Abstract
Reduced suppression of the auditory P50 event-related potential has long been associated with schizophrenia, but the mechanisms associated with the generation and suppression of the P50 are not well understood. Recent investigations have used spectral decomposition of the electroencephalograph (EEG) signal to gain additional insight into the ongoing electrophysiological activity that may be reflected by the P50 suppression deficit. The present investigation extended this line of study by examining how both a traditional measure of sensory gating and the ongoing EEG from which it is extracted might be modified by the presence of concurrent visual stimulation - perhaps better characterizing gating deficits as they occur in a real-world, complex sensory environment. The EEG was obtained from 18 patients with schizophrenia and 17 healthy control subjects during the P50 suppression paradigm and while identical auditory paired-stimuli were presented concurrently with affectively neutral pictures. Consistent with prior research, schizophrenia patients differed from healthy subjects in gating of power in the theta range; theta activity also was modulated by visual stimulation. In addition, schizophrenia patients showed intact gating but overall increased power in the gamma range, consistent with a model of NMDA receptor dysfunction in the disorder. These results are in line with a model of schizophrenia in which impairments in neural synchrony are related to sensory demands and the processing of multimodal information.
Keywords: schizophrenia, EEG, P50, spectral analysis
Introduction
In physiological investigations of sensory processing among patients with schizophrenia, impaired suppression of the P50 event-related potential (ERP; Bramon et al., 2004; Heinrichs, 2004) provides a strong conceptual framework for understanding early deficits. P50 suppression is postulated to index sensory gating or filtering whereby inhibitory processes are activated as a means of regulating the flow of incoming information. The P50 paradigm involves presenting two identical auditory stimuli (“S1” and “S2”), spaced 500 ms apart. Research with humans and animals suggests that the S1 activates an inhibitory mechanism that protects processing of the initial stimulus from the potentially disruptive impact of the S2 (Freedman et al., 1996). By computing a ratio score of S2/S1, the magnitude of P50 suppression of S2 relative to the amplitude of S1 allows for inferences regarding the degree of inhibition, with better suppression reflected by lower ratio scores.
A central but understudied issue involves how sensory gating processes unfold when embedded in a more perceptually rich, naturalistic environment. In a sample of healthy individuals, Jin and Potkin (1996) found reduced P50 to S1, and a concomitant reduction in suppression ratio, when randomly flashing lights were introduced during the traditional paired-click task to simulate greater naturalistic complexity through engagement of multiple sensory modalities. The authors suggested that this finding may implicate a “sensory distraction” model of impaired sensory gating in schizophrenia whereby patients suffer from a chronic, basal deficit in attentional and perceptual resources. However, to date, only one study has investigated gating in schizophrenia patients within the context of greater sensory or perceptual demand. Tregellas and colleagues (2009) observed an increased hemodynamic response in schizophrenia patients relative to controls when participants listened to simulated urban noise. The magnitude of the response correlated positively with P50 suppression in patients. These results suggest that under more complex types of perceptual stimulation, patients’ disrupted gating may be associated with neural hyperactivity.
Given that P50 suppression may rely on precisely synchronized neural coordination, investigators have decomposed the EEG waveforms elicited during the P50 paradigm into their spectral components in order to characterize associated oscillatory activity. The greatest difference between healthy controls and schizophrenia patients has been observed in the gating of low-frequency (1–20 Hz) activity (Clementz & Blumenfeld, 2001; Johannesen et al., 2005; Brockhaus-Dumke et al., 2008; Hong et al., 2008). However, magnetoencephalography (MEG) data have implicated the gamma band (30–50 Hz) as the range of greatest difference in some studies (Clementz et al., 1997), but not in others (Popov et al., 2011). Furthermore, an EEG study revealed gamma suppression impairments to be most strongly associated with the presence of perceptual abnormalities (Johannesen et al., 2008), independent of psychiatric diagnosis. Thus, some uncertainty remains regarding the extent to which low- or high-frequency oscillations, or both, contribute to P50 suppression abnormalities in schizophrenia, and by extension, ambiguity persists regarding the associated neurocognitive processes. Furthermore, recent discoveries of heightened pre-stimulus theta and gamma power in schizophrenia patients (e.g., Winterer et al., 2000) and the resultant biasing of baseline-normalized post-stimulus spectral estimates (Spencer, 2012) warrant further investigation of oscillatory activity during P50 suppression, including its modulation by the demands of the broader perceptual environment.
