Abstract
Background
Individuals with schizophrenia have a life expectancy that is 20 years less than the general population along with high rates of obesity and cardiovascular disease (CVD) mortality.
Objective
This study assessed the 10-year general CVD risk and vascular ages of 106 obese schizophrenia spectrum patients and 197 demographically matched obese controls without severe mental illness (SMI) from the National Health and Nutrition Examination Survey (NHANES).
Methods
Vascular age and general CVD risk were calculated using the Framingham global CVD calculator which incorporates age, sex, total and HDL cholesterol levels, systolic blood pressure, smoking status, and diabetes or hypertension treatment.
Results
Obese schizophrenia spectrum patients had a mean vascular age that was 14.1 years older than their mean actual age, while obese NHANES participants had only a 6.7 year difference. The probability of experiencing a CVD event within the next ten years was 10.7% for obese patients and 8.5% for obese NHANES participants.
Conclusion
These findings suggest that schizophrenia spectrum patients experience increased metabolic risk independent of weight. Primary care clinicians can utilize general CVD risk and vascular age scores to communicate metabolic risk more easily and to help make treatment decisions.
Introduction
Cardiovascular disease (CVD) kills more than 2,200 Americans daily and costs the United States (US) approximately $286 billion annually (1). General CVD is a composite term that encompasses coronary heart disease, cerebrovascular disease, peripheral vascular disease, and heart failure. Certain risk factors—age, sex, smoking status, hypertension, diabetes, and dyslipidemia—aggregate to promote CVD risk (2). As a result, several multivariable risk assessment tools incorporating these risk factors were created to predict CVD risk (3–5). Among these, the Framingham general CVD risk score (6) provides a 10-year risk estimate for all CVD events and calculates a vascular age, which signifies the CVD risk of an individual transformed to the age of a person with the same CVD risk but with normal levels for all risk factors. General CVD risk and vascular age calculations can be easily implemented in clinical settings to communicate metabolic risk in a straightforward manner and to help guide treatment decisions.
One group that may benefit from the increased incorporation of the general CVD risk calculation in clinical care is schizophrenia spectrum patients. Schizophrenia patients have a life expectancy that is approximately two decades shorter than the general population, along with high rates of CVD mortality (7, 8). Schizophrenia is associated with an increased prevalence of obesity (9) and type 2 diabetes (10). Obesity itself may represent a major CVD risk factor; the age adjusted relative risk for CVD was increased by 64% for women and 46% for men among obese individuals in the Framingham Heart Study (11). In a follow-up examination of this study, obesity reduced life expectancy by approximately six years in 40 year old nonsmokers (12).
Schizophrenia patients’ unhealthy lifestyles significantly contribute to CVD risk. Individuals with schizophrenia tend to make poor nutritional decisions (13), to be less physically active (14), and are three times more likely to smoke cigarettes (7). Furthermore, some antipsychotic medications, especially the newer second generation drugs, promote weight gain, and these medications may also impair lipid and glucose regulation (15–18). Despite an increased likelihood for metabolic disorders, schizophrenia patients often have CVD risk factors that are left untreated. In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study, 30.2% of patients with diabetes, 62.4% of patients with hypertension, and 88% of patients with dyslipidemia were not receiving treatment for these disorders (19). While the health risks associated with both obesity and schizophrenia spectrum disorders individually are well documented, obese schizophrenia spectrum patients may have additional CVD risk above and beyond obese individuals without severe mental illness (SMI), possibly due to the combination of an unhealthy lifestyle and medications that disrupt metabolism.
The present study compared the 10-year general CVD risk and vascular ages of obese schizophrenia spectrum patients to values for demographically matched obese controls without SMI. We hypothesized that obese schizophrenia spectrum patients would have increased CVD risk above and beyond obese individuals without SMI.
