Nile virus in the Caribbean. Am J Trop Med Hyg. 2008;78:666–668. [PubMed] [Google Scholar]
- 15.Adrian Diaz L, Komar N, Visintin A, Dantur Juri MJ, Stein M, Lobo Allende R, Spinsanti L, Konigheim B, Aguilar J, Laurito M, Almiron W, Contigiani M. West Nile virus in birds, Argentina. Emerg Infect Dis. 2008;14:689–691. doi: 10.3201/eid1404.071257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrocal L, Pena J, Gonzalez M, Mattar S. West Nile virus; ecology and epidemiology of an emerging pathogen in Colombia. Rev Salud Publica (Bogota) 2006;8:218–228. doi: 10.1590/s0124-00642006000200010. [DOI] [PubMed] [Google Scholar]
- 17.Mattar S, Edwards E, Laguado J, Gonzalez M, Alvarez J, Komar N. West Nile virus antibodies in Colombian horses. Emerg Infect Dis. 2005;11:1497–1498. doi: 10.3201/eid1109.050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goez-Rivillas Y, Taborda N, Diaz FJ, Gongora A, Rodas JD, Ruiz-Saenz J, Osorio JE. Antibodies to West Nile virus in equines of Antioquia and Meta, Colombia 2005–2008. Rev Colomb Cienc Pecu. 2010;23:462–470. [Google Scholar]
- 19.Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, Gutierrez G, Pigretti S, Menchaca H, Garrido N, Taylor N, Fernandez F, Levis S, Enria D. West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis. 2006;12:1559–1561. doi: 10.3201/eid1210.060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC . In: Epidemic/Epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. Services USDoHaH, editor. Fort Collins, CO: Centers for Disease Control and Prevention; 2003. p. 75. [Google Scholar]
- 21.Gaunt MW, Gould EA. Rapid subgroup identification of the flaviviruses using degenerate primer E-gene RT-PCR and site specific restriction enzyme analysis. J Virol Methods. 2005;128:113–127. doi: 10.1016/j.jviromet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Farfan-Ale JA, Lorono-Pino MA, Garcia-Rejon JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, Beaty BJ, Lanciotti RS, Blitvich BJ. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- 23.Xia X, Xie Z. DAMBE: software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 24.Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134:183–189. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Davis CT, Beasley DW, Guzman H, Siirin M, Parsons RE, Tesh RB, Barrett AD. Emergence of attenuated West Nile virus variants in Texas, 2003. Virology. 2004;330:342–350. doi: 10.1016/j.virol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 28.Crespo R, Shivaprasad HL, Franca M, Woolcock PR. Isolation and distribution of West Nile virus in embryonated chicken eggs. Avian Dis. 2009;53:608–612. doi: 10.1637/8829-040209-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 29.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz LA, Nemeth NM, Bowen RA, Almiron WR, Contigiani MS. Comparison of Argentinean St. Louis encephalitis virus non-epidemic and epidemic strain infections in an avian model. PLoS Negl Trop Dis. 2011;5:e1177. doi: 10.1371/journal.pntd.0001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baleotti FG, Moreli ML, Figueiredo LT. Brazilian flavivirus phylogeny based on NS5. Mem Inst Oswaldo Cruz. 2003;98:379–382. doi: 10.1590/s0074-02762003000300015. [DOI] [PubMed] [Google Scholar]
- 32.Iyer AV, Boudreaux MJ, Wakamatsu N, Roy AF, Baghian A, Chouljenko VN, Kousoulas KG. Complete genome analysis and virulence characteristics of the Louisiana West Nile virus strain LSU-AR01. Virus Genes. 2009;38:204–214. doi: 10.1007/s11262-008-0321-2. [DOI] [PubMed] [Google Scholar]
- 33.May FJ, Davis CT, Tesh RB, Barrett AD. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85:2964–2974. doi: 10.1128/JVI.01963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett AD. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]