In light of the fundamental principle that perceptual resources are of a limited, finite capacity which is split across different cognitive tasks and processes (Duncan, 1980; Fisher, 1982; Nuechterlein & Dawson, 1984), P50 suppression in both schizophrenia patients and control subjects should be attenuated (or further attenuated for patients) under conditions involving more complex sensory stimulation. The present study contrasted data obtained during the traditional P50 paradigm with responses recorded during concurrent presentation of visual images, to more closely approximate the complex, perceptual experiences encountered in the natural environment. To minimize affective modulation of brain activity, only images featuring neutral objects were utilized. Primary EEG spectral contributions to P50 suppression were assessed to evaluate large-scale neural circuit activity during sensory gating in schizophrenia. In keeping with the majority of published studies, low-frequency theta and alpha EEG gating was expected to differ most between patients and controls during the perceptually non-demanding (i.e., auditory only) paradigm. Consistent with the findings of Jin & Potkin (1996), reductions in gating of low-frequency activity was predicted among healthy controls during the more perceptually demanding, multisensory condition, with levels comparable to the baseline ‘distractibility’ of patients during the traditional paradigm. We anticipated even further degradation of patient gating as their attentional resources became further compromised during the multisensory condition. Given uncertainty about measurement of spectral activity using traditional baseline normalization methodologies (Spencer, 2012), we utilized measures of absolute spectral power and predicted overall heightening of gamma band activity among schizophrenia patients across task conditions. Analysis of beta activity was undertaken on an exploratory basis due to the relative paucity of reports on this frequency in the auditory gating literature.
Research Design and Methods
Participants
Eighteen outpatients with schizophrenia, diagnosed using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1997) and rated on the 24-item Brief Psychiatric Rating Scale (BPRS: Lukoff et al., 1986; Ventura et al., 1993), were assessed along with 17 healthy control subjects, who were screened with the SCID for personal or family history of major psychiatric disorders. All patients were recruited from the UCLA Aftercare program and were clinically stable and receiving antipsychotic medications. To avoid anticholinergic effects on the dependent measures, antiparkinsonian medications were discontinued at least 24 hours before testing, and smokers refrained from cigarettes for at least 45 minutes prior to data acquisition. All participants were screened for mental retardation, past head trauma, history of loss of consciousness exceeding 5 minutes, CNS injury or neurological disorder, and significant alcohol or substance use disorder during the past 6 months. Demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographics and Clinical Characteristics
| Healthy Comparison | Schizophrenia | Analysis | ||||
|---|---|---|---|---|---|---|
| Subjects (N = 17) | Patients (N = 18) | |||||
| Characteristic | Mean | SD | Mean | SD | t | p |
| Age (years) | 23.24 | 4.59 | 28.11 | 7.67 | 2.265 | 0.03 |
| N | N | χ2 | p | |||
| Gender | 0.229 | 0.632 | ||||
| Male | 11 | 13 | ||||
| Female | 6 | 5 | ||||
| Ethnicity | 8.89 | 0.114 | ||||
| African-American | 1 | 4 | ||||
| Asian-American | 0 | 1 | ||||
| Caucasian | 4 | 8 | ||||
| Hispanic | 5 | 4 | ||||
| Mixed | 5 | 1 | ||||
| Declined to report | 2 | 0 | ||||
| Medication | ||||||
| Traditional Antipsychotics | 1 | |||||
| Atypical Antipsychotics | 17 | |||||
| BPRS Total Score (24 item) | 38.22 | 8.00 | ||||
Mean age of members of each cohort revealed a significant difference in the average age of patients vs. controls. The addition of age as a covariate to each of the reported analyses did not affect the final results. No significant differences were found in the frequency of gender or ethnicity between groups.
Materials and Procedure
Participants were fitted with an EEG cap containing 124 Ag-AgCl sintered electrodes, along with electrooculogram (EOG) electrodes placed above and below the right eye. Electrodes were also placed on each earlobe and re-referenced offline to an averaged-ears montage. All data were collected with an initial bandpass filter of 0.5 to 200 Hz (+/− 24 dB/oct) and sampled at 1000 Hz.
Sound thresholds were determined for each ear separately, and paired stimuli consisting of amplified white noise were then presented at 55-dB SPL above each ear’s threshold for 3 ms, with an interstimulus interval (ISI) of 500 ms. Hearing thresholds did not differ significantly between groups (patients: M = 27.33 dB, SD = 4.22; controls: M = 26.34 dB, SD = 4.81, p = 0.52). During both the traditional and modified P50 suppression paradigms, 80 pairs of auditory stimuli were presented, separated by a variable intertrial interval (9–12 s). From these 80 pairs of stimuli, comparable numbers of useable trials were retained between groups (patients: M = 77.67, SD = 8.66; controls: M = 78.29, SD = 8.78, p = 0.83) following artifact rejection (described below). The traditional P50 suppression condition was always presented first. The picture component of the study involved pseudo-randomized presentation of 14 images from the International Affective Picture System (IAPS; Lang et al., 2005), all of which were rated as neutral in valence and low in arousal by the IAPS standardization sample. The pictures depicted household objects or vegetation, and each was presented for 60 s on a computer screen located 1 meter from the participant, with a 75 Hz refresh rate in 32 bit color at a resolution of 1024 × 768 pixels. Picture onset and offset were timed such that no picture changes occurred within 1000 ms of the auditory P50 stimuli. While affectively non-neutral pictures were presented to subjects in another part of this experiment, they were done so in separate, counterbalanced blocks so as not to contaminate responses during neutral picture viewing. Those data are not included in the present report. Behavioral responses were not required in either experimental condition.
