Abstract
Skin cancer is the most common malignancy in organ transplant recipients, causing serious morbidity and mortality. Preventing and treating skin cancer in these individuals has been extraordinarily challenging. Following organ transplantation, cyclosporin A (CsA) has been used as an effective immunosuppressive to prevent rejection. Therefore immunosuppression has been widely assumed to be the major cause for increased skin carcinogenesis. However, the mechanism of skin carcinogenesis in organ transplant recipients has not been understood to date; specifically, it remains unknown whether these cancers are immunosuppression-dependent or -independent. Here, using both immunocompromised nude mice which are defective in mature T lymphocytes as an in vivo model and human keratinocytes as an in vitro model, we showed that CsA impairs genomic integrity in the response of keratinocytes to UVB. Following UVB radiation, CsA inhibited UVB-induced DNA damage repair by suppressing the transcription of the DNA repair factor xeroderma pigmentosum C (XPC). In addition, CsA compromised the UVB-induced checkpoint function by up-regulating the molecular chaperone protein cyclophilin A (CypA). XPC mRNA levels were lower, whereas CypA mRNA and protein levels were higher in human skin cancers than in normal skin. CsA-induced PI3K/AKT activation was required for both XPC suppression and CypA up-regulation. Blocking UVB damage or inhibiting the PI3K/AKT pathway prevented CsA-sensitized skin tumorigenesis. Our findings identified deregulation of XPC and CypA as key targets of CsA, and UVB damage and PI3K/AKT activation as two principal drivers for CsA-sensitized skin tumorigenesis, further supporting an immunosuppression-independent mechanism of CsA action on skin tumorigenesis.
Keywords: XPC, CypA, Cyclosporin A, UVB, DNA repair, checkpoint
Introduction
Skin cancer in organ transplant recipients (OTRs) is a rising and devastating disease burden threatening their long-term survival (1–3). In these patients, squamous cell carcinoma (SCC) is the most common skin cancer, occurring 65–250 times as frequently as in the general population, causing serious morbidity and mortality (1, 2). Many OTRs are given immunosuppressive agents, including cyclosporin A (CsA), to suppress the host immune response. The primary mediator of immunosuppression by CsA is cyclophilin A (CypA), a member of a phylogenetically conserved cyclophilin family that potentially regulates protein folding in cells (4). This CsA-CypA complex binds to and inhibits calcineurin (5, 6), a serine/threonine phosphatase dephosphorylating family of transcription factor NFATs, which mediates an immune response (7).
Therefore it has been generally assumed that increased skin cancer risk is caused by immune suppression. However, this assumption is challenged by the following epidemiological findings: (a) in patients with acquired immunodeficiency syndrome (AIDS), who have severe damage to their immune systems, Kaposi’s sarcoma, cervical cancer, and lymphomas are the most common malignancies, while the risk of skin cancer only shows a marginal increase (1- to 3-fold) (8–13); (b) in contrast, in organ transplant recipients, skin cancer derived from epidermal keratinocytes is the most common malignancy (65–250 fold relative risk for SCC); and (c) skin cancer mostly occurs in sun-exposed areas, depends on the duration of immunosuppressive treatment and UV exposure history after (or even before) transplantation (3), and harbors mutations in tumor suppressor genes including p53 (14–16) and xerodermal pigmentosum C (XPC) (17). These findings suggest that CsA directly targets epidermal keratinocytes for tumorigenic transformation in an immunosuppression-independent but UV-dependent manner.
CsA may be linked to keratinocyte transformation at several levels. CsA may interfere with DNA damage repair and/or checkpoint function, processes activated by UVB-induced DNA damage to prevent genetic mutations. In response to DNA damage, the cells activate a specific DNA repair mechanism, i.e., global genome nucleotide excision repair (GG-NER), (18–21). In addition, the damaged cells activate the DNA damage response (DDR) signal-transduction pathway to coordinate cell-cycle transitions, DNA replication, DNA repair, and apoptosis to prevent cancer development (22–27).
Although immunosuppression has been thought to be the major factor in the increased skin cancer risk in OTRs, the mechanism of skin carcinogenesis in organ transplant recipients has not been understood to date; specifically, it remains unknown whether these cancers are immunosuppression-dependent or -independent. Here, using both immunocompromised nude mice as an in vivo model and human keratinocytes as an in vitro model, we have shown that, following UVB-induced DNA damage, CsA inhibited DNA repair through PI3K/AKT-dependent XPC down-regulation, and DNA damage response through PI3K/AKT-dependent CypA up-regulation. Either blocking UVB radiation or inhibiting the PI3K/AKT pathway prevented CsA-promoted skin tumorigenesis.
Materials and methods
Human normal and tumor samples
All human specimens were studied after approval by the University of Chicago Institutional Review Board. Frozen tissues were obtained under consent (Department of Medicine, University of Chicago). Protein lysate was used to determine mRNA levels of XPC and CypA by real-time PCR and CypA protein levels by Western blotting.
