Abstract
High-risk (HR) human papillomavirus (HPV) prevalence has been shown to correlate well with cervical cancer incidence rates. Our study aimed to estimate the prevalence of HR-HPV and cervical intraepithelial neoplasia (CIN) in China and indirectly inform on the cervical cancer burden in the country. 30,207 women from 17 population-based studies throughout China were included. All women received HPV DNA testing (HC2, Qiagen), visual inspection with acetic acid, and liquid-based cytology. Women positive for any test received colposcopy-directed or 4-quadrant biopsies. 29,579 women had HR-HPV testing results, of whom 28,761 had biopsy-confirmed (9019, 31.4%) or assumed (19,742, 68.6%) final diagnosis. Overall crude HR-HPV prevalence was 17.7%. HR-HPV prevalence was similar in rural and urban areas but showed dips in different age groups: at age 25–29 years (11.3%) in rural and at age 35–39 (11.3%) in urban women. In rural and urban women, age-standardized CIN2 prevalence was 1.5% (95%CI: 1.4%–1.6%) and 0.7% (95%CI: 0.7%–0.8%), and CIN3+ prevalence was 1.2% (95%CI: 1.2%–1.3%) and 0.6% (95%CI: 0.5%–0.7%), respectively. Prevalence of CIN3+ as a percentage of either all women or HR-HPV positive women steadily increased with age, peaking in 45–49 year-old women. High prevalence of HR-HPV and CIN3+ was detected in both rural and urban China. The steady rise of CIN3+ up to the age group 45–49 is attributable to lack of lesion removal through screening. Our findings document the inadequacy of current screening in China while indirectly raising the possibility that the cervical cancer burden in China is under-reported.
Keywords: Cervical Cancer, Human Papillomavirus, Cervical Intraepithelial Neoplasia, Prevalence, China
Introduction
Cervical cancer is the third most common cancer in women with an estimated 530,000 new cases and 275,000 deaths worldwide per year.1 Corresponding estimates for the People’s Republic of China are approximately 75,500 new cases (14% of all cervical cancer worldwide) and 34,000 deaths (12%).1,2 Despite these substantial disease numbers, the age-standardized cervical cancer incidence rate in China (9.6 per 100,000 women) is lower than the world average (15.2 per 100,000 women).1,3 However, estimates of cervical cancer incidence in China derive from incidence-mortality ratios based on cancer registries.1 These cancer registries include less than two percent of the Chinese population and are located in urban areas.4 Conversely, 70% of the Chinese population resides in rural areas,5 where 90% of incident cervical cancer cases are estimated to occur.6
High-risk human papillomavirus (HR-HPV) is the necessary cause of precancerous cervical intraepithelial neoplasia (CIN) and invasive cervical cancer.7 HR-HPV prevalence has been found to correlate well with cervical cancer risk in corresponding populations, particularly in middle-age women.8,9 In addition, the positive correlation between HPV prevalence and the age-standardized incidence of cervical cancer by subcontinent has been reported recently.10 China’s rapid industrialization and urbanization during the last three decades have coincided with an epidemic of sexually transmitted disease,11 which may correlate with an increase in HR-HPV and CIN prevalence as well.
Aiming to determine more realistic rates of HR-HPV, CIN, and cervical cancer prevalence in China, we performed a pooled analysis on individual data from 10 years of cervical cancer screening projects, which included 30,207 women from several rural and urban areas throughout China.
Materials and Methods
From 1999 to 2008, the Cancer Institute/Hospital of the Chinese Academy of Medical Sciences (CICAMS; Beijing, China), in collaboration with the Cleveland Clinic (Cleveland, OH, USA), International Agency for Research on Cancer (IARC; Lyon, France) and PATH (Seattle, WA, USA) screened 30,207 women in population-based, cross-sectional cervical cancer screening studies in five urban and nine rural areas of China, throughout nine Han majority provinces. Eligible women were sexually active, not pregnant, had an intact uterus, and had no history of treatment on the cervix. Recruitments for all the studies were based on community lists to minimize the selection bias. Most study women had never been screened for cervical cancer, and none had been screened in the last 5 years. Informed consent was obtained from all women. The study methodologies have been outlined in detail in the previously published works.12– 19 The human subjects review boards of CICAMS and Cleveland Clinic or PATH or IARC approved these studies.
Study Population
Information about individual studies is listed in Table 1 and has been published recently in great detail. 19
Table 1.
