Abstract
Aims
A non-neuronal cholinergic system has been described in epithelial cells including that of the urinary bladder (urothelium) and the upper gastrointestinal tract (esophagus). Epithelial dysfunction has been implicated in the pathophysiology of persistent pain conditions such as painful bladder syndrome as well as functional heartburn. For example, alterations in the ability to synthesize and release acetylcholine may contribute to changes in epithelial sensory and barrier function associated with a number of functional genitourinary and intestinal disorders.
Main methods
We examined using immunoblot, acetylcholine (ACh)-synthesis and release components in cat esophageal mucosa and whether elements of these components are altered in a naturally occurring model of chronic idiopathic cystitis termed feline interstitial cystitis (FIC).
Key findings
We identified proteins involved in ACh synthesis and release (high affinity choline transporter, CHT1; ACh synthesizing enzymes choline acetyltransferase ChAT and carnitine acetyltransferase CarAT; vesicular ACh transporter VAChT and the organic cation transporter isoforms 1-3 or OCT1-3) in cat esophageal mucosa. Significant alterations in CHT, ChAT, VAChT and OCT-1 were detected in the esophageal mucosa from FIC cats. Changes in the vesicular nucleotide transporter (VNUT) and the junctional protein pan-cadherin were also noted.
Significance
Taken together, these findings suggest that changes in the non-neuronal cholinergic system may contribute to alterations in cell-cell contacts and possibly communication with underlying cells that may contribute to changes in sensory function and visceral hyperalgesia in functional esophageal pain.
Keywords: esophageal mucosa, barrier function, signaling function
Introduction
Gastro-oesophageal reflux disease (GERD) is a common and well characterized disease in which reflux of hydrochloric acid from the stomach into the esophagus results in sensitization of visceral afferent pathways at the primary afferent and spinal level (with or without associated epithelial damage), and which is associated with symptoms of acid regurgitation and burning retrosternal pain, characteristically in the form of heartburn (Hershcovici and Fass, 2007; Orlando, 2008). While some patients exhibit evidence for epithelial injury in the form of erosions and ulcerations, there is also a non-erosive form of the disease where affected patients exhibit symptoms of heartburn and esophageal hypersensitivity without any visible signs of esophageal epithelial injury or erosions. When the symptoms of heartburn occur in the absence of any epithelial injury and in the absence of abnormal acid reflux, the syndrome is referred to as reflux negative heartburn or functional heartburn (Tack and Fass, 2004; Long and Orlando, 2007; Hershcovici and Fass, 2010). Functional heartburn often co-exists in the same patient with other persistent pain disorders, including functional dyspepsia, irritable bowel syndrome (IBS) and interstitial cystitis (Gasiorowska et al., 2009). Even though central mechanisms of pain amplification have been implicated as an important pathophysiological factor in functional heartburn and related disorders (Mayer and Bushnell, 2009), recent findings suggest a possible role of alterations in epithelial signaling and barrier function (Farre et al., 2007; Orlando et al., 2010). Changes in barrier function with a corresponding loss of epithelial integrity may result in leakage of irritating substances into the underlying tissues (including nerves, muscle) that can result in symptoms of hypersensitivity and pain.
The mechanisms underlying these changes in epithelial sensory and barrier function are not well understood. In some pathological conditions, alterations in levels of chemical mediators such as ATP have been linked with changes in epithelial function and/or integrity (Burnstock 2008). Another prominent example is the transmitter acetylcholine, which plays a significant role in maintaining a number of cellular functions. There is substantial support that cells outside the nervous system express the machinery to both synthesize and release acetylcholine (Wessler and Kirkpatrick, 2008). The enzymes necessary for synthesis (choline acetyltransferase) and metabolism (acetylcholinesterase) have been identified in human esophageal epithelium (Nguyen et al., 2000). Dysfunction in synthesis or release mechanisms associated with the non-neuronal cholinergic system has been associated with pathogenesis in a number of diseases (Gwilt et al., 2007; Kawashima and Fujii, 2008; Wessler and Kirkpatrick, 2008).
