Abstract
One of the fascinating properties of the central nervous system is its ability to learn: the ability to alter its functional properties adaptively as a consequence of the interactions of an animal with the environment. The auditory localization pathway provides an opportunity to observe such adaptive changes and to study the cellular mechanisms that underlie them. The midbrain localization pathway creates a multimodal map of space that represents the nervous system's associations of auditory cues with locations in visual space. Various manipulations of auditory or visual experience, especially during early life, that change the relationship between auditory cues and locations in space lead to adaptive changes in auditory localization behavior and to corresponding changes in the functional and anatomical properties of this pathway. Traces of this early learning persist into adulthood, enabling adults to reacquire patterns of connectivity that were learned initially during the juvenile period.
The location of a sound source does not project directly onto the sensory surface of the ear. Therefore, the central auditory system must infer the location of a stimulus from acoustic cues created by the interaction of the incoming sound with the head and ears. Interaural time difference (ITD), which results from a difference in the distance that sound travels to reach the near versus far ear, is the dominant cue for the horizontal (azimuthal) position of a sound source (1, 2). Other cues, such as interaural level differences (ILDs) and monaural spectral cues, which result from the frequency-dependent directional properties of the head and ears, have more complicated relationships with the horizontal and vertical locations of a sound source (3, 4). These cues are used for localizing sounds in both azimuth and elevation (2, 5).
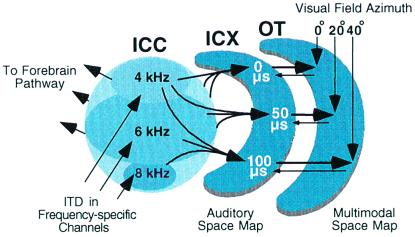
The central auditory system processes localization cues in parallel pathways in the midbrain and forebrain (6). The midbrain localization pathway (Fig. 1) branches from the main, tonotopic pathway at the level of the central nucleus of the inferior colliculus (ICC). This pathway leads to the optic tectum (called the superior colliculus in mammals), a primary function of which is to orient an animal's gaze toward interesting stimuli (9, 10). The forebrain localization pathway branches from the tonotopic pathway at the level of the primary auditory field in the forebrain (11). This pathway leads to various forebrain areas subserving a variety of behaviors, including auditory spatial memory and gaze control (12).
Figure 1.
The midbrain auditory localization pathway. For clarity, not all connections or pathways are shown. ITD is initially measured and mapped in frequency-specific channels in the nucleus laminaris, the avian equivalent of the mammalian medial superior olive (7). This information ascends to the ICC (arrows). From the ICC, information proceeds to the auditory thalamus in the forebrain and to the ICX in the midbrain pathway. In the projection from the ICC to the ICX, information about cue values, including ITD, converges across frequency channels, resulting in spatially restricted auditory receptive fields and a map of space. The auditory map of space merges with a visual map of space in the optic tectum. Tectal neurons receive visual inputs directly from the retina and indirectly from the forebrain. The optic tectum also projects topographically back to the ICX (8). OT, optic tectum.
To guide behavior accurately, the midbrain and forebrain localization pathways must associate values of localization cues with appropriate locations in space. The establishment and maintenance of an accurate representation of space is complicated by variability in the correspondences of cues with locations across sound frequencies and across individuals (3, 13). Moreover, the encoding of cue values by nerve impulses will vary with changes in the relative sensitivities of the ears and with the development and aging of the nervous system. The midbrain and forebrain pathways deal with the variability in cue-location correspondences by adjusting cue-location associations based on experience (14–16). The structural and functional changes that underlie these experience-driven adjustments are the subject of this review.
The shaping influence of experience on the auditory localization pathway has been documented most thoroughly in the midbrain pathway of the barn owl, a nocturnal predator that depends on sound localization for survival. Therefore, this review focuses primarily on these data. It is likely, however, that the principles of adaptive plasticity that operate in the midbrain pathway of barn owls operate in other pathways and in other species as well.