Methods
Setting and participants
This study utilized the baseline data of 115 outpatients aged 30 to 70 years (BMI ≥ 28) with a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (20) diagnosis of schizophrenia (n = 58) or schizoaffective disorder (n =48) from a lifestyle modification weight loss study. After excluding nine individuals with incomplete or unverified medication lists, we included 106 patients in our analyses. Clinicians referred individuals that were on a stable dose of antipsychotic medication for at least one month prior to baseline assessment. Exclusion criteria included pregnancy, history of dementia, major medical illness, or English non-fluency. Research assistants interviewed participants to obtain demographic information, and the treating clinician verified participants’ lists of current medications. The Human Investigation Committee of Yale University School of Medicine approved the study protocol, and potential subjects completed a ten question quiz to assess their understanding of the study before providing written informed consent.
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to evaluate and assess the health and nutritional status, disease risk factors, and disease prevalence for individuals within the United States. Two hundred thirty age, gender, race, and body mass index (BMI) matched individuals were selected using stratified random sampling from the 2005–2008 NHANES. After excluding 30 individuals with incomplete laboratory values and 3 individuals taking an antipsychotic medication, we analyzed a sample of 197 obese control patients.
Vascular age and general CVD risk calculation
The 10-year probability of developing overall CVD was calculated using a sex-specific multivariable risk factor algorithm that is available on the Framingham Heart Study website http://www.framinghamheartstudy.org/risk/gencardio.html# (6). This risk estimate incorporates age, total and HDL cholesterol levels, systolic blood pressure, smoking status, and treatment for diabetes or hypertension, and the calculator is validated for individuals between the ages of 30 and 74. Vascular age represents the CVD risk of an individual transformed to the age of a person with the same CVD risk but with normal levels for all risk factors (non-diabetic, non-smoker, and untreated systolic blood pressure of 125 mmHg, total cholesterol levels of 180 mg/dL, and HDL cholesterol levels of 45 mg/dL).
Metabolic syndrome
Metabolic syndrome inclusion criteria were the presence of at least three of the following five risk factors (21):
Elevated waist circumference > 88 cm for women or > 102 cm for men.
Elevated fasting plasma glucose levels > 100 mg/dL or undergoing pharmacotherapy for hyperglycemia.
Elevated blood pressure > 130/85 mmHG or undergoing pharmacotherapy for hypertension.
Elevated fasting triglyceride levels > 150 mg/dL or undergoing pharmacotherapy for hypertriglyceridemia
Reduced HDL cholesterol levels < 40 mg/dL for men or < 50 mg/dL for women or undergoing pharmacotherapy for reduced HDL levels.
Anthropometric measurements
Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg on a calibrated Health O Meter scale (Pelstar LLC, Alsip, IL). Waist circumference was measured around the trunk in a horizontal plane just above the uppermost lateral border of the right ilium to the nearest 0.1 cm. Blood pressure was measured using a calibrated wrist monitor (Oregon Scientific, Portland, OR) after participants sat quietly for five minutes.
Metabolic measurements
Plasma glucose concentration was analyzed using the glucose oxidase method (Yellow Springs Instruments, Ohio), and cholesterol levels were determined using standard enzymatic methods (Sigma, St. Louis, MO).
Statistical analyses
Independent two tailed t-tests were used to compare differences in general CVD risk and vascular age between obese individuals with and without SMI. Chi-square analyses were used for categorical variable comparisons between groups. Linear regression was used to determine the effects of income, waist circumference, or a schizophrenia spectrum diagnosis on the variation in the difference between mean vascular ages and mean actual ages after accounting for age, sex, race, and BMI. All analyses were performed using SPSS, version 17.0 for Windows (SPSS Inc., Chicago, IL), and statistical significance was set at p < 0.05.