In this study, we examined the presence of components involved in the synthesis and release of non-neuronal acetylcholine in esophageal mucosa from healthy cats and from cats diagnosed with feline IC, a naturally occurring form of interstitial cystitis (Buffington CA, 2004; Birder et al., 2010). Studies support a role for non-neuronal acetylcholine in epithelial signaling, barrier function and maintenance of cell-cell contacts. One may speculate that alterations in the non-neuronal cholinergic system may play a role in the integrity of the esophageal mucosa in a variety of functional gastrointestinal disorders, including functional heartburn and IBS.
Materials and Methods
Animals
All procedures were conducted in accordance with Institutional Animal Care and Use policies at each institution (University of Pittsburgh; Ohio State University). Both adult healthy cats (n=8) and cats diagnosed with irritative cystitis (hereafter referred to as feline interstitial cystitis or FIC, n=9) were used for this study. All cats with FIC were obtained as donations from clients due to a history of chronic recurrent stranguria, hematuria, pollakiuria, and/or urination in inappropriate locations and were evaluated at the OSU Veterinary Teaching Hospital as described previously (Birder et al., 2010). Healthy, age-matched cats obtained from commercial vendors and determined to be free of disease and signs referable to the lower urinary tract according to the same diagnostic criteria as cats with FIC were used as controls. All cats were housed in stainless steel cages and allowed to acclimatize to their environment for at least 3 months prior to study. Esophageal (proximal) tissue was dissected from deeply anesthetized cats (induction and maintenance with isoflurane). In a limited number of experiments, proximal esophageal tissue was also dissected from anesthetized Sprague Dawley rats.
Tissue preparation and western immunoblotting
Esophageal (proximal) mucosa (cat; rat) was dissected away from underlying smooth muscle and homogenized in HBSS (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5.6 mM glucose, and 10 mM Hepes, pH 7.4) containing complete protease inhibitor cocktail 1 tablet/10 ml, Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Sigma, 1:100). The homogenate was centrifuged (13,000g; 15 min). The membrane protein fraction was prepared by suspending the membrane pellets in lysis buffer containing 0.3 M NaCl, 50 mM Tris-HCl (pH 7.6), and 0.5% Triton-X100—and the same concentration of protease inhibitors as above. The suspensions were incubated on ice and centrifuged (13,000 rpm; 15 min at 4° C). The protein concentrations of the combined supernatants were determined using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL). Whole cell protein lysates were obtained and proteins were analyzed for relative expression levels using a standard immunoblotting protocol. Densitometry was performed using a Personal Densitometer SI (Molecular Probes). The membranes were stripped (membrane recycling kit from Alpha Diagnostic International, San Antonio, TX) and re-probed overnight with anti-β-actin (37 kDa; Cell Signaling Technology, Danvers MA) as a loading control. In a limited number of experiments, esophageal epithelial cells were also isolated and cultured according to previously described methods (Mihara et al., 2011) and prepared in a similar manner as mucosal tissue for western immunoblotting. The antibodies to the high affinity choline transporter (CHT, 63kDa), choline acetyltransferase (ChAT, 75kDa), the vesicular acetylcholine transporter (VAChT) and acetylcholinesterase (AChE, 68kDa) were purchased from Millipore. Antibodies for members of the organic cation transporter family (OCT-1/2/3, 60–66kDa) were purchased from Alpha Diagnostics International. In addition, antibodies for carnitine transferase (CarAT, 51kDa), pan-cadherin (140kDa), and VNUT (47kDa) were purchased from Aviva Systems Biology, Cell Signaling Technology, and Medical & Biological Laboratories, respectively. Single immunoreactive bands were observed for the targets aforemementioned. In order to verify the presence of VAChT in the esophageal mucosa, a immunizing peptide was obtained (CEDDYNYYSRS, American Peptide Company) and used to block a single immunoreactive band at 57kDa in primary esophageal epithelial cells obtained from rat and a single immunoreactive band at 65–70kDa in cat esophageal mucosa, which most likely represents post-translational glycosylation of the protein in native tissue. Peptides for the muscarinic receptors M2 (Calbiochem) and M3 (BioTrend) antibodies were available and blocked a single immunoreactive band for each at 66kDa and 90kDa, respectively, and have been fully characterized previously with their respective peptides and knock-out animals (Zarghooni et al., 2007). The observed immunoreactive band for M3AChR, at 90kDa, most likely represents a dimer of the receptor whose expected size is 45kDa.