Physiological Traces of Learning
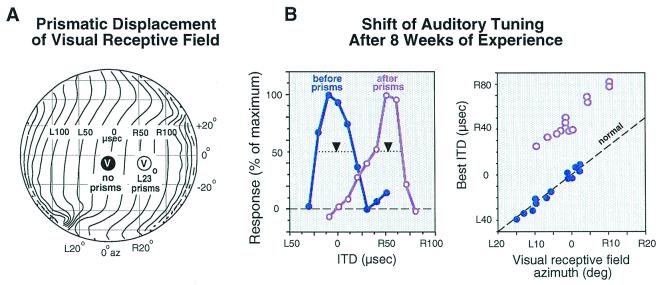
Behavioral adjustments to manipulations of auditory or visual experience leave clear physiological traces in the midbrain localization pathway of owls and ferrets (17, 18). In the optic tectum, auditory space is represented topographically, and the tuning of neurons for sound localization cues is sharp. Many tectal neurons also respond to visual stimuli, and their auditory and visual spatial receptive fields are mutually aligned. The location of a tectal neuron's visual receptive field indicates with high precision the values of sound localization cues to which a neuron should be tuned normally and, therefore, can be used to detect changes in the tuning of tectal neurons that result from abnormal sensory experience (Fig. 2).
Figure 2.
Functional traces of learning in the optic tectum of the barn owl. Experience with a prismatically displaced visual field causes adaptive adjustment of ITD tuning in the auditory space map. (A) The effect of L23°, horizontally displacing prisms on a visual receptive field location (encircled “V”). The globe represents space in front of the owl relative to the line of sight. The contour lines indicate the correspondence of ITD values (for 6 kHz sounds) with locations in space. (B) The ITD tuning of tectal neurons before and after prism rearing. The visual receptive fields of both neurons are centered at 0° azimuth. Before prism experience (blue symbols), the neuron is tuned to 0 μs ITD. After the owl has experienced L23° prisms for 8 weeks, the ITD tuning of the neuron from a very similar site has shifted to 50 μs right-ear leading (purple symbols). Downward arrows indicate the best ITD for each site. Best ITD is the midpoint of the range of ITDs that elicit more than 50% of the maximum response for that neuron. (C) Eight weeks of prism experience causes the relationship between best ITD and visual receptive field azimuth to be shifted (purple symbols) systematically from normal (blue symbols). The dashed line indicates the normal regression of best ITD on visual receptive field azimuth. The location of visual receptive fields, measured with prisms removed, is not altered in the tectum by prism experience. L, left-ear leading; R, right-ear leading. Data are from Brainard and Knudsen (29).
Abnormal Auditory Experience.
Chronic occlusion of one ear in young barn owls leads to adaptive adjustments in auditory localization behavior and in auditory spatial tuning in the optic tectum (19). By changing the timing and level of sound reaching the eardrum, an earplug alters the relationship of ITDs and ILDs with locations in space. Initially, monaurally occluded owls mislocalize sounds toward the side of the unplugged ear. After experiencing an earplug for a period of months, however, owls recover accurate auditory orienting responses. In the optic tecta of these animals, neurons exhibit altered tuning for ITD and ILD so that their auditory receptive fields align with their visual receptive fields despite the presence of the earplug (20, 21). Analogous adaptive adjustments in ITD and ILD tuning can also result from experimental alterations of the directional properties of the external ears in young and adult owls (22).
Adaptive adjustments to abnormal auditory experience are carried out in a frequency-dependent manner in the midbrain pathway. Values of ITD and ILD can be altered independently for different frequencies of sound by installing an acoustic filtering device in one ear (23). Acute insertion of such a device shifts the locations of tectal auditory receptive fields in different directions and by different amounts, depending on the frequency of the sound used to measure the receptive fields. After young owls experience such a device for a period of months, auditory receptive fields measured with different frequencies of sound are realigned with each other and with visual receptive fields, as long as the device is in the ear. The basis for this adaptive adjustment is a frequency-specific change in the tuning of tectal neurons for ITD and ILD that reflects the acoustic effects of the device (24).
Abnormal Visual Experience.
Auditory orienting behavior and auditory spatial tuning in the optic tectum also are adjusted adaptively in response to optical displacements of the visual field (17). At first, it may seem surprising that auditory responses should shift as a result of a shift in the visual field. Recall, however, that a primary function of the optic tectum is to orient gaze toward interesting stimuli. Prisms do not affect the movement required to bring a visual stimulus (seen through the prisms) onto the center of gaze. In contrast, prisms do alter the movement required to bring an auditory stimulus onto the center of gaze. Realignment of auditory receptive fields with optically displaced visual receptive fields in the tectum results in orienting movements that enable the owl to foveate the source of the auditory stimulus through the prisms. Because the eyes of a barn owl do not move substantially relative to the head (movement < 2°), auditory receptive fields must change to realign with optically displaced visual receptive fields.