Signal Processing
Data were filtered between 1 and 70 Hz, visually inspected for artifact leading to the removal of gross movement disturbances, and subjected to independent component analysis (ICA) for removal of ocular artifact (Jung et al., 2001). Average waveforms were computed separately for the P50 during the baseline and picture-viewing conditions. ERP computation included a 500 ms pre-stimulus baseline correction and a bandpass filter of 10 to 50 Hz that was not used for spectral analyses. The P50 component was identified at Cz as the most positive peak between 40 and 70 ms after stimulus onset. P50 amplitude was measured as the difference between the P50 peak and its negative predecessor, the N40. Data from two control subjects and one patient were excluded because a reliable P50 exceeding 0.5 µvolts to S1 could not be detected.
Spectral analyses of the EEG data used the single-trial complex Morlet Wavelet approach described by Brockhaus-Dumke et al. (2008), whereby a window of 50 ms flanked each P50 response to determine the spectral makeup of that window from 25 to 75 ms. Spectral decomposition relied on wavelets calculated in 1 Hz steps, ranging from 3 to 50 Hz, using 3 cycles at the lowest frequency and up to 6 cycles at the highest. Consistent with Brockhaus-Dumke and colleagues, frequency definitions were: theta (4–6 Hz), alpha (9–11 Hz), and beta (14–25) Hz, thereby allowing for a conservative estimate of frequency contributions despite wavelet analysis smoothing procedures. However, gamma was defined at 30–50 (rather than 60) Hz to guard against influence from 60 Hz line noise. The dependent variables for each frequency bin reflected amplitude in microvolts squared. Recent data have shown the non-independence of pre-stimulus and baseline-normalized evoked spectral power on estimates of differences between controls and schizophrenia patients, particularly in the gamma band (Spencer, 2012). Thus, absolute power rather than baseline normalized spectra was utilized.
Statistical Analysis
To obtain a difference or “subtraction” measure of P50 suppression from S1 to S2, group differences in P50 amplitude were examined using repeated-measures ANOVA with group as the between-subjects factor and stimulus (S1 vs. S2) and condition (baseline vs. picture-viewing) as within-subject factors. A one-way ANOVA was used to determine group differences with the traditional S2/S1 parameter. To investigate the effects of group and condition on suppression within specific frequency bands, ANOVAs were computed for each of the four frequency bins, using group as the between-subject factor and condition and stimulus as repeated-measure factors. T-tests were used at a 95% level of confidence to determine the loci of significant main effects and interactions.
Results
P50 ERP Amplitudes and P50 Suppression Ratio
Table 2 shows mean amplitudes and suppression ratios by group. A significant main effect of stimulus was observed, F(1, 30) = 55.46, p < 0.001, but no other differences emerged, suggesting P50 suppression occurred without variation across groups and conditions. Similarly, there were no significant interaction effects involving group, stimulus or condition (p’s > 0.05). Comparable results were obtained when the P50 ratio score was the dependent variable. (See Supplemental Figure 1 for waveforms.)
Table 2.
P50 Amplitudes and Suppression Ratios
| Condition | Measure | Healthy Comparison | Schizophrenia | ||
|---|---|---|---|---|---|
| Subjects (N = 15) | Patients (N = 17) | ||||
| Mean | SD | Mean | SD | ||
| Baseline P50 | S1 | 3.74 | 2.11 | 3.57 | 1.87 |
| S2 | 1.51 | 1.12 | 1.45 | 0.87 | |
| S2/S1 | 0.44 | 0.36 | 0.47 | 0.32 | |
| Ratio | |||||
| Picture P50 | S1 | 3.37 | 2.02 | 3.20 | 1.20 |
| S2 | 1.70 | 1.13 | 1.74 | 0.96 | |
| S2/S1 | 0.51 | 0.18 | 0.58 | 0.30 | |
| Ratio | |||||
Mean S1 and S2 P50 amplitudes and S2/S1 ratios for each group.
