A 76-year-old woman with type II diabetes and hypertension was diagnosed with gastrointestinal stromal tumor. At the time of diagnosis, she was on insulin glargine 10 U daily and atenolol 50 mg daily. She had a blood pressure (BP) of 104/58 mm Hg, serum creatinine (SCr) of 1.2 mg per 100 ml, and no proteinuria. For treatment of the gastrointestinal stromal tumor, she was started on imatinib mesylate. Sixteen months into this therapy, the tumor progressed. At that point, she had a BP of 130/78 mm Hg, weight of 53.4 kg, SCr of 1.2 mg per 100 ml, and still no proteinuria. Imatinib was replaced with sunitinib 37.5 mg daily (Sutent).
Over the next 20 weeks, her BP gradually rose to 166/76 mm Hg. A total of 30 weeks after initiation of sunitinib, her BP was 142/49 mm Hg, weight was 59.2 kg, SCr was 2.4 mg per 100 ml, and protein/creatinine ratio was 7.2 (g/g). When she was first seen in the renal clinic 3 months later, her BP was 156/69 mm Hg, weight was 59.4 kg, jugular venous pressure was 6 cm, cardiopulmonary examination was unremarkable, renal artery bruits were not appreciated, but 1+ pitting pedal edema was present. Urinalysis showed 3+ protein with a bland sediment. SCr was 1.8 mg per 100 ml and urine protein/creatinine was 10.4 (g/g) (Figure 1). Serum complement and protein electrophoreses were normal; ANA, cryoglobulin, and rheumatoid factor were negative; and there was no evidence of microangiopathic hemolysis.
Figure 1. Development and resolution of proteinuria during sunitinib treatment.

Patient 2 from a published case series (1) had no proteinuria after 1 month of therapy but developed a protein/creatinine ratio of 7.2 (g/g) after 30 weeks of therapy and 10.4 (g/g) after 44 weeks. Proteinuria, hypertension and acute kidney injury completely resolved within 24 weeks of stopping sunitinib.
What is the diagnosis?
The clinical diagnosis was a preeclampsia-like syndrome associated with the anti-angiogenic agent sunitinib. Thrombocytopenia prohibited renal biopsy. Sunitinib was discontinued, and within 24 weeks, proteinuria had resolved completely and SCr had returned to 1.2 mg per 100ml (Figure 1).
Sunitinib is a member of a novel class of multitargeted, small molecule tyrosine kinase inhibitors that block the activity of multiple enzymes, including the vascular endothelial growth factor receptor (VEGF-R; Table 1). These chemotherapies are also associated with development of hypertension, proteinuria, and azotemia in at least 2.3% of patients receiving them.1 This clinical syndrome is similar to preeclampsia, in which disordered VEGF signaling plays a crucial role.2 The renal pathology in most patients reported to date is that of thrombotic microangiopathy, and genetic evidence in a mouse model implicates glomerular VEGF inhibition in the pathogenesis of this condition (Figure 2).3 The renal toxicities of molecule tyrosine kinase inhibitors add to growing evidence that VEGF is required for endothelial cell homeostasis, especially in the glomerular endothelial cell bed.4
Table 1. FDA-approved anti-angiogenic therapies.
| Agent | Class | Mechanism of action | FDA approval |
|---|---|---|---|
| Sunitinib | Small molecule | Tyrosine kinase inhibitor of VEGFR, PDGFR | RCC, GIST |
| Sorafenib | Small molecule | Tyrosine kinase inhibitor of VEGFR, PDGFR, and Ras | RCC, HCC |
| Bevacizumab | Monoclonal antibody | VEGF-depleting antibody | Colon cancer, NSCLC, breast cancer |
GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; NSCLC, non-small-cell lung cancer; PDGFR, platelet-derived growth factor receptor; RCC, renal cell carcinoma; VEGFR, vascular endothelial growth factor receptor.
Figure 2. Mechanisms of vascular endothelial growth factor (VEGF) inhibition by anti-angiogenic therapies in the glomerulus.
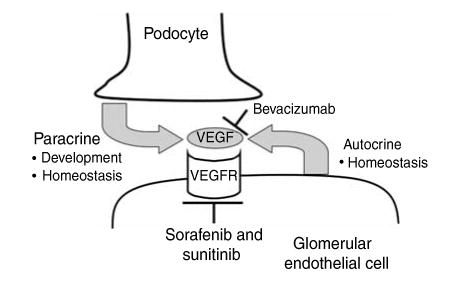
VEGF is produced by podocytes, where it may act in a paracrine fashion on glomerular capillary endothelial cells. VEGF is also produced by endothelial cells, where it signals in an autocrine manner to maintain endothelial cell differentiation. Bevacizumab binds and depletes free, circulating VEGF but does not inhibit autocrine VEGF signaling pathways that do not involve extracellular VEGF secretion. Sorafenib and sunitinib inhibit the intracellular tyrosine kinase activity of the VEGF receptor, among other tyrosine kinases, and therefore disrupt both paracrine and autocrine VEGF signaling.
Our experience suggests that some patients can be maintained on anti-angiogenic therapies despite development of hypertension and proteinuria. Tight control of BP with inhibitors of angiotensin signaling, diuretics, and, if necessary, calcium channel blockers may allow patients to continue anti-angiogenic therapy. However, the long-term renal consequences of anti-angiogenic therapy in patients who do develop hypertension and/or proteinuria, remain unknown. If, as in this case, nephrotic-range proteinuria, hypertension with end-organ consequences, or renal failure develops, dose reduction, extended washout, or discontinuation of therapy should be considered. Before prescribing immunosuppressive medications or in cases with atypical clinical features, an active urinary sediment or positive laboratory findings, renal biopsy should be performed to exclude other renal diseases.
Acknowledgments
Dr. Obhrai was supported by grant from NIAID (K08A1076631).
References
- 1.Patel TV, Morgan JA, Demetri GD, et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 2.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and Angiogenic Imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 3.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