Results
Table 1 shows the demographic composition of both study groups, and table 2 provides a list of psychiatric and somatic medications for the obese schizophrenia spectrum group. Sixty-seven percent of obese schizophrenia spectrum patients and 46% of obese controls had metabolic syndrome (χ2 = 12.0, p = .001) (Table 3). Mean vascular age was significantly higher (t = −13.7, p ≤ .001) than mean actual age for both patients (14.1 years) and obese controls (6.7 years). However, the difference between mean vascular age and mean actual age for obese schizophrenia spectrum patients was significantly greater than this age difference for obese controls (t = 5.33, p ≤ .001). Obese schizophrenia spectrum patients had a significantly higher probability for a general CVD event within the next 10 years (general CVD risk) than obese controls (t = 2.34, p = 0.02) (Figure 1). Fifty-seven percent of obese schizophrenia spectrum patients and 34% of obese controls had a vascular age that was more than ten years higher than their actual age. While, only 24% of obese schizophrenia spectrum patients had a vascular age that was within five years of their actual age, whereas 54% of participants in the obese control group was within this range.
Table 1.
Study sample characteristics
| Variable | Obese schizophrenia spectrum patients (n= 106) | Obese NHANES controls (n=197) | t or χ2 | p |
|---|---|---|---|---|
| Female (%) | 61 | 57 | 0.57 | 0.45 |
| AA/C/H (%) | 55/41/4 | 50/41/9 | 2.83 | 0.42 |
| Smokers (%) | 49 | 23 | 20.8 | ≤ 0.001 |
| Hypertension treatment (%) | 46 | 35 | 3.99 | 0.05 |
| *Diabetes (%) | 48 | 15 | 38.1 | ≤ 0.001 |
| Hypercholesterolemia treatment (%) | 33 | 19 | 7.71 | 0.005 |
| BMI (mean± SD), (kg/m2) | 37.9 ± 7.8 | 37.1 ± 6.8 | 1.02 | 0.31 |
| Waist circumference (mean± SD), (cm) | 118.4 ± 16.0 | 114.2 ± 14.5 | 2.27 | 0.02 |
| Men | 120.1 ± 17.8 | 115.2 ± 14.1 | 1.68 | 0.10 |
| Women | 117.4 ± 14.7 | 113.4 ± 14.8 | 1.71 | 0.09 |
| SBP (mean± SD), (mmHg) | 127 ± 17 | 124 ± 15 | 1.49 | 0.14 |
| Total cholesterol levels (mean± SD), (mg/dL) | 181 ± 43 | 198 ± 42 | −3.41 | 0.001 |
| HDL cholesterol levels (mean± SD), (mg/dL) | 47 ± 14 | 50 ± 14 | −1.93 | 0.06 |
| LDL cholesterol levels (mean± SD), (mg/dL) | 107 ± 39 | 119 ± 36 | −2.42 | 0.02 |
| Triglycerides (mean± SD), (mg/dL) | 131 ± 69 | 149 ± 115 | −1.36 | 0.18 |
| Actual age (mean± SD), (years) | 47.5 ± 8.3 | 47.7 ± 8.5 | −0.24 | 0.82 |
| Vascular age (mean± SD), (years) | 61.6 ± 14.3 | 54.5 ± 15.6 | 4.03 | ≤ 0.001 |
Represents the number of individuals diagnosed with Type 2 diabetes or met criteria with impaired fasting glucose.