Animal Treatments
All animal procedures have been approved by the University of Chicago Institutional Animal Care and Use Committee. Nude mice were obtained from Harlan. Mice were exposed to UVB (100 mJ/cm2, dose selected to avoid visible sunburn) dorsally or sham-irradiated, three times a week for up to 25 weeks to monitor tumor formation and growth. Mice were treated with vehicle (olive oil) or CsA (20 mg/kg) daily by gavage. Sunscreen was topically applied to the mouse dorsal skin prior to each UVB exposure. The sunscreen was composed of titanium dioxide (7.5%) and zinc oxide (7.5%) with a SPF factor of 60. LY (in 200 μl acetone) or vehicle (acetone alone) was applied topically to the mouse dorsal skin one hour prior to each UVB irradiation. Mouse skin samples were fixed in formalin for histological analysis or immunohistochemical analysis for Ki67-positive cells (Immunohistochemistry Core facility), or snap-frozen for immunoblotting analysis. Mice were housed five animals per cage, and there was no evidence of dorsal wounds caused by fighting or sunburn.
Statistical analyses
Statistical analyses were performed using Prism 5 (GraphPad software, San Diego, CA). Data were expressed as the mean of three independent experiments and analyzed by Student’s t-test and ANOVA. Log-rank tests were used to evaluate the tumor onset in mice. A P value of less than 0.05 was considered statistically significant.
The details for UVB treatment, cell culture, siRNA and XPC transfection, cytosolic and nuclear fractionation, Western blotting, immunohistochemical analysis, real-time PCR, luciferase reporter assays, and DNA repair analysis can be found in SI Appendix.
Results
CsA increases UVB tumorigenesis but is not tumorigenic by itself
To determine the role of CsA in skin carcinogenesis, we used immunocompromised nude mice and a UVB skin carcinogenesis model. Nude mice are defective in the T-cell mediated immune system, which is a target of CsA for immunosuppression treatment in OTRs. In addition, nude mice have been widely used for xenograft models in investigating immunosuppression-independent mechanisms of CsA tumorigenesis in our work (28) and others (29–31). Mice were treated with CsA (20 mg/ml) daily by gavage for one week prior to the initial UVB exposure and throughout the experiment, as in our previous studies (28), and irradiated with UVB (100 mJ/cm2) three times a week for 25 weeks. This UVB radiation (100 mJ/cm2) is the maximal exposure that does not cause sunburn in nude mice.
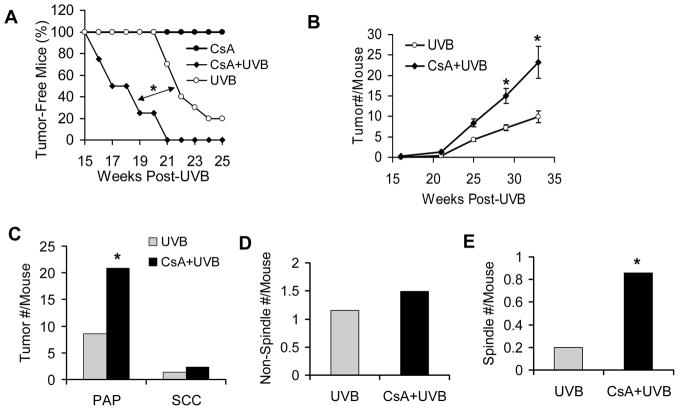
Compared to the UVB-irradiated vehicle group, CsA treatment caused early onset of tumorigenesis (Fig. 1A; P < 0.05, log-rank test) and increased tumor multiplicity (Fig. 1B; P < 0.05, Student’s t-test). However, CsA alone without UVB treatment was not tumorigenic (Fig. 1A). The number of both papillomas (PAP) and squamous cell carcinomas (SCC) per mouse was higher in CsA-treated mice than in their vehicle counterparts (Fig. 1C). While formation of the differentiated non-spindle type of SCC was similar in both groups, the incidence of spindle cell SCC, the most aggressive form, was increased in the CsA-treated group as compared with the vehicle group (Fig. 1D–E). Taken together, these data indicate that, while CsA alone is not tumorigenic, it increases UVB tumorigenesis in different stages, including tumor onset, multiplicity, and aggressiveness, resembling the observations made in human organ transplant recipients (3).
Fig. 1. CsA increases UVB tumorigenesis but is not itself tumorigenic.
A, percent (%) of tumor-free nude mice in CsA-treated (CsA, n=10), vehicle-treated UVB-irradiated (UVB, n = 10), and CsA-treated UVB-irradiated (CsA+UVB) groups (n = 10). *, P < 0.05, significant difference between indicated groups. B, number (#) of tumors per mouse in UVB and CsA+UVB groups. *, P < 0.05, significant difference between UVB and CsA+UVB groups. C, number (#) of papillomas and SCC per mouse in UVB and CsA+UVB groups. D, number (#) of non-spindle cell SCC per mouse in UVB and CsA+UVB groups. E, number (#) of spindle cell SCC per mouse in UVB and CsA+UVB groups.