Results
Feline esophageal mucosa expresses the cholinergic enzymes ChAT, CarAT and AChE and the cholinergic muscarinic receptor subtypes M2 and M3
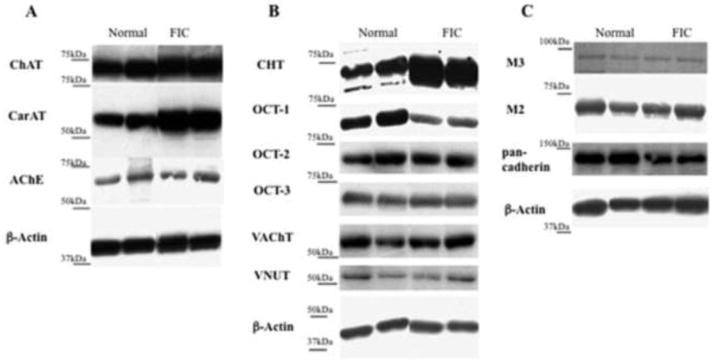
Expression of proteins involved in the synthesis of acetylcholine including choline acetyltransferase (ChAT) and carnitine acetyltransferase (CarAT) was observed in feline esophageal mucosa (Figure 1). As compared to esophageal mucosa from healthy cats, ChAT protein expression was significantly decreased in FIC esophageal mucosa (Table 1, p<0.05). There was no significant difference in protein expression of CarAT or acetylcholinesterase (AChE) between healthy and FIC esophageal mucosa. We also observed both cholinergic muscarinic receptor subtypes M2 and M3 are expressed in esophageal mucosa as shown in Figure 1 (as well as in primary esophageal epithelial cultures from rat mucosa). No significant differences in levels of expression for either subtype were found in healthy versus FIC esophageal mucosa (Table 1).
Figure 1.
Representative western blot analysis of components of the non-neuronal cholinergic system from normal and FIC esophageal mucosa tissue extracts. A, Expression of enzymes involved in acetylcholine synthesis (choline acetyltransferase, ChAT; carnitine acetyltransferase, CarNT) and degradation (acetylcholinesterase, AChE). B, Expression of molecular transporters including choline transporter (CHT), members of the organic cationic transporter family (OCT-1, OCT-2, OCT3), the vesicular acetylcholine transporter (VAChT) and VNUT, a transporter responsible for filling vesicles with ATP. C, Expression of pan-cadherin, an adhesion molecule, and muscarinic receptor type 2 (M2) and type 3 (M3). Shown in each panel is the loading control β-actin.
Table 1.