Horizontally displacing prisms cause a horizontal shift of auditory receptive fields in the optic tecta of young owls (25). This shift in auditory spatial tuning is caused by a shift in the tuning of tectal neurons for ITD (Fig. 2), the dominant cue for azimuth. The dynamics of this shift in tuning can be observed with high resolution in the tectum (26). Learned, adaptive neuronal responses gradually increase over a period of weeks after mounting prisms. With additional experience, normal responses are gradually eliminated and the remaining responses become sharply tuned for learned values of ITD. Thus, a shift in ITD tuning involves two components: (i) acquisition of neuronal responses to values of ITD that are adaptive with the prisms in place (“learned responses”) and (ii) elimination of responses to values of ITD that previously were effective but are no longer appropriate with the prisms in place (“normal responses”).
Site of Plasticity.
Abnormal auditory or visual experience leads to adaptive changes at the same site in the midbrain localization pathway: the external nucleus of the inferior colliculus (ICX). The ICX is the site in the pathway where the auditory system creates a map of space by merging information across frequency channels (Fig. 1). The frequency-specific information about cue values is provided by inputs from neurons in the ICC. By combining information across frequency channels, ICX neurons eliminate much of the spatial ambiguity that is inherent in individual, frequency-specific cues (27, 28).
In owls that have adjusted to either optical prisms or an acoustic filtering device, the adaptive functional changes in the auditory space map observed in the optic tectum also are observed in the ICX (16, 29). In contrast, the representations of cue values in the ICC, in both cases, remain unaltered. Normal representations in the ICC followed by adaptively shifted representations in the ICX suggest, but do not prove, that a site of experience-driven plasticity is the ICX. Proof that the ICX is a site of plasticity is based on the anatomical and pharmacological traces of learning discussed below.
That adaptive adjustments to abnormal auditory experience are made at the level of the ICX makes sense because the adjustments that need to be made are different for different frequency channels; therefore, the adjustments must be made before information is merged across frequency channels. Adaptive adjustments in response to optical prisms may occur at the same site simply because the nervous system normally uses vision to calibrate changing and unpredictable auditory cues and has not evolved to deal with large, sustained displacements of the visual field.
Anatomical Traces of Learning
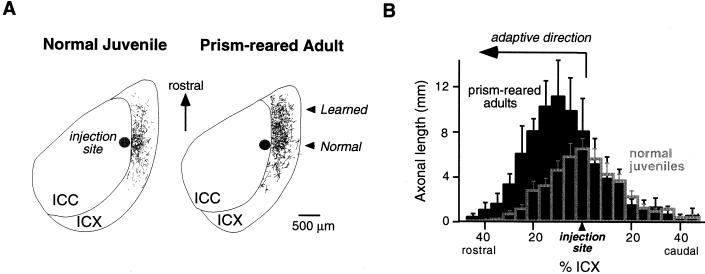
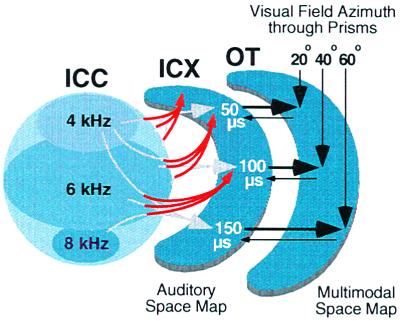
Adaptive adjustments to prisms involve anatomical changes in the midbrain localization pathway (ref. 30 and W.M.D., D. Feldman, and E.I.K., unpublished data). Normally, neurons located in the lateral regions of the ICC send axons radially into the ICX (Fig. 3A Left). This topographic projection forms the basis of the space map (Fig. 1). In prism-reared owls that have acquired a shifted map of ITD in the ICX, this topographic projection is altered in an adaptive manner (Fig. 4).
Figure 3.