Spectral Amplitudes
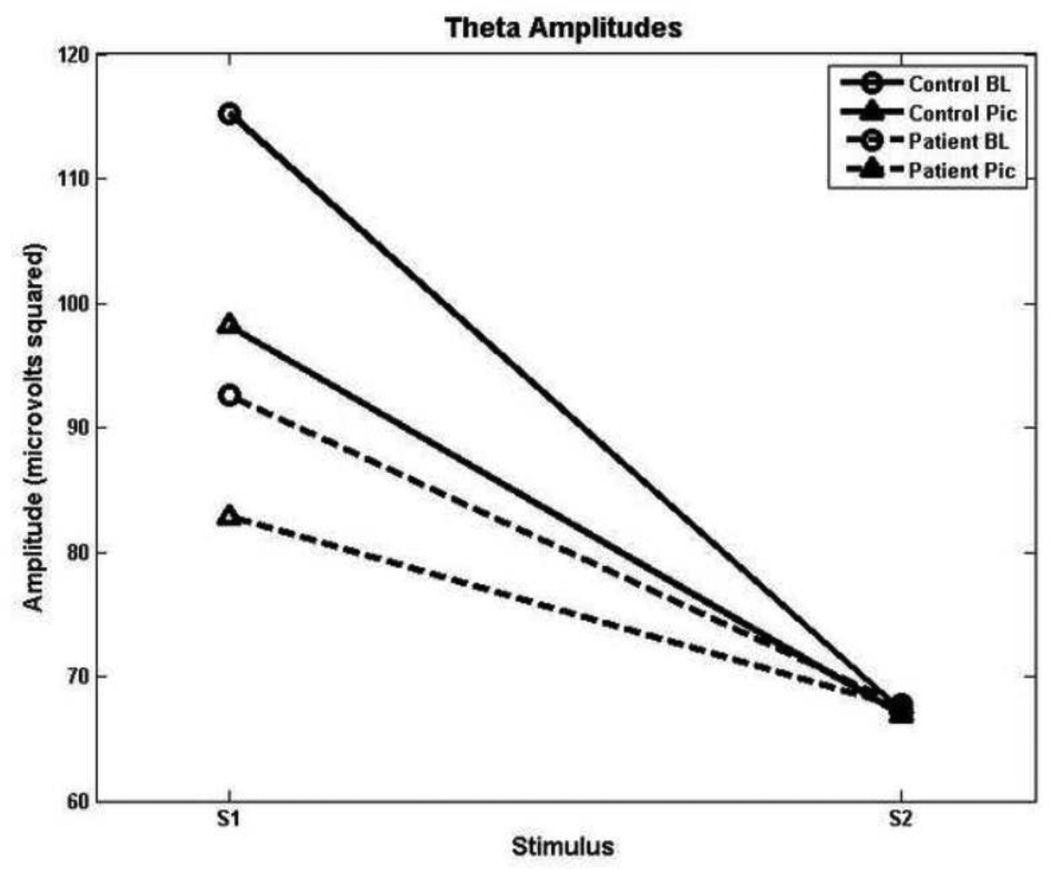
Table 3 shows mean spectral amplitudes by group. In the theta band, a significant main effect of stimulus, F(1, 33) = 40.71, p < 0.001, was modified by a significant group by stimulus interaction, F(1, 33) = 4.68, p = 0.038. Post hoc analyses determined that consistent with prior research, schizophrenia patients showed decrements in theta to S1 with reduced activity extending across both conditions. A significant main effect of condition, F(1, 33) = 5.39, p = 0.027, and a significant condition by stimulus interaction, F(1, 33) = 7.41, p = 0.01, revealed that across groups, there was an overall dampening of theta activity to S1 during the picture-viewing condition (see Figure 1).
Table 3.
Spectral Amplitudes
| Condition | Measure | Healthy Comparison | Schizophrenia | ||
|---|---|---|---|---|---|
| Subjects (N = 17) | Patients (N = 18) | ||||
| Mean | SD | Mean | SD | ||
| Baseline Theta | S1 | 115.25 | 46.34 | 92.59 | 28.65 |
| S2 | 67.23 | 11.19 | 67.75 | 13.51 | |
| Picture Theta | S1 | 98.15 | 29.75 | 82.84 | 16.59 |
| S2 | 66.95 | 8.33 | 67.48 | 16.76 | |
| Baseline Alpha | S1 | 84.95 | 30.32 | 77.90 | 15.24 |
| S2 | 59.99 | 17.79 | 58.43 | 16.03 | |
| Picture Alpha | S1 | 76.91 | 17.69 | 69.55 | 9.76 |
| S2 | 59.62 | 11.15 | 62.12 | 14.12 | |
| Baseline Beta | S1 | 65.98 | 9.19 | 63.89 | 7.32 |
| S2 | 57.03 | 8.50 | 57.80 | 8.08 | |
| Picture Beta | S1 | 59.26 | 10.12 | 61.91 | 7.91 |
| S2 | 59.00 | 9.94 | 60.10 | 8.06 | |
| Baseline Gamma | S1 | 52.25 | 10.43 | 58.27 | 16.10 |
| S2 | 50.01 | 8.89 | 54.70 | 15.00 | |
| Picture Gamma | S1 | 50.75 | 11.31 | 58.69 | 10.77 |
| S2 | 49.13 | 6.49 | 54.59 | 9.28 | |
Mean S1 and S2 spectral amplitudes for each group.
Figure 1.
Theta amplitudes as a function of group, condition and stimulus. Patients show decreased suppression from S1 to S2 irrespective of condition.