Expression levels of components of the non-neuronal cholinergic system in esophageal mucosa isolated from feline control versus idiopathic cystitis (FIC).
| Enzymes | Control | Feline Interstitial Cystitis |
|---|---|---|
| ChAT | 3.58 ± 1.65* | 0.89 ± 0.08* |
| CarNT | 112.50 ± 11.37 | 150.10 ± 20.29 |
| AChE | 24.01 ± 7.90 | 25.76 ± 4.12 |
| Transporters | ||
| CHT | 174.90 ± 36.65* | 297.20 ± 32.59* |
| OCT-1 | 135.60 ± 39.04* | 48.69 ± 5.26* |
| OCT-2 | 0.91 ± 0.25 | 0.84 ± 0.23 |
| OCT-3 | 28.00 ± 4.72 | 21.42 ± 1.93 |
| VAChT | 30.41 ± 10.16* | 71.17 ± 9.57* |
| VNUT | 0.43 ± 0.21 | 0.23 ± 0.05 |
| Adhesion molecule | ||
| Pan-cadherin | 0.58 ± 0.15 | 0.29 ± 0.09 |
| Receptors | ||
| M2 | 60.22 ± 18.80 | 62.54 ± 3.72 |
| M3 | 4.60 ± 0.62 | 3.95 ± 0.33 |
P ≤ 0.05 (Student’s t test).
Feline esophageal mucosa expresses cholinergic (and purinergic) transporters
Feline esophageal mucosa was found to express members of the organic cation transporter (OCT) family (OCT-1, -2 and -3), which are thought to mediate the non-vesicular release of acetylcholine (Figure 1). Only the OCT-1 was significantly decreased in FIC as compared to healthy or control mucosa (Table 1). In addition, the choline transporter (CHT1) was expressed in feline esophageal mucosa (Figure 1) and this was significantly elevated in FIC. The vesicular acetylcholine transporter (VAChT, shown in Figure 1) was localized in feline esophageal mucosa and this expression was decreased in FIC esophageal mucosa. VAChT may not be expressed in all types of epithelial cells, thus we also explored expression using esophageal epithelial cells isolated from another species (rat). Here, we also found expression of VAChT protein that was blocked using a peptide against VAChT. In addition, feline esophageal mucosa expresses the transporter thought to be responsible for filling vesicles with ATP (VNUT, Figure 1). There was a trend toward decreased expression (though not significant) in FIC esophageal mucosa as compared to healthy controls (Table 1).
Feline FIC esophageal mucosa exhibits a trend toward decreased expression of the adhesion molecule pan-cadherin
The adhesion molecule cadherin exhibits multiple functions, including regulation of epithelial junctional integrity. The expression of pan-cadherin (shown in Figure 1) was decreased (though not significant) in FIC esophageal mucosa as compared to healthy control (Table 1).
Discussion
The findings of this study revealed that feline esophageal mucosa expresses all of the components of the acetylcholine synthesis and release machinery. This includes components involved in cellular uptake for choline (CHT1), ACh synthesis (ChAT and CarNT) and release (VAChT; OCT1, -2 and -3) as well as metabolism (AChE). Our findings also reveal a number of alterations in uptake, synthesis and release in a naturally occurring feline model of interstitial cystitis. Given that all components of the non-neuronal cholinergic system may be altered in pathology, our findings may point to involvement of this system in upper GI tract function in health and disease.
Patients that exhibit reflux symptoms often present with a hypersensitivity to physical and chemical stimuli, even in the absence of epithelial damage or irritation. In GERD, decreased integrity of the esophageal mucosa (termed dilated intercellular space or DIS) has been reported (Orlando and Orlando, 2009), and it has been postulated that intraluminal acid gains access to afferent nerve terminals through these spaces. Alterations in epithelial permeability have also been described in functional GI disorders, even though the underlying mechanisms are not known. Such types of changes are not limited to the esophagus as similar alterations in epithelial barrier function have been reported in patients diagnosed with other disorders including inflammatory bowel disorders, asthma and even overactive bladder syndrome and painful bladder syndrome / interstitial cystitis (PBS/IC) (Birder and Chapple, 2009; Catalioto et al., 2011). For example, in patients diagnosed with PBS/IC as well as in animal models for this syndrome, epithelial alterations including hypersensitivity to cholinergic stimuli and changes in barrier function have been reported (Lavelle et al., 2000; Parsons, 2007; Gupta et al., 2009). A systematic study of all the components of the non-neuronal cholinergic system in a variety of diseases has not been achieved. However, evidence supports a role for non-neuronal acetylcholine in sensation, cell-cell signaling, proliferation and cell growth as well as maintaining barrier function (Wessler and Kirkpatrick, 2008). Thus given the associations with a number of gastro-intestinal and urinary functional syndromes, we examined the expression levels of components of the non-neuronal cholinergic system in esophageal epithelium (mucosa) in a feline animal model for interstitial cystitis.