Anatomical traces of learning in the ICX of the barn owl. (A) Digital image drawings of labeled axons in 40-μm thick, horizontal sections through the optic lobe of a normal juvenile (Left) and a prism-reared adult (Right). To visualize the pattern of axonal projection from the ICC to the ICX, the anterograde tracer, biocytin, was injected into the ICC at a site representing 20 μs ITD contralateral ear leading. The location of the injection site in the ICC is indicated as a dark circle. Labeled axons in the ICX are shown as thin lines. In a normal juvenile, the axonal projection field in the ICX is spatially restricted, symmetric, and centered around the rostral-caudal level of the injection site. In contrast, in a prism-reared adult expressing a rostral map shift, there is a dramatic expansion of the projection field in the rostral portion of the ICX. The direction of this axonal expansion is adaptive for the direction of prismatic displacement. Thus, these axons represent the learned projection field. (B) Composite spatial distributions of axons for normal juveniles (empty gray bars) and prism-reared adults (solid black bars). To quantify the spatial distribution for each case, the ICX was subdivided into measurement zones oriented orthogonal to the rostral-caudal axis. Each measurement zone corresponded to 5% of the rostral-caudal extent of the ICX. Total axonal length in each measurement zone was determined by computer analysis of the digital image drawings. Histograms were aligned relative to the rostral-caudal level of the injection site. Composite distributions were obtained by averaging the individual cases. In prism-reared adults with rostral map shifts (n = 4), the axonal density on the rostral flank of the distribution is significantly greater than in normal juveniles (n = 7; ANOVA, P < 0.0001), indicating that remodeling occurs by a net elaboration of axons. In contrast, the axonal density within the normal projection field, located at the rostral-caudal level of the injection site, is not significantly changed by prism experience. Data are from W.M.D., D. Feldman, and E.I.K., unpublished data.
Figure 4.
Schematic representation of the change in the anatomical projection from the ICC to the ICX that results from early prism experience, as indicated by the data shown in Fig. 3. For each direction of prismatic displacement, an abnormal rostralward projection of ICC axons (red arrows) appears on one side of the brain and a caudalward projection appears on the other (data not shown). In addition, the normal projection persists (gray arrows). For additional details, see Fig. 1.
Anatomical remodeling has been demonstrated with both retrograde and anterograde tracers. Retrograde tracers injected into the ICX label large numbers of neuronal somata not only in the normal location in the ICC, but also in an abnormal location in the ICC where, normally, few somata are labeled (30). ITD tuning at this location in the ICC matches the adaptively shifted ITD tuning expressed at the site of injection in the ICX. Consistent with this result, anterograde tracers injected into the ICC label numerous, branching, bouton-laden axons not only in the normal projection zone in the ICX, but also in a zone of the ICX that normally contains few labeled processes (Fig. 3A Right). The adaptively shifted ITD tuning of ICX neurons in this zone matches the tuning at the site of the ICC injection.
The experience-dependent change in the axonal projection from the ICC to the ICX could result either from differential sculpting of an initially broad and dense projection field or from axonal elaboration in the adaptive portion of the projection field. Injections of anterograde tracer into the ICC of normal juveniles (at the age when prism experience begins) reveal that the projection in juveniles is indeed broader than in adults (W.M.D., D. Feldman, and E.I.K., unpublished data). However, the distribution of axonal densities in the juvenile projection field is far from sufficient to account for the axonal density in the adaptive portion of the projection field in prism-reared adults (Fig. 3B). Therefore, new axons must be formed. Moreover, because all axons in the adaptive portion of the projection field are studded with synaptic boutons, prism experience must result in synaptogenesis as well as in axonal elaboration.
The acquisition of the learned, adaptive circuitry does not seem to come at the expense of the normal circuitry: The density of axons in the normal portion of the projection field is about the same in prism-reared and normal owls (Fig. 3B). Thus, at the anatomical level, the normal circuitry is able to coexist with the newly acquired circuitry.
Pharmacological Traces of Learning
Pharmacological experiments, in which specific neurotransmitter receptors are blocked in the ICX of prismed-reared owls, confirm that the ICX is a site of plasticity and reveal the roles of certain classes of receptors in the adaptive, functional changes that take place.