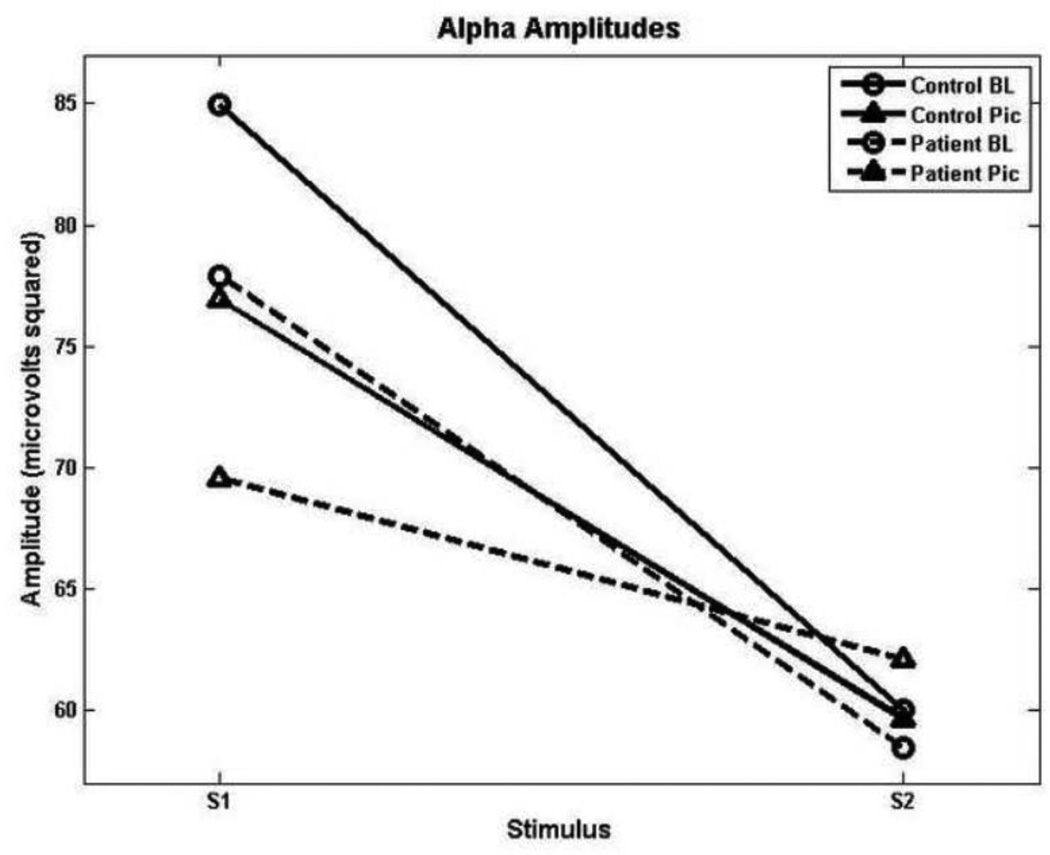
In the alpha range, a significant effect for stimulus, F(1, 33) = 37.32, p < 0.001 and a condition by stimulus interaction, F(1, 33) = 7.47, p = 0.01, were observed. Thus, alpha suppression was present across groups, with stronger gating during baseline relative to when neutral images were presented concurrently (see Figure 2).
Figure 2.
Alpha amplitudes, depicting reduced gating in both groups during the picture-viewing condition.
In the beta range, a significant effect for stimulus, F(1, 33) = 8.55, p = 0.001, and a condition by stimulus interaction, F(1, 33) = 12.07, p = 0.001, were observed, pointing again to reductions in beta gating for both groups upon introduction of pictures.