Characteristics of pooled studies
| No | Study Name | Study Year & location | # Screened |
Age (years) |
Screening Tests | Follow-up procedure | Histology/Cytology location and review |
|---|---|---|---|---|---|---|---|
| 1 | SPOCCS I | 1999 Xiangyuan County, Shanxi Province (Rural) |
1997 | 35–45 | HC2 (self, physician), fluorescence test, LBC, VIA, colposcopy |
All women received 4-quadrant biopsies and ECC under colposcopy. |
CICAMS. Blinded International Review |
| 2 | SPOCCS II | 2001–2002Xiangyuan & Yangcheng County, Shanxi Province (Rural) |
8497 | 35–50 | HC2 (self, physician), LBC, VIA, AFB |
Positive VIA, self- or physician-test for high-risk HPV, or an abnormal AFB, or a positive Pap test(ASCUS or worse): 4-quadrant biopsies and ECC. |
CICAMS |
| 3 | SPOCCS III-(1) | 2006 Xiangyuan County, Shanxi Province (Rural) |
884 | 16–54 | HC2 (self, physician), LBC, VIA |
(1)Positive VIA or positive self- HC2: colposcopy and directed biopsy, ECC if necessary; (2)positive physician-collected HC2 or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, ECC if necessary. |
CICAMS; Blinded International Review (only histology) |
| 4 | SPOCCS III-(2) | 2006Beijing City (Urban) | 795 | 16–54 | HC2, LBC, VIA | (1)Positive VIA: colposcopy and directed biopsy, ECC if necessary; (2) Positive physician-collected HC2 and ASC-US on LBC or ≥ASC-H: colposcopy and 4-quadrant biopsies, ECC if necessary |
Peking University People's Hospital; Blinded International and CICAMS Review |
| 5 | SPOCCS III-(3) | 2006Ximi, Henan Provence (Rural) |
879 | 16–54 | HC2 (self, physician), LBC, VIA |
Same as SPOCCS III-(1) | CICAMS; Blinded International Review (only histology) |
| 6 | SPOCCS III-(4) | 2007 Shanghai City (Urban) |
774 | 16–54 | HC2, LBC, VIA | (1)Positive VIA: colposcopy and directed biopsy, ECC if necessary; (2) Positive physician-collected HC2 or ≥ASC-H: colposcopy and 4-quadrant biopsies, ECC if necessary |
Shanghai; Blinded International and CICAMS Review (for cytology, just Blinded CICAMS Review) |
| 7 | START 2003 | 2003Xiangyuan County, Shanxi Province (Rural) |
2005 | 30–49 | HC2, LBC, VIA | Positive VIA,HC2 positive or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies and ECC. |
CICAMS; Blinded International Review |
| 8 | START 2004 | 2004Xiushui County, Jiangxi Province (Rural) |
2499 | 30–49 | HC2, LBC, VIA, VILI, colposcopy | (1)Positive VIA, VILI or colposcopy: 4-quadrant biopsies and ECC. (2)Negative VIA/VILI but HC2 positive or ≥ASC-H on LBC: Repeat colposcopy and 4-quadrant biopsies and ECC. |
Jiangxi; Blinded International and CICAMS Review |
| 9 | START 2005 | 2005 Wudu County, Gansu Province (Rural) |
2053 | 30–49 | HC2, LBC, VIA, VILI | (1)Positive VIA or VILI: colposcopy and directed biopsy, and ECC if necessary. (2)Negative VIA/VILI but HC2 positive or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies and ECC. |
Histology: Gansu Cancer Hospital; Blinded International and CICAMS Review Cytology: CICAMS; Blinded International Review |
| 10 | START 2006 | 2006 Qinxian County, Shanxi Province (Rural) |
2500 | 30–49 | HC2, LBC, VIA, VILI, colposcopy | Same as START 2005 | CICAMS; Blinded International Review |
| 11 | START 2007 | 2007Xiangyuan & Wuxiang County, Shanxi Province (Rural) |
2530 | 30–54 | HC2, careHPV™, LBC, VIA, colposcopy | (1)Positive VIA or colposcopy: directed biopsy, and ECC if necessary. (2)Negative VIA but HC2 or careHPV™ positive or ≥ASC-H on LBC: Repeat colposcopy and 4-quadrant biopsies and ECC if necessary. |
CICAMS; Blinded International Review |
| 12 | IARC-(1) | 2005 Shenzhen city, Guangdong Province (Urban) |
1137 | 15–59 | HC2, LBC, VIA, colposcopy | (1)Positive colposcopy: directed biopsy, and ECC if necessary; (2) Negative colposcopy, but HC2 positive and ASC-US, or ≥LSIL on LBC: Repeat colposcopy with directed biopsies, and ECC if necessary; |
Shenzhen; Blinded CICAMS Review |
| 13 | IARC-(2) | 2004 Yangcheng County, Shanxi Province (Rural) |
745 | 15–59 | HC2, fluorescence test, LBC, VIA, VILI, colposcopy |
(1)Positive colposcopy or fluorescence test: directed biopsy, and ECC if necessary; (2)Negative colposcopy,but HC2 positive and ASC-US, or ≥LSIL on LBC: Follow up one year later. |
Shanxi Provincial Cancer Hospital. Blinded International and CICAMS Review (for histology, only Blinded CICAMS Review). |
| 14 | IARC-(3) | 2005Shenyang, Liaoning Province(Urban) |
719 | 15–59 | VIA, VILI, LBC | (1)VIA or VILI positive: directed biopsies, and ECC if necessary; (2) Women with negative VIA and VILI, or negative colposcopy during the 1st visit, but abnormal cytology (>=ASC-H) during callback: 4-quadrant biopsies, and ECC if necessary |
CICAMS |
| 15 | FastHPV trial | 2007Qinxian County, Shanxi Province (Rural) |
818 | 30–50 | HC2, careHPV™, LBC, VIA, VILI | (1) Either VIA or VILI or careHPV™ was positive: colposcopy and directed biopsy, and ECC if necessary; (2) VIA and VILI and careHPV™ were negative, or colposcopy was negative, but HC2 positive or ≥LSIL on cytology: 4-quadrant biopsies, and ECC if necessary. |