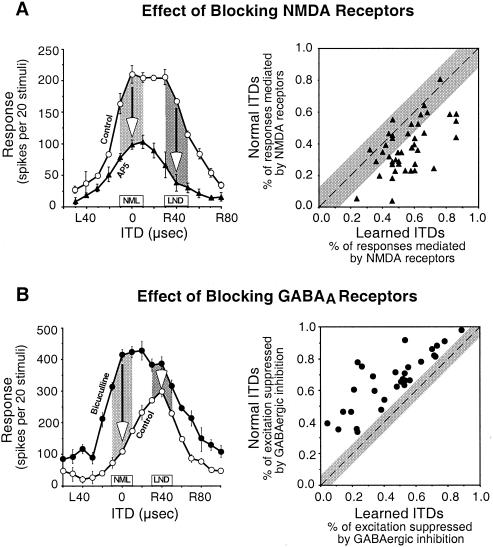
The N-methyl-d-aspartate (NMDA) subtype of glutamate receptors is particularly important for the expression of newly learned responses in the ICX. In the ICX of normal owls, blocking NMDA receptors, with iontophoretic injections of d,l-2-amino-5-phosphonopentanoic acid (AP5), reduces auditory responses by more than 50%, on average, across the entire range of effective ITDs (32). In the ICX of prisms-reared owls in which learned responses are becoming strong, blocking NMDA receptors has a differentially greater effect on the newly learned responses and sometimes completely eliminates them (Fig. 5A) (33, 34). In contrast, nonspecific glutamate receptor antagonists do not have this differential blocking effect on the expression of learned responses.
Figure 5.
Pharmacological traces of learning in the ICX of the barn owl. (A) Newly learned responses are differentially mediated by NMDA receptors. (Left) Effect of iontophoretic application of AP5, a specific antagonist for NMDA receptors, on the ITD tuning of an ICX site that was at an early stage of learning. Arrows indicate the AP5-induced decrease in responses to the normal (NML) and learned (LND) ITDs, respectively; learned ITDs were 40 μs away toward right-ear leading from normal ITDs. (Right) Comparison of the proportion of the responses mediated by NMDA receptors, calculated as (control response − response with AP5)/control response, between normal and learned ITDs for all ICX sites (n = 41) that were at an early stage of learning. The diagonal dashed line denotes equal percentage of NMDA-receptor-mediated responses to normal and learned ITDs. The gray region represents the mean ± 2 SD of the difference in the percentage of NMDA-receptor-mediated responses to ITDs ± 40 μs away from the best ITD on each side of ITD tuning curves in normal owls. Data are from Feldman and Knudsen (34). (B) Normal responses are selectively suppressed by GABAergic inhibition. (Left) Effect of iontophoretic application of bicuculline, a specific antagonist for GABAA receptors, on the ITD tuning of an ICX site that was at a late stage of learning. Arrows indicate the amount of excitation that was suppressed by GABAergic inhibition at the normal (NML) and learned (LND) ITDs, respectively. (Right) Comparison of the percentage of excitation that was suppressed by GABAergic inhibition, calculated as (response with bicuculline − control response)/response with bicuculline, between normal and learned ITDs for all ICX sites (n = 30) that were at a late stage of learning. The diagonal dashed line denotes an equal percentage of GABAergic suppression for the normal and learned ITDs. The gray region represents the mean ± 2 SD of the difference in percentage of GABAergic suppression of excitation for ITDs ± 40 μs away from the best ITD on each side of ITD tuning curves in normal owls. Data are from Zheng and Knudsen (35).
The A subtype of γ-aminobutyric acid (GABA) receptors plays an essential role in suppressing inappropriate normal responses in an ICX that is expressing a fully shifted map of space (35). To enable a full, adaptive shift of an ICX tuning curve, responses to normal ITDs must be eliminated. Iontophoretic injection of the GABAA receptor blocker bicuculline at an ICX site that is expressing a fully shifted ITD tuning curve results in an immediate reexpression of strong normal responses and a shift of the tuning curve back toward normal values of ITD (Fig. 5B). Thus, consistent with the preservation of normal anatomical circuitry in prismed-reared owls discussed above, functional connections mediating normal responses remain strong (although slightly weaker than normal) in an ICX that is expressing a shifted map of ITD, but responses to those inputs are differentially suppressed by GABAergic inhibition.
Traces of Juvenile Learning Endure into Adulthood
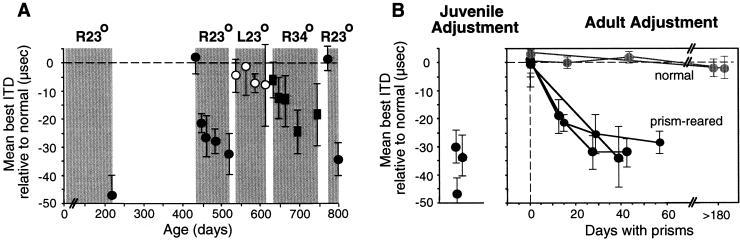
Large changes in auditory spatial tuning are induced readily by manipulation of experience in young animals (36). In adult animals, adaptive adjustments may occur, but the range of adjustment is restricted. The period during development when large-scale changes occur in response to prism experience is referred to as the sensitive period for this manipulation (36). Within the sensitive period, horizontally displacing prisms can cause shifts of up to 70 μs in the ITD tuning of tectal neurons. For owls past the sensitive period, the magnitude of adjustment for first-time exposure to identical prisms is substantially lower: Mean shifts in ITD tuning rarely exceed 10 μs in naïve adults (Fig. 6B, normal).