Sunscreen effectively prevents CsA promoted UVB-induced tumorigenesis
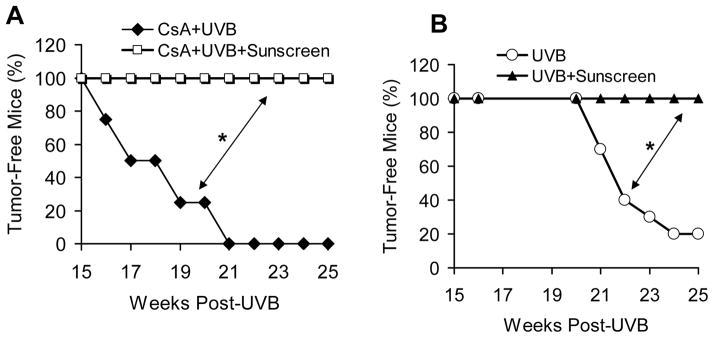
To determine the role of UV in CsA-sensitized skin tumorigenesis, we applied sunscreen topically to the mouse dorsal skin prior to each UVB irradiation. Sunscreen completely prevented the skin tumorigenesis induced by UVB and CsA (Fig. 2A; P < 0.05, log-rank test). As expected, sunscreen also completely blocked UVB-induced skin tumorigenesis (Fig. 2B; P < 0.05, log-rank test). These data further demonstrate that UVB radiation is the trigger for CsA-sensitized skin carcinogenesis.
Fig. 2. Sunscreen effectively prevents CsA-UVB tumorigenesis.
A, percent (%) of tumor-free nude mice treated with CsA+UVB with or without sunscreen application (n = 10). B, representative mouse pictures from CsA+UVB and CsA+UVB+Sunscreen groups. C, percent (%) of tumor-free mice treated with UVB with or without sunscreen (n = 10). *, P < 0.05, significant difference between indicated groups.
CsA compromises UVB-induced DNA damage repair through reducing XPC availability
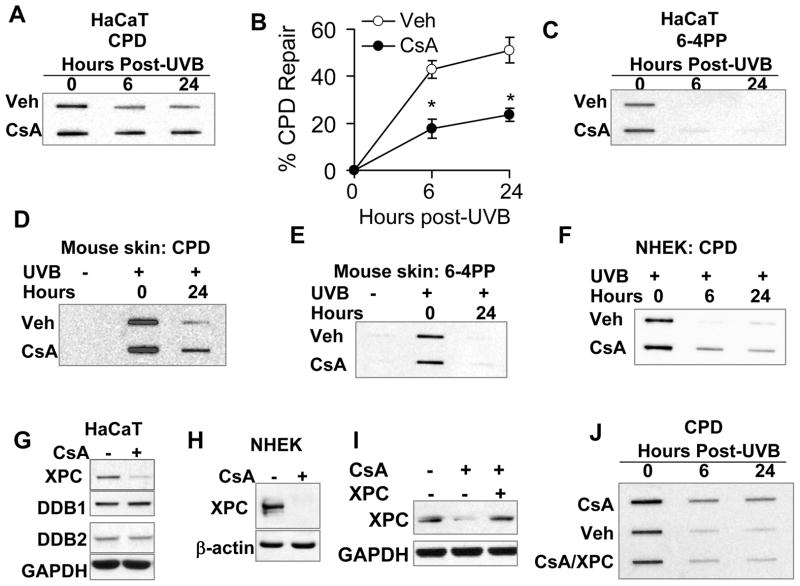
To determine the mechanism of CsA’s cocarcinogenic action, we analyzed the impact of CsA on repairing UVB-induced DNA damage products, cyclobutane pyrimidine dimers (CPD) and pyrimidine(6–4)pyrimidone dimers (6–4PP) (32, 33). In HaCaT keratinocytes, CsA significantly inhibited CPD repair (Fig. 3A–B), while it had no effect on 6–4PP repair (Fig. 3C). A similar effect of CsA on CPD and 6–4PP repair was detected in mouse skin and NHEK cells post-UVB irradiation (Fig. 3D–F). To determine the molecular target of CsA, we measured the protein levels of key factors in global genome nucleotide excision repair (GG-NER), including xeroderma pigmentosum C (XPC) and DNA damage-binding proteins 1 and 2 (DDB1 and DDB2) (18–21). CsA down-regulated the XPC levels in both HaCaT and NHEK cells while it had no effect on either DDB1 or DDB2 (Fig. 3G–H). Adding XPC to CsA-treated HaCaT cells restored the DNA repair capacity (Fig. 3I–J). These findings indicate that CsA impairs UVB-induced DNA damage repair through suppressing XPC.
Fig. 3.