Abbreviations: AA, African American; C, Caucasian; H, Hispanic; BMI, body mass index; SBP, systolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Psychiatric and somatic medications used by obese schizophrenia spectrum patients (n= 106)
| Antipsychotic medications | n (%) |
|---|---|
| Risperidone | 22 (20.8) |
| Aripiprazole | 21 (19.8) |
| Clozapine | 18 (17) |
| Fluphenazine | 15 (14.2) |
| Quetiapine | 15 (14.2) |
| Haloperidol | 13 (12.3) |
| Perphenazine | 12 (11.3) |
| Olanzapine | 10 (9.4) |
| Ziprasidone | 10 (9.4) |
| Paliperidone | 3 (2.8) |
| Thiothixene | 2 (1.9) |
| Mood stabilizer medications | |
| Divalproex Sodium | 20 (18.9) |
| Lamotrigine | 9 (8.5) |
| Gabapentin | 4 (3.8) |
| Valproic Acid | 2 (1.9) |
| Lithium | 1 (0.9) |
| Antidepressant medications | |
| Trazodone | 11 (10.4) |
| Sertraline | 8 (7.5) |
| Citalopram | 6 (5.7) |
| Paroxetine | 4 (3.8) |
| Mirtazapine | 2 (1.9) |
| Imipramine | 1 (0.9) |
| Venlafaxine | 1 (0.9) |
| Anxiolytic medications | |
| Clonazepam | 13 (12.3) |
| Lorazepam | 3 (2.8) |
| Diazepam | 1 (0.9) |
| Buspirone | 1 (0.9) |
Table 3.
Metabolic syndrome risk factors present for obese individuals with (n = 106) or without (n =197) a schizophrenia spectrum diagnosis
| Metabolic syndrome criteria | Obese schizophrenia spectrum patients: n (%) | Obese NHANES controls n (%) |
|---|---|---|
| Low HDL (< 40 mg/dL for men or < 50 mg/dL for women or undergoing pharmacotherapy for reduced HDL) | 55 (51.9) | 68 (34.5) |
| High Triglycerides (> 150 mg/dL or undergoing pharmacotherapy for hypertriglyceridemia) | 53 (50) | 58 (29.4) |
| High blood pressure (> 130/85 mmHG or undergoing pharmacotherapy for hypertension) | 73 (68.9) | 110 (55.8) |
| Impaired fasting Glucose (>100 mg/dL or undergoing pharmacotherapy for hyperglycemia) | 64 (60.4) | 79 (40.1) |
| High waist circumference (>88 cm for women or > 102 cm for men) | 100 (94.3) | 184 (93.4) |
|
| ||
| Number of risk factors present
| ||
| 0 | 1 (0.9) | 5 (2.5) |
| 1 | 9 (8.5) | 28 (14.2) |
| 2 | 25 (23.6) | 73 (37.1) |
| 3 | 22 (20.8) | 50 (25.4) |
| 4 | 26 (24.5) | 30 (15.2) |
| 5 | 23 (21.7) | 11 (5.6) |
Metabolic syndrome inclusion criteria were the presence of at least three of the five risk factors
Figure 1. Probability of a CVD event in 10 years.
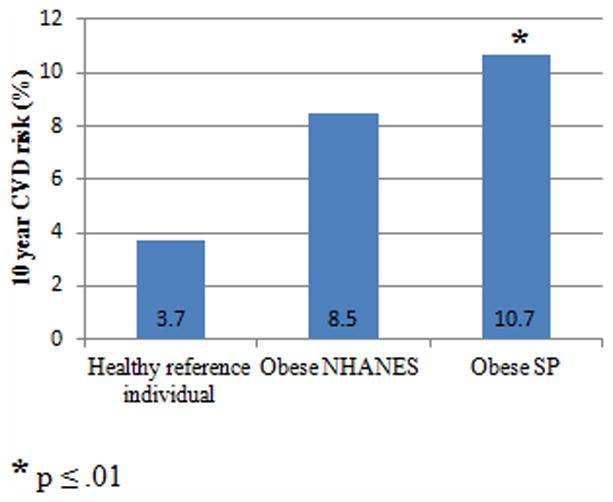
Abbreviations: SP, schizophrenia spectrum patients.
Normal reference percentage was calculated using values for a 47 year old female with no risk factors (systolic blood pressure = 125 mmHg, total cholesterol level = 180 mg/dl, and HDL cholesterol level = 45 mg/dl).
Obese schizophrenia spectrum patients had significantly higher waist circumference values (t = 2.27, p = 0.02), lower total cholesterol levels (t = −3.41, p = 0.01), and lower LDL cholesterol levels (t = −2.42, p = 0.02) than the matched NHANES group. The obese control group tended to have higher HDL cholesterol levels (t = −1.93, p = 0.06) than schizophrenia spectrum patients. There were no significant differences in blood pressure between groups.