Figure 6.
Endurance of the traces of learning in the midbrain auditory localization pathway of the barn owl. Juvenile owls adjusted to R23° prisms. The prisms were then removed for a period of at least 6 months. The ability of the ITD map in the optic tectum to shift in response to prisms was tested again after the owls reached adulthood. Barn owls reach adulthood at about 7 months old. (A) Adjustments of tectal ITD tuning (measured as shown in Fig. 2C) in an adult owl that had adjusted to R23° prisms as a juvenile. The plot shows mean and standard deviation of the shift in best ITDs relative to predicted normal, measured at various ages. Shaded regions represent periods of experience with prisms of different strength or direction, as indicated above. Adaptive adjustment to L23° prisms would have been above the 0-μs line. Regardless of the strength or direction of the prisms, adult adjustment is limited to the range of juvenile adjustment. (B) Shift of ITD tuning in three prism-reared (black) versus two normal (gray) adults. The two normal adults had no prior experience with prisms. The three prism-reared adults had adjusted to R23° prisms previously as juveniles (Left). All adult owls were over 1 year old. Data are from Knudsen (37).
In contrast, prism-reared adults exhibit an enhanced capacity for adaptive adjustments. Restoration of normal experience (by prism removal) leads to the recovery of normal ITD tuning in the optic tectum, and this capacity to recover normal tuning persists throughout life (Fig. 6A and ref. 36). In addition, reexposure to prisms leads to the reexpression of the adaptive responses that were learned during the sensitive period (37). For example, in an adult in which mean ITD tuning had shifted by more than 30 μs toward left-ear leading values as a juvenile, and had subsequently shifted back to near 0 μs after a period of normal experience, prism reexposure leads to a shift in mean ITD tuning of 30 μs toward left-ear leading values (Fig. 6A). This enhanced capacity of plasticity persists well into adulthood. However, adult experience with a larger prismatic displacement does not lead to tuning shifts to larger left-ear leading values, and adult experience with prisms that displace the visual field in the opposite direction does not lead to tuning shifts to right-ear leading values (Fig. 6A). Thus, the acquisition of alternative tuning during the sensitive period leaves an enduring trace in this pathway that enables substantial functional plasticity in adults. This trace of learning specifically reflects the range of tuning states acquired during the juvenile period.
The persistence of a trace of learning in the midbrain localization pathway is characteristic of sensitive period learning, as evidenced by similar effects of song learning in song birds (38), imprinting in birds and mammals (39), and language learning in humans (40).
Comparison with Plasticity in Other Systems
Functional and anatomical plasticity, analogous to that described here for the midbrain localization pathway of the barn owl, has been observed in many other pathways and in many other species. In the hippocampus (41), visual and somatosensory cortices (42–46), and optic tectum of other species (47), for example, NMDA receptor-mediated excitation is critically involved in the acquisition of new responses, GABAergic inhibition regulates the plasticity, and patterns of axonal projection can be modified.
In most of these systems, the degree to which the plastic changes are adaptive is unclear. Abnormal experience often takes the form of deprivation, denervation, or excessive use (42, 46). Although the plastic changes that result from such manipulations are likely to be adaptive, the degree to which this is true is difficult to ascertain, because the importance of the neuronal changes for behavior is unknown.
In contrast, the adaptive nature of the plasticity in the midbrain localization pathway is clear. The role of the midbrain pathway in mediating orienting behavior is established (48). Moreover, orienting behavior is readily quantified, and the tuning of neurons for specific auditory cues is both precise and predictable (e.g., Fig. 2). Together, these properties allow quantitative links to be formed between behavioral learning and neuronal plasticity. These links reveal the adaptive nature of the functional and anatomical changes in this system. In addition, unlike plasticity reduced by deprivation, denervation, or excessive use (which may result simply from the induced imbalances in afferent activity and may be explained on the basis of self-organizational principles), the plasticity described here for the auditory localization pathway is instructed (49). Analogous to instructed motor learning in the cerebellum (50), an instructive signal (visually based, in the case of prism experience) controls the adaptive adjustment of the auditory space map in the midbrain.