We found the choline transporter, CHT1, is expressed in feline esophageal mucosa and expression was significantly elevated in cats diagnosed with FIC. This finding implies a significant increase or uptake of the ACh precursor choline in FIC esophageal mucosa as compared to normal controls. Despite the enhanced expression of CHT1 in feline IC, the enzyme responsible for converting choline into acetylcholine, ChAT, was significantly down-regulated which may suggest a decrease in ACh production. Acetylcholinesterase (AChE), responsible for the breakdown of ACh and may also be involved in cell division and differentiation (Balasubramanian and Bhanumathy, 1993), was expressed though not significantly changed from control. Alteration in ACh synthesis and release machinery is likely to have multiple effects on cellular function including signaling pathways mediated via n- and mACh receptor subtypes. For example, stimulation of n- or mAChRs in esophageal epithelium may play a role in epithelial integrity, wound-healing as well as provide a target for effects of nicotine (Nguyen et al., 1999; Wessler and Kirkpatrick, 2008).
Release of mediators via n- and mAChRs exerts multiple effects including regulating the excitability of underlying cells including nerves, immune and smooth muscle. For example, stimulation of mAChRs in urinary bladder urothelium results in release of ATP which plays an important role in signaling within the bladder wall (Ikeda and Kanai, 2008; Kullmann et al., 2008). There is a great deal of evidence supporting a role for epithelial-derived release of mediators such as ATP in both autocrine and paracrine signaling in viscera including the urinary bladder, colon and esophagus (Burnstock, 2008; 2011). Stimulation of muscarinic or nicotinic ATP released from epithelial cells during a mechanical or chemical stimulus can activate purinergic receptors on nearby afferent nerves triggering sensations including fullness, discomfort and pain. A role for this type of chemical communication in visceral function is supported by studies using knockout mice whereby deletion of P2X3 receptors can lead to a decrease in urinary bladder distension as well as gastroesophageal sensation (Cockayne et al., 2000; Mcilwrath et al., 2009). Though ATP may be released via multiple pathways, recent reports have identified a candidate vesicular nucleotide transporter or VNUT specialized to transport nucleotides such as ATP (Sawada et al., 2008). This transporter is thought to play an important role in uptake, storage and possibly release of ATP in a number of ATP-secreting cells including esophageal epithelium. In the present studies we show that VNUT is expressed in feline esophageal mucosa and is decreased in esophageal mucosa from FIC cats in comparison to control animals. Altered VNUT expression could lead to changes in ATP storage and release that in turn, could impact on sensation as well as epithelial proliferation, differentiation and the integrity of the epithelial barrier. Further studies are needed to fully examine the role of mediators such as ATP and associated transporters on sensory and barrier function in esophageal mucosa.