Linear regression analyses showed that a schizophrenia spectrum diagnosis (t = −2.31, p = .022) and an elevated waist circumference (t = 4.85, p ≤ .001) explained a significant amount of the variation in the difference between mean vascular age and mean actual age after accounting for age, sex, race, and BMI.
Effect of antipsychotic medication on vascular age, CVD risk, and metabolic syndrome incidence
Chlorpromazine equivalents were used to compare antipsychotic medication dose (22). Mean antipsychotic medication dose was 645 mg/day and median antipsychotic medication dose was 600 mg/day. Vascular age and general CVD risk were assessed between individuals receiving more than 600 mg/day of antipsychotic medication and those receiving less. There was no difference in vascular age, general CVD risk, or metabolic syndrome incidence between individuals in either the low or high dose medication groups. There was also no difference in the duration of schizophrenia spectrum diagnosis (mean 21± 11 years) between individuals with or without metabolic syndrome.
Discussion
Obese schizophrenia spectrum patients had a significantly higher mean 10-year general CVD risk score and mean vascular age than age, gender, race, and BMI matched controls without SMI. Although the mean actual age of the schizophrenia spectrum patients was only 47, the mean vascular age for this sample indicates that they have the same general CVD risk as a 61 year old individual with normal risk factors. These results are in accordance with those found in the Cardiovascular, Lipid, and Metabolic Outcomes Research in Schizophrenia Study (CLAMORS), which reported that schizophrenia patients treated with antipsychotic medications develop CVD risk factors 10–15 years before the general population (23). Obese schizophrenia spectrum patients had an estimated 23% higher CVD risk than matched controls, which is consistent with results from previous versions of the Framingham risk score that found schizophrenia patients had increased heart disease risk (24, 25). The mean general CVD risk for obese schizophrenia spectrum patients (10.7%) was almost three times greater than the reference value for a healthy, 47 year old woman without metabolic risk factors (3.7%). Similarly, obese controls had a mean general CVD risk score (8.5%) that was more than twice as high as the reference value. The increased CVD risk in our schizophrenia spectrum sample is striking since we have previously shown that first episode psychosis patients with less than eight weeks of antipsychotic treatment do not have higher CVD risk than matched controls without SMI (26). Taken together, these results suggest that obesity alone does not fully account for increased CVD risk.
Sixty-seven percent of obese schizophrenia spectrum patients and 46% of obese controls had metabolic syndrome, while 48% of patients and 15% of controls were diabetic. Our patient values are much higher than the 40.9% metabolic syndrome and 13% diabetes incidences reported in the CATIE sample (24, 27). One reason for this discrepancy is the sampling bias in the current study resulting from the BMI ≥ 28 inclusion criteria. Another reason for the different reported incident rates is that the average age of patients in the current study was seven years older than the mean age of subjects in the CATIE sample. Several risk factors for metabolic syndrome are co-morbid with obesity and increased age represents more exposure to these risk factors. Obese schizophrenia spectrum patients had a significantly higher mean waist circumference than the matched obese sample. While the significance of waist circumference as an independent predictor of CVD is controversial (28), schizophrenia patients with visceral abdominal obesity are less likely to reverse metabolic syndrome (29).
High smoking rates provide another prominent CVD risk factor for the obese schizophrenia spectrum sample. Forty-nine percent of sampled patients smoke cigarettes, while only 23% of the matched sample and 20% of American adults smoke (1). A large percentage of the excess mortality in schizophrenia is possibly smoking related (30). Additionally, smoking is reported to be a significant risk factor for type 2 diabetes (31) and to limit the effectiveness of interventions designed to reduce health inequalities that are associated with socioeconomic status (32). Smoking represents a modifiable risk factor that should be targeted to reduce CVD risk.