One unusual property of the plasticity in the midbrain localization pathway is the low level of competition between alternative afferent pathways to the ICX. In the visual and somatosensory cortices of mammals, afferent inputs compete anatomically and physiologically for representation (43, 44, 46). In contrast, axonal projections corresponding to normal and learned inputs coexist in an ICX expressing a fully shifted map of space (Fig. 3B), and excitatory drive conveyed through both of these inputs is strong (35). The low level of competition between alternative afferent pathways may be critical for allowing the long-term retention of alternative maps despite disuse, enabling them to be restored at a later time should they become adaptive (Fig. 6).
A close parallel to the functional plasticity described here for the midbrain localization pathway in barn owls is found in the analogous pathway in mammals. In mammals, the homologue of the optic tectum, the superior colliculus, guides orienting movements of the eyes and head (9, 48). As in owls, it contains a multimodal map of space, and the auditory space map is shaped by experience during early life (18, 31). In ferrets, the auditory space map is altered adaptively in response to abnormal auditory experience or surgical rotation of the eye (analogous in most respects to prism experience). Although the sites and mechanisms of plasticity in this pathway in mammals are as yet unknown, it is likely that they share many features in common with those described here for the barn owl.
The properties of the midbrain localization pathway in the barn owl allow questions about the characteristics and mechanisms of learning to be addressed at many levels, from behavioral to molecular. Because of the tight links in this system among behavior, neuronal function, and neuronal structure, behavioral results can directly inform physiological and anatomical experiments, and vice versa. Consequently, a multilevel approach in this system greatly facilitates the search for principles and mechanisms of learning.
Acknowledgments
We are grateful for the substantial contributions of Drs. Michael Brainard and Dan Feldman to the work described here. We thank Phyllis Knudsen for technical assistance during many of these projects and for help in preparing the figures, and P. Hyde, B. Linkenhoker, and Dr. Y. Gutfreund for helpful comments on the manuscript. This work is supported by the National Institute of Deafness and Other Communication Disorders, National Institutes of Health Grant RO1 DC00155–18 and by a McKnight Senior Investigator Award.
Abbreviations
- ITD
interaural time difference
- ILD
interaural level difference
- ICC
the central nucleus of the inferior colliculus
- ICX
the external nucleus of the inferior colliculus
- NMDA
N-methyl-d-aspartate
- AP5
d,l-2-amino-5-phosphonopentanoic acid
- GABA
γ-aminobutyric acid
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Wightman F L, Kistler D J. J Acoust Soc Am. 1992;91:1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen E I, Konishi M. J Comp Physiol. 1979;133:13–21. [Google Scholar]
- 3.Middlebrooks J C, Makous J C, Green D M. J Acoust Soc Am. 1989;86:89–108. doi: 10.1121/1.398224. [DOI] [PubMed] [Google Scholar]
- 4.Keller C H, Hartung K, Takahashi T T. Hear Res. 1998;118:13–34. doi: 10.1016/s0378-5955(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 5.Middlebrooks J C. J Acoust Soc Am. 1992;92:2607–2624. doi: 10.1121/1.404400. [DOI] [PubMed] [Google Scholar]
- 6.Cohen Y E, Knudsen E I. Trends Neurosci. 1999;22:128–135. doi: 10.1016/s0166-2236(98)01295-8. [DOI] [PubMed] [Google Scholar]
- 7.Carr C E, Konishi M. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyde P S, Knudsen E I. J Comp Neurol. 2000;421:146–160. doi: 10.1002/(sici)1096-9861(20000529)421:2<146::aid-cne2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Freedman E G, Stanford T R, Sparks D L. J Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen E I, Knudsen P F. Exp Brain Res. 1996;108:23–32. doi: 10.1007/BF00242901. [DOI] [PubMed] [Google Scholar]
- 11.Rauschecker J P. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen E I, Knudsen P F. Nature (London) 1996;383:428–431. doi: 10.1038/383428a0. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen E I, Esterly S D, du Lac S. J Neurosci. 1991;11:1727–1747. doi: 10.1523/JNEUROSCI.11-06-01727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofman P M, Van Riswick, Jos G A, Van Opstal A J. Nat Neurosci. 1998;1:417–421. doi: 10.1038/1633. [DOI] [PubMed] [Google Scholar]