CsA impairs UVB-induced DNA damage repair through down-regulating XPC. A, slot blot analysis of CPD levels in vehicle (Veh)- or CsA-treated HaCaT cells at 0, 6, and 24 h post-UVB (20 mJ/cm2). B, quantification of A as percentage of CPD repair. C, slot blot analysis of 6–4PP levels in Veh- or CsA-treated HaCaT cells at 0, 6, and 24 h post-UVB (20 mJ/cm2). D and E, slot-blot analysis of the CPD (D) and 6–4PP (E) levels in mouse skin treated with sham-irradiation or UVB irradiation (100 mJ/cm2) at 0 or 24 h. F, slot blot analysis of CPD levels in Veh- or CsA-treated NHEK cells at 0, 6, and 24 h post-UVB (20 mJ/cm2). G, immunoblot analysis of XPC, DDB1, DDB2 and GAPDH in HaCaT cells treated with or without CsA (250 ng/ml). H, immunoblot analysis of XPC and GAPDH in NHEK cells treated with or without CsA (250 ng/ml). I, immunoblot analysis of XPC and GAPDH in vehicle or CsA- HaCaT cells transfected with empty vector (−) or XPC plasmid. J, slot blot analysis of CPD levels of Veh-treated, CsA-treated, and CsA-treated/XPC-transfected HaCaT cells. ). *, P < 0.05, significant difference between vehicle and CsA-treated cells.
PI3K/AKT activation mediates CsA-induced XPC down-regulation
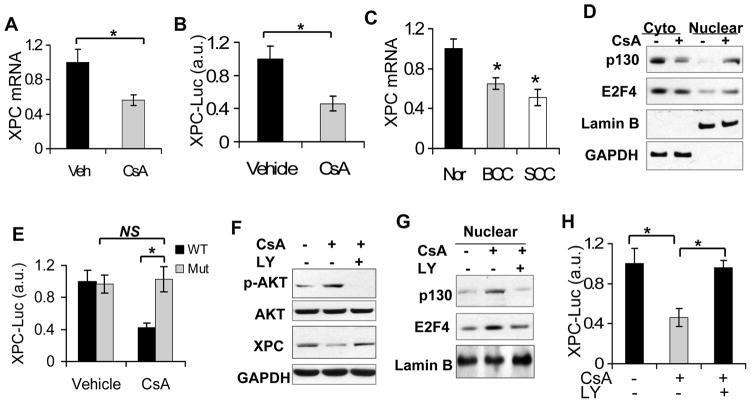
To determine how CsA reduces XPC protein levels, we ascertained whether it occurs at the mRNA and/or transcriptional level. CsA significantly reduced the mRNA levels of XPC and the transcriptional activity of the XPC promoter (Fig. 4A–B). As compared with normal human skin, XPC mRNA levels were significantly down-regulated in human SCCs and basal cell carcinomas (BCCs) (Fig. 4C; P < 0.05, Student’s t-test). To determine how CsA decreases XPC transcription, we assessed the effect of CsA on the nuclear accumulation of E2F4/p130, the repressor complex for XPC transcription (34). CsA increased the nuclear localization of both p130 and E2F4 (Fig. 4D). Removing the active E2F binding site in the XPC promoter diminished the inhibitory effect of CsA on XPC transcription (Fig. 4E). Inhibiting PI3K/AKT activation by LY294002 (LY, 10 μM) restored XPC protein levels (Fig. 4F), reduced the nuclear E2F4 level (Fig. 4G), and increased XPC transcription (Fig. 4H). These data indicate that CsA suppresses XPC transcription through PI3K/AKT-dependent activation of the E2F4/p130 nuclear localization.
Fig. 4.
CsA suppresses XPC transcription through AKT-dependent nuclear localization of E2F4/p130. A, a real-time PCR analysis of the XPC mRNA levels in vehicle and CsA-treated HaCaT cells. B, XPC promoter luciferase reporter assay of vehicle and CsA-treated HaCaT cells. *, P < 0.05, significant difference between comparison groups. C, real-time PCR analysis of XPC mRNA levels in normal human skin (Nor), BCC and SCC specimens. *, P < 0.05, significant difference between normal human skin and human SCC. D, immunoblot analysis of p130, E2F4, Lamin B and GAPDH in cytosolic and nuclear fractions from vehicle and CsA-treated HaCaT cells. E, promoter reporter assay of wild-type (WT) and E2F-inactive mutant (Mut) XPC promoter in vehicle and CsA-treated HaCaT cells. F, immunoblot analysis of p-AKT, AKT, XPC and GAPDH in vehicle-treated, CsA-treated, and CsA-treated/LY-treated HaCaT cells. G, immunoblot analysis of p130, E2F4, GAPDH and Lamin B in nuclear fractions from cells as treated in F. H, XPC promoter luciferase reporter assay of vehicle-treated, CsA-treated, and CsA-treated/LY-treated HaCaT cells. *, P < 0.05, significant difference between comparison groups.