Surprisingly, obese schizophrenia spectrum patients had significantly lower mean total cholesterol and LDL cholesterol values than obese controls. One possible reason for this reduction was that one-third of sample patients received pharmacotherapy for hypercholesterolemia, while only 19% of the matched sample received this treatment. Another possible reason is that the most prescribed antipsychotic medication for sample patients, risperidone, was associated with a reduction in total cholesterol levels during the CATIE study (33). Although it did not reach statistical significance, obese schizophrenia spectrum patients tended to have lower mean HDL levels than controls. Epidemiological evidence suggests that low HDL values contribute to elevated CVD risk (34). Increased physical activity coupled with dietary modification and modest weight losses provide numerous improvements to lipid and glucose metabolism that could reduce excess CVD risk for schizophrenia spectrum patients (35–37).
This study has some limitations that must be acknowledged. First, this was a cross-sectional analysis, with a limited sample size, so results should be interpreted carefully. Since sample patients were volunteers for a weight loss research study, our results may not be generalized to all individuals with SMI, but this study provides insight into the prevalence of CVD events for obese schizophrenia spectrum patients seeking weight loss treatment. Second, the Framingham general CVD risk calculator has not been validated yet for individuals with a schizophrenia spectrum diagnosis. Finally, longitudinal studies are necessary to determine the relationship between estimated CVD risk and CVD mortality in this population. A previous study using the Framingham 10-year coronary heart disease calculator (38) reported that schizophrenia patients from the CATIE study had higher risk than controls (24), so the general CVD risk calculator, which represents an update to this tool, likely provides similar validity. The results from this study could be used to help develop specific CVD risk calculators for individuals with SMI. For example, incorporating socioeconomic status may improve CVD risk estimates within this population. Strengths of this study include a large schizophrenia spectrum sample and the use of a BMI matched control group.
Schizophrenia impacts one percent of the population and many of the individuals affected by this disease are obese. Obese schizophrenia spectrum patients have significantly increased general CVD risk and higher vascular ages than matched controls without SMI. Schizophrenia often limits future educational prospects and socioeconomic status by occurring at an early age. These patients have less knowledge of how to reduce heart disease risk by modifying dietary intake and physical activity (39). Patients’ diet, physical activity, and medications should be monitored diligently by clinicians (40). A complete metabolic screening, including a fasting glucose measurement and lipid panel, are essential for improving CVD outcomes in this population. Vascular age represents an easier way to communicate CVD risk, and the 10-year general CVD risk calculation allows clinicians to assess several risk factors simultaneously to guide their treatment decisions.
Footnotes
Disclosure
This study was supported by a National Institutes of Health R01 award (MH080048-02) to Dr. Cenk Tek, as well as partial support from the State of Connecticut, Department of Mental Health, and Addiction services. The sponsor of the study had no role in the collection, analysis, and interpretation of data; in the writing of this report; and in the decision to submit the paper for publication. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics—2011 Update. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson R, Lawes CMM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005 Feb 29;365(9457):434–41. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 3.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Buring JE, Rifai N, Cook NR. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women. JAMA. 2007 Feb 14;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007 Jul 21;335(7611):136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General Cardiovascular Risk Profile for Use in Primary Care. Circulation. 2008 Feb 12;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 7.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005 Dec;150(6):1115–21. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Chang C-K, Hayes RD, Perera G, Broadbent MTM, Fernandes AC, Lee WE, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt RIG, Peveler RC. Obesity, serious mental illness and antipsychotic drugs. Diabetes, Obesity & Metabolism. 2009 Jul;11(7):665–79. doi: 10.1111/j.1463-1326.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- 10.Sukdeb M. High prevalence of type II diabetes in schizophrenic patients. Schizophr Res. 1995 Apr;15(1–2):195. [Google Scholar]