- 15.Miller G L, Knudsen E I. J Neurosci. 1999;19:2326–2336. doi: 10.1523/JNEUROSCI.19-06-02326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold J I, Knudsen E I. J Neurosci. 2000;20:3469–3486. doi: 10.1523/JNEUROSCI.20-09-03469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen E I. J Comp Physiol. 1999;185:305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- 18.King A J. Exp Physiol. 1993;78:559–590. doi: 10.1113/expphysiol.1993.sp003708. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen E I, Knudsen P F, Esterly S D. Nature (London) 1982;295:238–240. [Google Scholar]
- 20.Knudsen E I. Science. 1983;222:939–942. doi: 10.1126/science.6635667. [DOI] [PubMed] [Google Scholar]
- 21.Mogdans J, Knudsen E I. J Neurosci. 1992;12:3473–3484. doi: 10.1523/JNEUROSCI.12-09-03473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen E I, Esterly S D, Olsen J F. J Neurophysiol. 1994;71:79–94. doi: 10.1152/jn.1994.71.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Gold J I, Knudsen E I. J Neurophysiol. 1999;82:2197–2209. doi: 10.1152/jn.1999.82.5.2197. [DOI] [PubMed] [Google Scholar]
- 24.Gold J I, Knudsen E I. J Neurosci. 2000;20:862–877. doi: 10.1523/JNEUROSCI.20-02-00862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen E I, Brainard M S. Science. 1991;253:85–87. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- 26.Brainard M S, Knudsen E I. J Neurophysiol. 1995;73:595–614. doi: 10.1152/jn.1995.73.2.595. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Konishi M. J Neurosci. 1986;6:3413–3422. doi: 10.1523/JNEUROSCI.06-12-03413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brainard M S, Knudsen E I, Esterly S D. J Acoust Soc Am. 1992;91:1015–1027. doi: 10.1121/1.402627. [DOI] [PubMed] [Google Scholar]
- 29.Brainard M S, Knudsen E I. J Neurosci. 1993;13:4589–4608. doi: 10.1523/JNEUROSCI.13-11-04589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman D E, Knudsen E I. J Neurosci. 1997;17:6820–6837. doi: 10.1523/JNEUROSCI.17-17-06820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King A J, Hutchings M E, Moore D R, Blakemore C. Nature (London) 1988;332:73–76. doi: 10.1038/332073a0. [DOI] [PubMed] [Google Scholar]
- 32.Feldman D E, Knudsen E I. J Neurosci. 1994;14:5939–5958. doi: 10.1523/JNEUROSCI.14-10-05939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman D E, Brainard M S, Knudsen E I. Science. 1996;271:525–528. doi: 10.1126/science.271.5248.525. [DOI] [PubMed] [Google Scholar]
- 34.Feldman D E, Knudsen E I. J Neurosci. 1998;18:3073–3087. doi: 10.1523/JNEUROSCI.18-08-03073.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, Knudsen E I. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- 36.Brainard M, Knudsen E. J Neurosci. 1998;18:3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knudsen E I. Science. 1998;279:1531–1533. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- 38.Doupe A J, Kuhl P K. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 39.Hess E H. Imprinting: Early Experience and the Developmental Psychobiology of Attachment. New York: Van Nostrand Reinhold; 1973. [Google Scholar]
- 40.Tong Y C, Busby P A, Clark G M. J Acoust Soc Am. 1988;84:951–962. doi: 10.1121/1.396664. [DOI] [PubMed] [Google Scholar]
- 41.Martin S J, Grimwood P D, Morris R G M. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 42.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 43.Daw N W. Invest Ophthalmol Visual Sci. 1994;35:4168–4179. [PubMed] [Google Scholar]
- 44.Katz L C, Shatz C J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 45.Feldman D E. Nature Neurosci. 2000;3:303–304. doi: 10.1038/73849. [DOI] [PubMed] [Google Scholar]
- 46.Kaas J H. The New Cognitive Neuroscience. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 47.Udin S B, Grant S. Prog Neurobiol. 1999;59:81–106. doi: 10.1016/s0301-0082(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 48.Stein B, Meredith M. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 49.Knudsen E I. J Neurosci. 1994;14:3985–3997. doi: 10.1523/JNEUROSCI.14-07-03985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond J L, Lisberger S G, Mauk M D. Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]