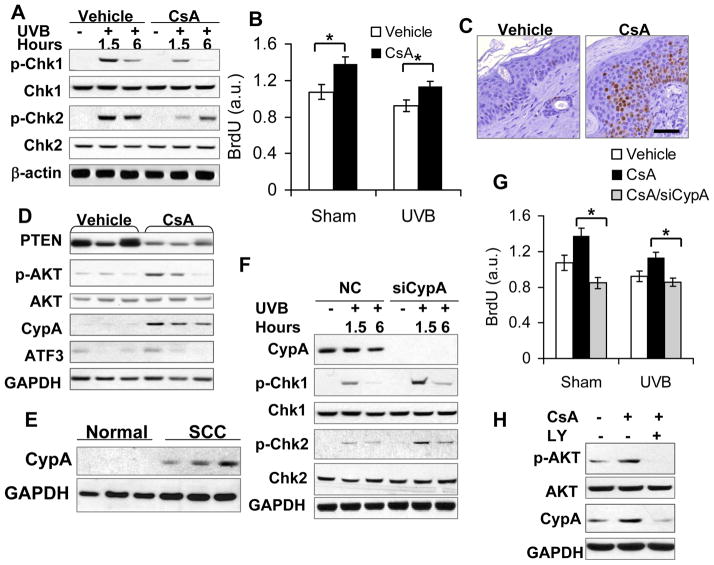
CsA impairs DNA damage response by inhibiting Chk1/2 activation through PI3K/AKT-mediated CypA up-regulation
In addition to repairing DNA damage, a proper DNA damage response is also vital for maintaining genomic integrity following UVB damage. To determine the role of CsA on the DNA damage response, we analyzed the role of CsA in UVB-induced activation of checkpoint kinase-1 (Chk1) (35, 36) and checkpoint kinase-2 (Chk2) (37–40), two pathways critical for DNA damage response and checkpoint function. UVB alone activated both Chk1 and Chk2 (Fig. 5A), while CsA inhibited UVB-induced activation of Chk1 and Chk2 (Fig. 5A). CsA increased BrdU incorporation in both un-irradiated cells and UVB-irradiated cells (Fig. 5B), indicating that CsA promotes cell proliferation and impairs checkpoint function. In UVB-irradiated mouse skin, CsA increased the incidence of Ki67-positive cells (Fig. 5C), suppressed PTEN expression (Fig. 5D, s1), and increased AKT activation (Fig. 5D), consistent with our recent in vitro findings (28). In addition, in mouse skin, HaCaT and NHEK cells, CsA also increased the levels of cyclophilin A (CypA) (Fig. 5D, s2A–C). As compared with normal human skin, CypA mRNA and protein levels were increased in human SCCs and BCCs (Fig. 5E, s2D), suggesting that CypA is an oncogene in human skin cancer. In CsA-treated cells, knockdown of CypA using siRNA increased the activation of Chk1 and Chk2 (Fig. 5F) and reduced BrdU incorporation in both un-irradiated and UVB-irradiated cells (Fig. 5G), whereas it had no effect on XPC down-regulation, AKT activation (Fig. s3A), or UVB-induced DNA damage repair (Fig. s3B). CsA had no effect on ATF3, which has been shown to be induced by calcineurin inhibition (31) (Fig. s4A). Knockdown of Calcineurin B (CnB1) up-regulated ATF3, while it did not affect XPC and CypA levels (Fig. s4B), UVB-induced DNA damage repair (Fig. s4C), or the activation of Chk1 and Chk2 (Fig. s4D). However, inhibiting the PI3K/AKT pathway by LY (10 μM) suppressed CsA-induced CypA up-regulation (Fig. 5H), indicating that CypA is a downstream factor for PI3K/AKT. However, overexpression of AKT by infecting HaCaT cells with constitutively active AKT (A+) had no effect on CypA expression (Fig. s2E), indicating that PI3K/AKT activation is required but not sufficient for CsA-induced CypA up-regulation. Other molecular pathways altered by CsA may play an important role. These data indicate that the negative impact of CsA on UVB-induced checkpoint function is mediated through PI3K/AKT-dependent CypA up-regulation.
Fig. 5.
CsA disrupts UVB-induced checkpoint activation through AKT-dependent CypA up-regulation. A, immunoblot analysis of p-Chk1 (serine 345), Chk1, p-Chk2 (Threonine 68), Chk2, and β-actin in vehicle- and CsA-treated HaCaT cells at 1.5 and 6 h post-UVB (20 mJ/cm2). B, BrdU incorporation ELISA assay of vehicle- and CsA-treated HaCaT cells post-sham or -UVB irradiation. *, P < 0.05, significant difference between vehicle and CsA treatment. C, immunohistochemical analysis of Ki67-positive cells in vehicle and CsA-treated non-tumor mouse skin irradiated with UVB three times a week for 25 weeks. Scale Bar: 50 μm. D, immunoblot analysis of PTEN, p-AKT, AKT, CypA, ATF3 and GAPDH in vehicle and CsA-treated mouse skin as in C. E, immunoblot analysis of CypA protein levels in normal human skin and SCC specimens. F, immunoblot analysis of CypA, p-Chk1, Chk1, p-Chk2, Chk2, GAPDH in CsA-treated HaCaT cells transfected with negative control (NC) siRNA or siRNA targeting CypA (siCypA) at 1.5 or 6 h post-UVB (20 mJ/cm2). G, BrdU incorporation ELISA assay of vehicle-treated, CsA-treated, CsA-treated/siCypA-transfected HaCaT cells post-sham or -UVB irradiation. *, P < 0.05, significant difference in CsA-treated HaCaT cells between NC and siCypA treatment. H, immunoblot analysis of p-AKT, AKT, CypA and GAPDH in vehicle-treated, CsA-treated, and CsA/LY-treated HaCaT cells.