- 11.Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and Obesity as Determinants of Cardiovascular Risk: The Framingham Experience. Arch Intern Med. 2002 Sep 9;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 12.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Mamun AA, Bonneux L, et al. Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Ann Intern Med. 2003 Jan 7;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 13.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- 14.Vancampfort D, De Hert M, Skjerven LH, Gyllensten AL, Parker A, Mulders N, et al. International Organization of Physical Therapy in Mental Health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio-metabolic risk in patients with schizophrenia. Disabil Rehabil. 2012;34(1):1–12. doi: 10.3109/09638288.2011.587090. [DOI] [PubMed] [Google Scholar]
- 15.Simon V, Van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041–50. doi: 10.4088/jcp.08r04392. [DOI] [PubMed] [Google Scholar]
- 16.Chaggar PS, Shaw SM, Williams SG. Effect of Antipsychotic Medications on Glucose and Lipid Levels. J Clin Pharmacol. 2011 May 1;51(5):631–638. doi: 10.1177/0091270010368678. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009 Jan 1;119(1):4–14. doi: 10.1111/j.1600-0447.2008.01317.x. [DOI] [PubMed] [Google Scholar]
- 18.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Endocrinol Rev. 2011 doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 19.Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: Data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006 Sep;86(1–3):15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2007. revision. [Google Scholar]
- 21.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 22.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 23.Bobes J, Arango C, Aranda P, Carmena R, Garcia-Garcia M, Rejas J. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: Results of the CLAMORS Study. Schizophr Res. 2007;90(1–3):162–73. doi: 10.1016/j.schres.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005 Dec 1;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Cohn T, Prud’homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: High prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49(11):753–60. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- 26.Phutane VH, Tek C, Chwastiak L, Ratliff JC, Ozyuksel B, Woods SW, et al. Cardiovascular risk in a first-episode psychosis sample: A “critical period” for prevention? Schizophr Res. 2011;127(1–3):257–61. doi: 10.1016/j.schres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005 Dec 1;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schorr SG, Slooff CJ, Bruggeman R, Taxis K. The incidence of metabolic syndrome and its reversal in a cohort of schizophrenic patients followed for one year. J Psychiatr Res. 2009;43(13):1106–11. doi: 10.1016/j.jpsychires.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Wildgust HJ, Beary M. Are there modifiable risk factors which will reduce the excess mortality in schizophrenia? J Psychopharmacol (Oxford, England) 2010;24(4 Suppl):37–50. doi: 10.1177/1359786810384639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 32.Gruer L, Hart CL, Gordon DS, Watt GCM. Effect of tobacco smoking on survival of men and women by social position: A 28 year cohort study. BMJ. 2009;338(7695) doi: 10.1136/bmj.b480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieberman JA, Scott Stroup T, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 34.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J. 2011;32(11):1345–61. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M-K, Wang C-K, Bai Y-M, Huang C-Y, Lee S-D. Outcomes of Obese, Clozapine-Treated Inpatients With Schizophrenia Placed on a Six-Month Diet and Physical Activity Program. Psychiatr Serv. 2007 Apr 1;58(4):544–50. doi: 10.1176/ps.2007.58.4.544. [DOI] [PubMed] [Google Scholar]
- 36.Kwon JS, Choi J-S, Bahk W-M, Kim CY, Kim CH, Shin YC, et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. J Clin Psychiatry. 2006;67(4):547–53. doi: 10.4088/jcp.v67n0405. [DOI] [PubMed] [Google Scholar]
- 37.De Hert M, Schreurs V, Vancampfort D, Van Winkel R. Metabolic syndrome in people with schizophrenia: A review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998 May 19;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 39.Osborn DPJ, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):787–93. doi: 10.1007/s00127-007-0247-3. [DOI] [PubMed] [Google Scholar]
- 40.Tek C, Ratliff JC, Chwastiak L. Pharmacological treatment of obesity. Psychiatr Ann. 2011;41(10):489–95. [Google Scholar]