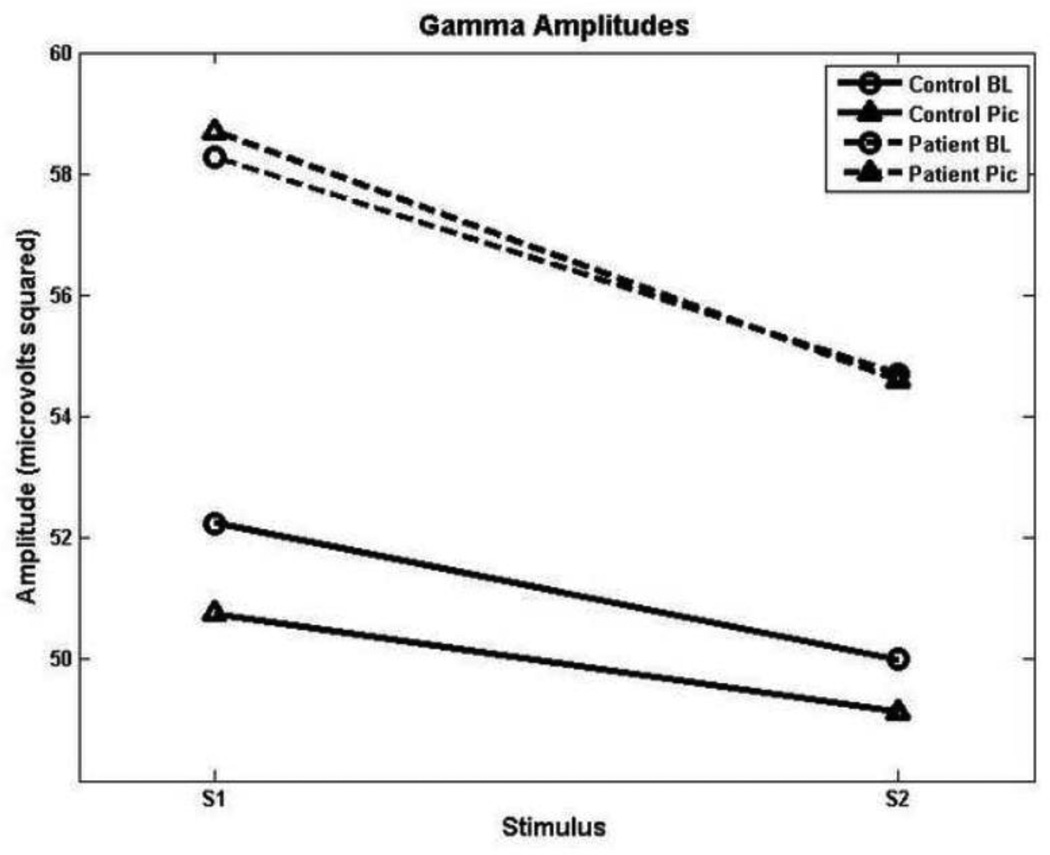
In the gamma band, significant effects of group, F(1, 33) = 5.23, p = 0.029, and stimulus, F(1,33) = 4.56, p = 0.04, were obtained. Thus, while gamma suppression was not statistically distinguishable between the two groups, patients showed higher gamma power overall (see Figure 3).
Figure 3.
Beta amplitudes, showing reduced gating in both groups during the picture-viewing condition.
Discussion
The present study examined event-related, oscillatory EEG activity associated with P50 suppression in schizophrenia patients and its modulation by the demands of the broader perceptual environment. In addition to supporting previous findings of abnormal modulation of low frequency activity in patients with schizophrenia, we also observed disruption to low frequency gating in healthy control subjects when viewing neutral pictures. These findings are consistent with the “sensory distraction” model of sensory gating abnormalities in schizophrenia (Jin & Potkin,1996). Furthermore, abnormalities in the gamma band, not in the suppression of activity but in the form of overall increased gamma power, were present in patients.
Theta band results from the baseline condition are consistent with a growing body of research showing that low-frequency oscillations can provide valuable information to complement the traditional P50 ERP ratio measure (Brockhaus-Dumke et al., 2008; Clementz & Blumenfeld, 2001; Johanessen et al., 2008). Hong and colleagues (2008), for instance, found that low-frequency suppression during the P50 paradigm exhibited a level of heritability nearly four times that of the traditional P50 score. Therefore, reduced low-frequency activity may be a more viable endophenotype while the traditional P50 ratio may be associated with clinical and experimental measures of attentional impairment (Cullum et al. 1993; Erwin et al., 1998; Lijffijt et al., 2009; Yee et al., 1998; 2010).
Theta activity has been associated with the sensory encoding function of the hippocampus (Buzsaki, 2002), a critical neural structure linked to P50 suppression along with the dorsolateral prefrontal cortex, superior temporal gyrus (STG), and thalamus (Tregellas et al., 2007; Williams et al., 2011). Similarly, activity in this frequency band may represent the attention-orienting response to novel stimuli given its peak upon initial presentation of stimuli and subsequent habituation over successive exposures (Dietl et al., 1999). Because increased perceptual demands may diminish the availability of cognitive resources required for processing efficiency (Duncan, 1980; Fisher, 1982; Nuechterlein & Dawson, 1984), findings from the present study of diminished theta gating, in patients and across groups within a richer perceptual environment, may both validate and provide a mechanistic account of the “sensory distraction” model of sensory gating impairments in schizophrenia.
Jin and Potkin (1996) modeled a reduced P50 response to S1, and thus S2/S1 ratio, of schizophrenia patients using non-ill individuals by introducing competing visual stimuli as a sensory distraction. The authors suggested that concurrent stimulation reduces the allocation of resources to the processing of novelty. The present study is the first to show similar levels of low-frequency gating between a resting baseline condition in schizophrenia patients and a sensory-distraction condition in healthy controls (see Figure 1).
Findings that link theta activity to long-range connectivity may also point to reduced theta as indicative of abnormal inter-areal communication between broad cortical regions (Cohen, 2011; Von Stein & Sarnthein, 2000), potentially contributing to complex, “downstream” functions such as top-down control of perception (Uhlhaas, 2008; Ford et al., 2002). Considering this perspective in light of present findings may suggest that a relatively broad, temporal lobe-centered circuit is impaired in the "gating in" of the initial stimulus in a sequence among schizophrenia patients (Brenner et al., 2009).