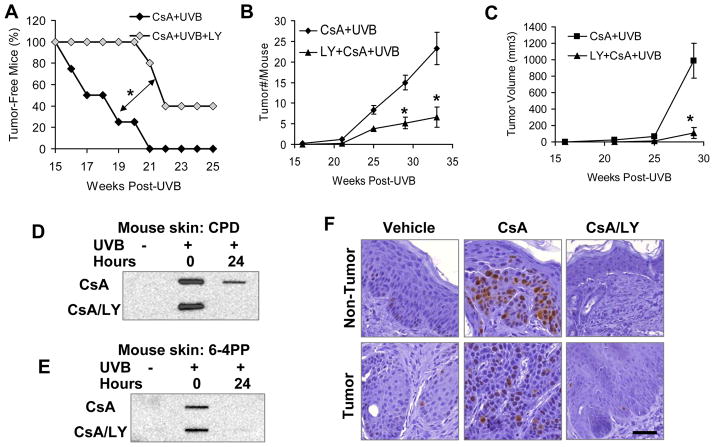
CsA-induced PI3K/AKT activation sensitizes mice to UVB-induced skin tumorigenesis
To determine the specific role of epidermal PI3K/AKT activation in CsA-sensitized skin tumorigenesis in mice, we topically applied the widely used PI3K inhibitor LY (10 nmol) one hour prior to each UVB exposure. LY delayed tumor onset in CsA- and UVB-treated nude mice (Fig. 6A; P < 0.05, log-rank test). In addition, LY also reduced the number of tumors (Fig. 6B; P < 0.05, Student’s t-test) and tumor volume (Fig. 6C; P < 0.05, Student’s t-test) per mouse. The incomplete preventive effect by LY might be due to its limited penetration into the mouse skin, especially when the epidermis became hyperplastic and hyperkeratotic following chronic UVB irradiation. In the skin from CsA-treated mice, LY enhanced CPD repair (Fig. 6D), while it did not affect 6–4PP repair, which was more efficient than CPD repair (Fig. 6E). In non-tumor skin from UVB-irradiated mice, LY decreased the incidence of Ki67-positive cells (Fig. 6F). These data demonstrate that PI3K/AKT activation drives the cocarcinogenesis of CsA by compromising DNA repair and cell growth control.
Fig. 6.
Inhibiting the AKT pathway prevents UVB/CsA-induced skin tumorigenesis. A, percent of tumor-free mice in CsA-treated and CsA/LY-treated mice at different times post-UVB irradiation three times a week for 25 weeks. *, P < 0.05, significant difference between indicated groups. B, tumor number (#) per mouse in CsA-treated and CsA/LY-treated mice at different times post-UVB irradiation three times a week for 25 weeks. *, P < 0.05, significant difference between CsA+UVB and CsA+UVB+LY groups. C, tumor volume (mm3) per mouse in CsA-treated and CsA/LY-treated mice at different times post-UVB irradiation three times a week for 25 weeks. *, P < 0.05, significant difference between CsA+UVB and CsA+UVB+LY groups. D and E, slot blot analysis of CPD (D) and 6–4PP (E) levels in vehicle-treated, CsA-treated, and CsA-LY-treated mouse skin at 0 and 24 h post-UVB (100 mJ/cm2) or sham-irradiation. F, immunohistochemical analysis of Ki67-positive cells in non-tumor skin and tumor samples from vehicle-treated, CsA-treated, and CsA/LY-treated mice. Scale Bar: 50 μm.
DISCUSSION
In CsA-treated transplant recipients, immunosuppression has been widely accepted as the major cause of increased skin carcinogenesis. However, consistent with epidemiological data supporting the critical role of UV radiation, we have used controlled laboratory in vitro and in vivo models to show that CsA impairs the DNA repair and DNA damage response in the response of keratinocytes to UVB. As a consequence, in immunocompromised nude mice, CsA increased skin tumorigenesis in which UVB damage and PI3K/AKT activation are required. At the molecular level, CsA down-regulates the DNA repair protein XPC and up-regulates the CsA-binding partner CypA through activating the PI3K/AKT pathway. Blocking UVB damage or inhibiting the PI3K/AKT pathway prevents CsA-sensitized skin tumorigenesis. Our findings further support a cell-autonomous action of CsA on skin tumorigenesis.