Alterations in non-neuronal ACh could also lead to cell-cell detachment, changes in the cytoskeleton as well as expression of adhesion or junctional markers (Kurzen et al., 2007; Wessler and Kirkpatrick, 2008). A characteristic of gastrointestinal reflux disease or GERD (even those with non-erosive reflux) is the presence of ultrastructural abnormalities termed dilated intercellular spaces as well as an increase in junctional permeability within the esophageal mucosa (Orlando, 2010; 2011). Though a number of junctional proteins may play a role in epithelial barrier function recent evidence has revealed that patients diagnosed with GERD exhibit a loss of e-cadherin within esophageal epithelium (Jovov et al., 2010). There is evidence in other types of epithelium that adhesion molecules such as members of the cadherin family play an important role in both establishing and maintaining epithelial-cell contacts (Nawijn et al., 2011). In the present study we also have observed a decrease in (pan)-cadherin suggesting that FIC esophageal epithelial integrity may also be compromised similar to patients with symptoms associated with esophageal reflux. In addition, while choline is a necessary precursor for acetylcholine, it is also a substrate for various cell membrane components. Thus our finding of an increase in choline transporter in FIC esophageal mucosa (with the likelihood of a corresponding increase in choline requirement) further supports a change in esophageal mucosal membrane in pathology. A change in membrane integrity or barrier function is likely to play a role in permitting noxious substances into the underlying spaces as well as impacting on the function of underlying cells including nerves and muscle.
It has been suggested that in some types of epithelia, in contrast to exocytosis of synaptic vesicle contents, the organic cation ion transporters (OCTs) may be more important in mediating ACh release (Wessler and Kirkpatrick, 2008). In the present study, we found that feline esophageal mucosa expresses all three subtypes of OCTs (OCT1, 2 and 3). These transporter proteins are widely expressed and various subtypes involved in uptake and/or release are likely to differ between organs. For example, studies have shown that epithelial ACh content was enhanced in OCT 1/2 double knockout mice supporting a role for OCTs in mediating non-neuronal acetylcholine release in the airways (Kummer et al., 2006). We found that OCT1 was significantly decreased in feline IC esophageal mucosa with no change in OCT subtypes 2 or 3 as compared to control. While the significance of this change is not known and may also reflect compensatory mechanisms, a decrease in OCT subtype may result in impaired ACh release and possibly increased ACh content. This is also supported by our findings that the vesicular acetylcholine transporter (VAChT) was significantly elevated in FIC as compared to normal control esophageal mucosa. VAChT, which is also expressed in airway bronchial epithelium, is responsible for packaging ACh into vesicles (Gwilt et al., 2007) and in some types of cells, may also play a role in secretion of acetylcholine.
Conclusion
To our knowledge, these findings are the first report that examines expression of the non-neuronal cholinergic synthesis and release machinery in non-human esophageal mucosa. We show that all components including synthesis, storage, release, inactivation as well as expression of mAChR subtypes are expressed within feline esophageal mucosa. Alteration in expression of components of the non-neuronal ACh machinery in pathology (feline interstitial cystitis) may contribute to epithelial hypersensitivity and barrier dysfunction that often occur in patients with various functional and inflammatory gastrointestinal esophageal symptoms. Thus, a more detailed understanding of the non-neuronal cholinergic system of the esophageal epithelium could potentially influence therapeutics for the clinical management of esophageal disorders. While it is not known whether similar changes occur in other tissues, alterations in epithelial barrier and sensory functions could have major implications for understanding of co-morbid disorders.