Turning to a higher EEG frequency range, reports of both abnormal reductions (e.g., Kwon et al., 1999; Light et al., 2006) and significant increases in gamma band power in schizophrenia patients (e.g., Baldeweg et al., 1998; Gordon et al., 2001; Lee et al., 2003a, 2003b) support the possibility that schizophrenia entails disrupted neural synchrony (Allen et al., 2011; Stephan et al., 2009), particularly at the level of relatively localized neural assemblies responsible for the encoding of sensory stimuli (Basar-Eroglu et al., 1996; Von Stein & Sarnthein, 2000; Allen et al., 2011). The present study’s finding of overall increased gamma power among patients may also be considered in light of current models of N-methyl-D-aspartate (NMDA) receptor dysfunction, in which NMDA receptor hypofunction reduces the excitation of parvalbumin-expressing inhibitory interneurons, postulated to be chief regulators of cortical gamma oscillations (Whittington & Traub, 2003; Sohal et al., 2009; Lewis et al., 2011). Therefore, down-regulation of inhibitory interneurons may be reflected in increased gamma band activity in patients (Spencer, 2012).
Generally heightened gamma activity in patients relative to healthy controls is also consistent with the proposition that schizophrenia may be associated with a chronic state of heightened tonic sensory demand (Jin & Potkin, 1996) and reductions in the availability of resources (e.g., Nuechterlein & Dawson, 1984). Suppression in this band was observed in both patients and control subjects, suggesting that gating of gamma band activity may be intact and potentially relying upon NMDA-receptor-independent mechanisms. A goal for future research will be to determine the functional corollaries of intact high-frequency gating relative to those of heightened overall gamma activity. It also bears noting that because all patients in this study were receiving antipsychotics, the influence of medication on present results cannot be accounted for entirely. The absence of associations between medication exposure and any of the key dependent variables suggests the lack of a major contributing influence although a more limited effect of medication remains possible.
Nevertheless, findings from the present study begin to extend our understanding of sensory gating abnormalities within the context of a more richly-detailed model of sensory demands and processing in schizophrenia. Moreover, they give additional credence to the notion of schizophrenia as a disorder of abnormal neural synchrony and more generally, dysregulated neurocognitive coordination.
Supplementary Material
ERP waveforms from site Cz showing comparable P50 suppression between patients and controls. An additional 30 Hz low pass filter was applied for visualization purposes in this figure.
Figure 4.
Gamma amplitudes showing intact sensory gating in both groups but heightened overall activity in patients.
Acknowledgments
The authors would like to thank Ty Cannon, Maria Jalbrzikowski, Ian Mathis, Gretchen Sholty, and Jane Sun for their helpful discussion throughout this study.
Role of funding source
This study was supported in part by grants MH57322, MH37705, and Center grant P50 MH066286 from the National Institute of Mental Health, Bethesda, MD. The NIMH had no further role in study design; collection, analysis or interpretation of data; or in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Mr. Moran contributed to data processing and analysis and wrote the first draft of the manuscript. Dr. Williams contributed to data collection and manuscript preparation. Drs. Bachman and Yee contributed to the interpretation of the results and manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflict of interests.
References
- Allen EA, Liu J, Kiehl KA, Gelernter J, Pearlson GD, Perrone-Bizzozero NI, Calhoun VD. Components of cross-frequency modulation in health and disease. Frontiers in Systems Neuroscience. 2011;5:59. doi: 10.3389/fnsys.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassnik T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. International Journal of Psychophysiology. 1996;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hasketh S, Sham P, Murray R, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP. Event-related potential abnormalities in schizophrenia: A failure to “gate in” salient information? Schizophrenia Research. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: Relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:1–20. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Experimental Brain Research. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. Neuroimage. 2011;55:1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Annen L, Masthof E, Hodiamont P. Audio-visual integration in schizophrenia. Schizophrenia Research. 2001;59:211–218. doi: 10.1016/s0920-9964(01)00344-9. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, de Jon SJ, Masthoff ED, Trompenaars FJ, Hodiamont P. Multisensory integration of emotional faces and voices in schizophrenics. Schizophrenia Research. 2004;72:195–203. doi: 10.1016/j.schres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan J. The demonstration of capacity limitation. Cognitive Psychology. 1980;12:75–96. [Google Scholar]
- Erwin R. P50 abnormalities in schizophrenia: Relationship to clinical and neuropsychological indices of attention. Schizophrenia Research. 1998;33:157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- Fisher DL. Limited-channel models of automatic detection: Capacity and scanning in visual search. Psychological Review. 1982;89:662–692. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBM. Structured clinical interview diagnostic (SCID) for DSM-IV axis I disorders-clinician version. American Psychiatric Press. 1997 [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Lacambre M, Pham B-T, Giersch A, Elliott MA. Low time resolution in schizophrenia Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophrenia Research. 2007;97:118–127. doi: 10.1016/j.schres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects: Human recordings, computer simulations, and an animal model. Archives of General Psychiatry. 1996;53:1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Gordon E, Williams LM, Haig AR, Bahramali H, Wright J, Meares R. Symptom profile and ‘gamma’ processing in schizophrenia. Cognitive Neuropsychiatry. 