Following UVB damage, CsA inhibited UVB-induced DNA damage repair through suppressing XPC transcription. Consistent with recent studies using human keratinocytes and fibroblasts (41–44), we found that CsA reduced DNA repair, especially CPD repair, in human keratinocytes and mouse skin. In renal OTRs, UV-type p53 mutations were found to be prevalent, further supporting the negative role of CsA on DNA repair of UVB-induced damage (16). Although CsA may affect many molecular targets (45), we found that PI3K/AKT-dependent E2F4/p130 nuclear accumulation is critical for CsA-induced XPC down-regulation. This is consistent with the effect of inhibiting PTEN by siRNA knockdown or by increasing its acetylation through SIRT1 inhibition on nucleotide excision repair (46, 47).
In addition to reducing DNA repair capacity, CsA also disrupted the UVB-induced DNA damage response. In keratinocytes, CsA not only inhibited UVB-induced activation of Chk1 and Chk2, but also bypassed UVB-induced proliferation control. At the molecular level, we found that CsA induced CypA up-regulation through a PI3K/AKT-dependent mechanism. The up-regulation of CypA was required for CsA-mediated inhibition of checkpoint function but not DNA repair. Interestingly, CypA is found to be over-expressed in many types of cancer cells and contributes to cell proliferation and survival (48, 49). We found that CypA expression is increased in human cutaneous SCCs and BCCs, the two most common skin malignancies derived from keratinocytes and mainly caused by UV damage, suggesting the oncogenic role of CypA in skin tumorigenesis. It is possible that through increasing CypA levels in keratinocytes, CsA predisposes skin to UVB-induced skin tumorigenesis by inhibiting the proper DNA damage response.
In summary, we have demonstrated that UVB damage and PI3K/AKT activation are the principle drivers for CsA-sensitized skin tumorigenesis and that deregulation of XPC and CypA compromises UVB-induced DNA damage repair and checkpoint function. Our findings not only identified immunosuppression-independent molecular and cellular mechanisms by which CsA compromises genomic integrity, but also revealed UV damage and PI3K/AKT activation as essential triggers that can be used to improve skin cancer prevention and treatment in CsA-treated patients.
Supplementary Material
Acknowledgments
This work was supported by NIH grant ES016936 (YYH), the University of Chicago Comprehensive Cancer Center Pilot program (P30 CA014599), the CTSA (NIH UL1RR024999), and UC Friends of Dermatology Research Funds. We thank Terri Li for the Ki67 immunohistochemistry, Dr. Pradip Raychaudhuri (University of Illinois at Chicago, Chicago, IL) for kindly providing the XPC promoter luciferase construct, Drs. Tom Gajewski and Ping Yu for helpful discussions, and Dr. Ann Motten for critical reading of the manuscript.
Abbreviations
- 6–4PP
pyrimidine(6–4)pyrimidone dimers
- AK
actinic keratosis
- AKT
a serine-threonine kinase, downstream of PI3K, also called protein kinase B
- ATM
ataxia-telangiectasia mutated
- ATR
ATM and RAD3-related
- BCC
basal cell carcinoma
- Chk1
checkpoint kinase 1
- Chk2
checkpoint kinase 2
- CnB1
calcineurin B1
- CPD
cyclobutane pyrimidine dimer
- CsA
cyclosporin A
- CypA
cyclophilin A
- LY
LY294002, a specific PI3K/AKT activation inhibitor
- NHEK
normal human epidermal keratinocytes
- NMSC
non-melanoma skin cancer
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- SCC
squamous cell carcinoma
- UVB
Ultraviolet B (280–315 nm)
- Veh
vehicle
- XP
xeroderma pigmentosum
- XPC
xeroderma pigmentosum C
Footnotes
Conflicts of Interest: Keyoumars Soltani serves on the board of directors in Elorac Pharma and receives stocks from Elorac Pharma, DUSA, Winston and Gideon Pharmaceuticals, but none of them are relevant to this study. Other authors declare no conflicts of interest.