Acknowledgments
The authors thank H. Braun and M. Perpetua for technical assistance. This work was supported by NIH grants R37 DK54824 and R01 DK57284 (LAB), P50 DK64539 (LAB, CAB, EM) and K01 DK80184 (AHM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian AS, Bhanumathy CD. Noncholinergic functions of cholinesterases. FASEB J. 1993;7:1354–1358. doi: 10.1096/fasebj.7.14.8224608. [DOI] [PubMed] [Google Scholar]
- Birder LA, Chapple CR. Visceral pain syndromes, chronic pelvic pain/interstitial cystitis. In: Mayer EA, Bushnell MC, editors. Functional pain syndromes: presentation and pathophysiology. Vol. 7. Seattle: IASP Press; 2009. pp. 151–168. [Google Scholar]
- Birder LA, Wolf-Johnston AS, Chib MK, Buffington CA, Roppolo JR, Hanna-Mitchell AT. Beyond neurons: involvement of urothelial and glial cells in bladder function. Neurourol Urodyn. 2010;29:88–96. doi: 10.1002/nau.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172:1242–8. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The journey to establish purinergic signaling in the gut. Neurogastroenterol Motil. 2008;20:8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Introductory overview of purinergic signaling. Front Biosci. 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- Catalioto RM, Maggi CA, Giuliani S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem. 2011;18:398–426. doi: 10.2174/092986711794839179. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Farre R, de Vos R, Geboes K, Verbecke K, Vanden Bergh P, Depoortere I, Biondeau K, Tack J, Sifrim D. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007;56:1191–7. doi: 10.1136/gut.2006.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiorowska A, Poh CH, Fass R. Gastroesophageal reflux disease (GERD) and irritable bowel syndrome (IBS)—is it one disease or an overlap of two disorders? Digestive Disease Science. 2009;54:829–1834. doi: 10.1007/s10620-008-0594-2. [DOI] [PubMed] [Google Scholar]
- Gupta GN, Lu SG, Gold MS, Chai TC. Bladder urothelial cells from patients with interstitial cystitis have an increased sensitivity to carbachol. Neurourol Urodyn. 2009;28:1022–7. doi: 10.1002/nau.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharm Ther. 2007;115:208–22. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Hershcovici T, Fass R. Are functional heartburn and functional dyspepsia one disorder? Nature Rev Gastroenterol Hepatol. 1020;7:71–72. doi: 10.1038/nrgastro.2009.233. [DOI] [PubMed] [Google Scholar]
- Hershcovici T, Fass R. Pharmacological management of GERD: where does it stand now? Trends Pharmacol Sci. 2011;32:258–264. doi: 10.1016/j.tips.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol. 2008;295:F454–461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovov B, Que J, Tobey NA, Djukic Z, Hogan BLM, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2010;106:1039–47. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Basic and clinical aspects on non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci. 2008;106:167–173. doi: 10.1254/jphs.fm0070073. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol. 2008;294:F971–81. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, Koepsell H, Habergerger RV, Lips KS. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res. 2006;7:65–77. doi: 10.1186/1465-9921-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Met Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol. 2000;278:F540–53. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- Long JD, Orlando RC. Nonerosive reflux disease. Minerva Gastro Dietol. 2007;53:127–41. [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC. Functional pain disorders: time for a paradigm shift? In: Mayer EA, Bushnell MC, editors. Functional pain syndromes: presentation and pathophysiology. Vol. 25. Seattle: IASP Press; 2009. pp. 531–541. [Google Scholar]
- Mcilwrath SL, Davis BM, Bielefeldt K. Deletion of P2X3 receptors blunts gastro-oesophageal sensation in mice. Neurogastro Motil. 2009;21:890–899. doi: 10.1111/j.1365-2982.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589.14:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn MC, Hackett TL, Postma DS, van Oosterhout AJM, Heijink IH. E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 2011;32:248–44. doi: 10.1016/j.it.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Hall LL, Gallacher G, Ndoye A, Jolkovsky DL, Webber RJ, Buchli R, Grando SA. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–949. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. 2008;42:584–588. doi: 10.1097/MCG.0b013e31815d0628. [DOI] [PubMed] [Google Scholar]
- Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Practice Res Clin Gastro. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando RC. Dilated intercellular spaces and chronic cough as an extra-oesophageal manifestation of gastroesophageal reflux disease. Pulm Pharmacol Ther. 2011;24:272–5. doi: 10.1016/j.pupt.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. PNAS. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Fass R. Review article: approaches to endoscopic-negative reflex disease: part of the GERD spectrum or a unique acid-related disorder? Aliment Pharm Ther. 2004;10:28–34. doi: 10.1111/j.0953-0673.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, Wess J, Kummer W, Lips KS. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci. 2007;80:2308–13. doi: 10.1016/j.lfs.2007.01.046. [DOI] [PubMed] [Google Scholar]