2001;6:7–20. [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: Variant evidence or evidence of variants? Neuroscience & Biobehavioral Reviews. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Archives of General Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley A. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophrenia Bulletin. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG. P50 changes with visual interference in normal subjects: A sensory distraction model for schizophrenia. Clinical Electroencephalography. 1996;27:151–154. doi: 10.1177/155005949602700308. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Bodkins M, O’Donnell BF, Shekhar A, Hetrick WP. Perceptual anomalies in schizophrenia co-occur with selective impairments in gamma frequency component of midlatency auditory ERPs. Journal of Abnormal Psychology. 2008;117:106–118. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O'Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, McKeown MJ, Bell AJ, Lee T, Sejnowski TJ. Imaging brain dynamics using independent component analysis. Proceedings of the IEEE. 2001;89:1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Induced gamma-band activity and human brain function. Neuroscientist. 2003;9:475–484. doi: 10.1177/1073858403259137. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM. Feature selection in the human brain: Electrophysiological correlates of sensory enhancement and feature integration. Brain Research. 2010;1313:172–184. doi: 10.1016/j.brainres.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Archives of General Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Instruction manual and affective ratings. Technical Report A-6. The Center for Research in Psychophysiology, University of Florida; 2005. International affective picture system (IAPS) [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspar M, Gordon E. Synchronous gamma activity: A review and contributions to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. Gamma (40 Hz) phase synchronicity and symptom dimension in schizophrenia. Cognitive Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G. Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Current Opinion in Neurobiology. 2011;21:866–872. doi: 10.1016/j.conb.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, Moeller FG, Swann AC. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46:1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. American Journal of Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biological Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale. Schizophrenia Bulletin. 1986;12:594–602. [Google Scholar]
- Mathalon DH, Heinks T, Ford JM. Selective attention in schizophrenia: Sparing and loss of executive control. American Journal of Psychiatry. 2004;161:872–881. doi: 10.1176/appi.ajp.161.5.872. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophrenia Bulletin. 2011;37:659–663. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Weisz N, Elbert T, Rockstroh B, Miller GA. Evoked and induced oscillatory activity contributes to abnormal auditory sensory gating in schizophrenia. NeuroImage. 2011;56:307–314. doi: 10.1016/j.neuroimage.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Quian Quiroga R, Schurmann M, Basar E. Bisensory stimulation increases gamma-responses over multiple cortical regions. Brain Research Cognitive Brain Research. 2001;11:267–279. doi: 10.1016/s0926-6410(00)00081-1. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Cognitive Brain Research. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Talsma D, Hermann CS, Woldorff MG. Multisensory processing and oscillatory gamma responses: Effects of spatial selective attention. Experimental Brain Research. 2005;166:411–426. doi: 10.1007/s00221-005-2381-z. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Diesseroth K. Parvalbumin neurons and gamma rhythms synergistically enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Frontiers in Human Neuroscience. 2012;5:1–7. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. Journal of Neuroscience. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in Schizophrenia: From abnormal synaptic plasticity to failures in self-monitoring. Schizophrenia Bulletin. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus, and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophrenia Research. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Ellis J, Shatti S, Du YP, Rojas DC. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. American Journal of Psychiatry. 2009;166:354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophrenia Bulletin. 2008;62:1–17. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas, Silverstein Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychological Bulletin. 2005;131:618–632. doi: 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nature Reviews: Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukeoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. A Brief Psychiatric Rating Scale (BPRS) expanded version: Scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993;3:227–243. [Google Scholar]
- Von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub R. Inhibitory interneurons and network oscillations in vitro. Trends in Neuroscience. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology. 2011;48:470–478. doi: 10.1111/j.1469-8986.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biological Psychiatry. 2003;54:1181–1192. doi: 10.1016/s0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Hermann WM, Coppola R. Schizophrenia: reduced signal-to-noise ratio an impaired phaselocking during information processing. Clinical Neurophysiology. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen J-M, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Yee CM, Nuechterlein KH, Morris SE, White PM. P50 Suppression in recent-onset schizophrenia: Clinical correlates and risperidone effects. Journal of Abnormal Psychology. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- Yee CM, Williams TJ, White PM, Nuechterlein KH, Wirshing DA, Subotnik KL. Attentional modulation of the P50 suppression deficit in recent-onset and chronic schizophrenia. Journal of Abnormal Psychology. 2010;119:31–39. doi: 10.1037/a0018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ERP waveforms from site Cz showing comparable P50 suppression between patients and controls. An additional 30 Hz low pass filter was applied for visualization purposes in this figure.