References
- 1.Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–86. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 2.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. quiz 8–20. [DOI] [PubMed] [Google Scholar]
- 3.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 4.Colgan J, Asmal M, Yu B, Luban J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. J Immunol. 2005;174:6030–8. doi: 10.4049/jimmunol.174.10.6030. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J, Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 7.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 8.Dal Maso L, Polesel J, Serraino D, Lise M, Piselli P, Falcini F, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100:840–7. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 11.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–9. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 12.Allardice GM, Hole DJ, Brewster DH, Boyd J, Goldberg DJ. Incidence of malignant neoplasms among HIV-infected persons in Scotland. Br J Cancer. 2003;89:505–7. doi: 10.1038/sj.bjc.6601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 14.McGregor JM, Berkhout RJ, Rozycka M, ter Schegget J, Bouwes Bavinck JN, Brooks L, et al. p53 mutations implicate sunlight in post-transplant skin cancer irrespective of human papillomavirus status. Oncogene. 1997;15:1737–40. doi: 10.1038/sj.onc.1201339. [DOI] [PubMed] [Google Scholar]
- 15.Noel JC, Heenen M. Posttransplant skin cancer: a possible role for p53 gene mutation but not for oncogenic human papillomaviruses. J Am Acad Dermatol. 1995;32:819–20. doi: 10.1016/0190-9622(95)91490-0. [DOI] [PubMed] [Google Scholar]
- 16.Queille S, Luron L, Spatz A, Avril MF, Ribrag V, Duvillard P, et al. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis. 2007;28:724–31. doi: 10.1093/carcin/bgl191. [DOI] [PubMed] [Google Scholar]
- 17.de Feraudy S, Ridd K, Richards LM, Kwok PY, Revet I, Oh D, et al. The DNA damage-binding protein XPC is a frequent target for inactivation in squamous cell carcinomas. Am J Pathol. 2010;177:555–62. doi: 10.2353/ajpath.2010.090925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–4. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–21. [PubMed] [Google Scholar]
- 20.Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, et al. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell. 1998;2:223–32. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 21.Sugasawa K. UV-induced ubiquitylation of XPC complex, the UV-DDB-ubiquitin ligase complex, and DNA repair. J Mol Histol. 2006;37:189–202. doi: 10.1007/s10735-006-9044-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Gene Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 23.Menoyo A, Alazzouzi H, Espin E, Armengol M, Yamamoto H, Schwartz S., Jr Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727–30. [PubMed] [Google Scholar]
- 24.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316:1289–94. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 25.Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–5. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 26.Hannan MA, Hellani A, Al-Khodairy FM, Kunhi M, Siddiqui Y, Al-Yussef N, et al. Deficiency in the repair of UV-induced DNA damage in human skin fibroblasts compromised for the ATM gene. Carcinogenesis. 2002;23:1617–24. doi: 10.1093/carcin/23.10.1617. [DOI] [PubMed] [Google Scholar]
- 27.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–32. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285:11369–77. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–4. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 30.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–72. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niggli HJ, Rothlisberger R. Cyclobutane-type pyrimidine photodimer formation and induction of ornithine decarboxylase in human skin fibroblasts after UV irradiation. J Invest Dermatol. 1988;91:579–84. doi: 10.1111/1523-1747.ep12477095. [DOI] [PubMed] [Google Scholar]
- 33.Vink AA, Berg RJ, de Gruijl FR, Roza L, Baan RA. Induction, repair and accumulation of thymine dimers in the skin of UV-B-irradiated hairless mice. Carcinogenesis. 1991;12:861–4. doi: 10.1093/carcin/12.5.861. [DOI] [PubMed] [Google Scholar]
- 34.Cam H, Balciunaite E, Blais A, Spektor A, Scarpulla RC, Young R, et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Gene Dev. 2003;17:615–28. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Gene Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–7. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–54. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–94. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–6. [PubMed] [Google Scholar]
- 41.Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125:1020–5. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- 42.Thoms KM, Kuschal C, Oetjen E, Mori T, Kobayashi N, Laspe P, et al. Cyclosporin A, but not everolimus, inhibits DNA repair mediated by calcineurin: implications for tumorigenesis under immunosuppression. Exp Dermatol. 2011;20:232–6. doi: 10.1111/j.1600-0625.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 43.Kuschal C, Thoms KM, Mori T, Kobayashi N, Boeckmann L, Laspe P, et al. Cyclosporin A, but not everolimus, inhibits DNA repair in human fibroblasts and lymphoblasts. Int J Clin Pharmacol Ther. 2009;47:38–40. doi: 10.5414/cpp47038. [DOI] [PubMed] [Google Scholar]
- 44.Kelly GE, Meikle W, Sheil AG. Scheduled and unscheduled DNA synthesis in epidermal cells of hairless mice treated with immunosuppressive drugs and UVB-UVA irradiation. Br J Dermatol. 1987;117:429–40. doi: 10.1111/j.1365-2133.1987.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 45.Baiao AM, Wowk PF, Sandrin-Garcia P, Junta CM, Fachin AL, Mello SS, et al. cDNA microarray analysis of cyclosporin A (CsA)-treated human peripheral blood mononuclear cells reveal modulation of genes associated with apoptosis, cell-cycle regulation and DNA repair. Mol Cell Biochem. 2007;304:235–41. doi: 10.1007/s11010-007-9505-7. [DOI] [PubMed] [Google Scholar]
- 46.Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, et al. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. 2011;71:5287–95. doi: 10.1158/0008-5472.CAN-10-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci U S A. 2010;107:22623–8. doi: 10.1073/pnas.1010377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J. Role of cyclophilin a during oncogenesis. Arch Pharm Res. 2010;33:181–7. doi: 10.1007/s12272-010-0200-y. [DOI] [PubMed] [Google Scholar]
- 49.Lee J, Kim SS. An overview of cyclophilins in human cancers. J Int Med Res. 2010;38:1561–74. doi: 10.1177/147323001003800501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.