CICAMS |
| 16 | Prevalence Survey | 2008 Binhai & Jintan County (Rural), Xuzhou city, Jiangsu Province (Urban) |
316 | 18–25 | HC2, LBC, VIA | (1)Positive VIA: colposcopy and directed biopsy, and ECC if necessary; (2)Negative VIA but ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, and ECC if necessary; (3)Negative VIA but HC2 positive and ≤ASC-US on LBC: colposcopy and directed biopsy, and ECC if necessary. |
CICAMS |
| 17 | HC2 trial | 2008 Xiangyuan County, Shanxi Province (Rural) |
1059 | 30–59 | HC2, LBC, VIA, VILI | ((1)Positive VIA or VILI: directed biopsy, and ECC if necessary; (2)Negative VIA or VILI but HC2 positive and ASC-US, or ≥ASC-H on LBC: colposcopy and 4-quadrant biopsies, and ECC if necessary; 3)Either HC2 positive or ASC-US on LBC: directed biopsy, and ECC if necessary. |
CICAMS |
Study Procedure
All participants underwent liquid-based cytology (LBC) (SurePath™, BD Diagnostics, Franklin Lakes, NJ USA or ThinPrep®, Hologic, Bedford, MA, USA), visual inspection with acetic acid (VIA) and high-risk HPV DNA testing (HC2, Qiagen Inc., Gaithersburg, MD, USA). All women in SPOCCS I and those positive for any of the three screening tests in the other studies received biopsy performed using a 2mm bronchoscopy biopsy instrument. Colposcopy-directed biopsy was used in cases of visible lesions. When study protocol included random (four-quadrant) punch biopsies (Table 1), biopsies were taken at positions of two, four, eight, and ten o’clock at the squamocolumnar junction. Details about study procedure have been published in detail in prior reports.19
HPV Testing
HPV DNA testing was performed using the high-risk probe set of Hybrid Capture 2 (HC2, Qiagen Inc., Gaithersburg, MD, USA), which detects 13 carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). All women received physician-collected HPV DNA sampling; for primary analyses, HPV DNA positivity was defined according to the manufacturer’s recommended positive cut-point of 1.0 relative light units per cutoff (RLU/Co)(≈pg DNA/mL), as previously described.12,20
Verification of Disease Status
In all studies, laboratory personnel performing HC2 were blinded to other test results, and cytopathologists and histopathologists made diagnoses without knowledge of other test results. Colposcopists were blinded to the results of all screening tests but were aware one test was positive, except in SPOCCS I, in which all women received colposcopy regardless of test positivity.12 In most studies, cytology and biopsy results were read at CICAMS, although some biopsies were read by local pathologists. Cytology results in 8 studies and biopsy results in 10 studies were reviewed for quality control by international experts (Table 1). In SPOCCS I and the START studies, all abnormal pathological slides and 10% of the negatives were reviewed by international clinical experts for re-interpretation. Comparatively, in SPOCCS III studies, 10%–35% abnormal pathological slides received international review while in the IARC Yangcheng study, all abnormal cytological slides and 20% of the negatives were reviewed by international experts.
Data was pooled using a uniform disease-status classification based on histological-confirmed biopsies. Women without biopsy results, but with negative HR-HPV DNA and negative or ASC-US at LBC results were considered to be true negatives, based on findings from SPOCCS I that found only one CIN2 case (1/1511, 0.07% of population) and no CIN3+ cases among those women.12 Women without biopsy but with negative cytology, positive HR-HPV DNA, and negative colposcopy were also categorized as negative. Women were considered as having incomplete data and excluded from the analysis if they had no biopsy but fell in one of these categories: (1) ASC-US and HR-HPV positive; (2) LSIL+ (including ASC-H, LSIL, HSIL, AGC, AIS, ADC and SCC); (3) negative cytology, HR-HPV positive and missing/positive colposcopy; (4) missing cytology.
Statistical Methods
Individual raw data from 17 studies were combined in order to estimate the prevalence of HR-HPV, different grades of CIN, and cervical cancer. Additionally, overall age-standardized prevalence was calculated using the world standard population reported by Doll and colleagues.21 Crude prevalence is reported when not otherwise specified. Prevalence stratified by age group (15–24, 25–29, 30–34, 35–39, 40–44, 45–49 and 50–59 years old) were also calculated for HR-HPV and CIN 1, 2, and 3 or worse (CIN3+), and Cochran–Armitage trend test was used to test the trend of age-stratified prevalence. Additionally, logistic regression model was used to explore the effects of age and geographic location on HPV infection status and to explore whether international quality control affected CIN2+ prevalence, adjusting for age and geographic location. Age-stratified CIN prevalence in all women and in HR-HPV positive women was restricted to rural women on account of the relatively low number of urban women in individual age groups
Results
Patient Characteristics
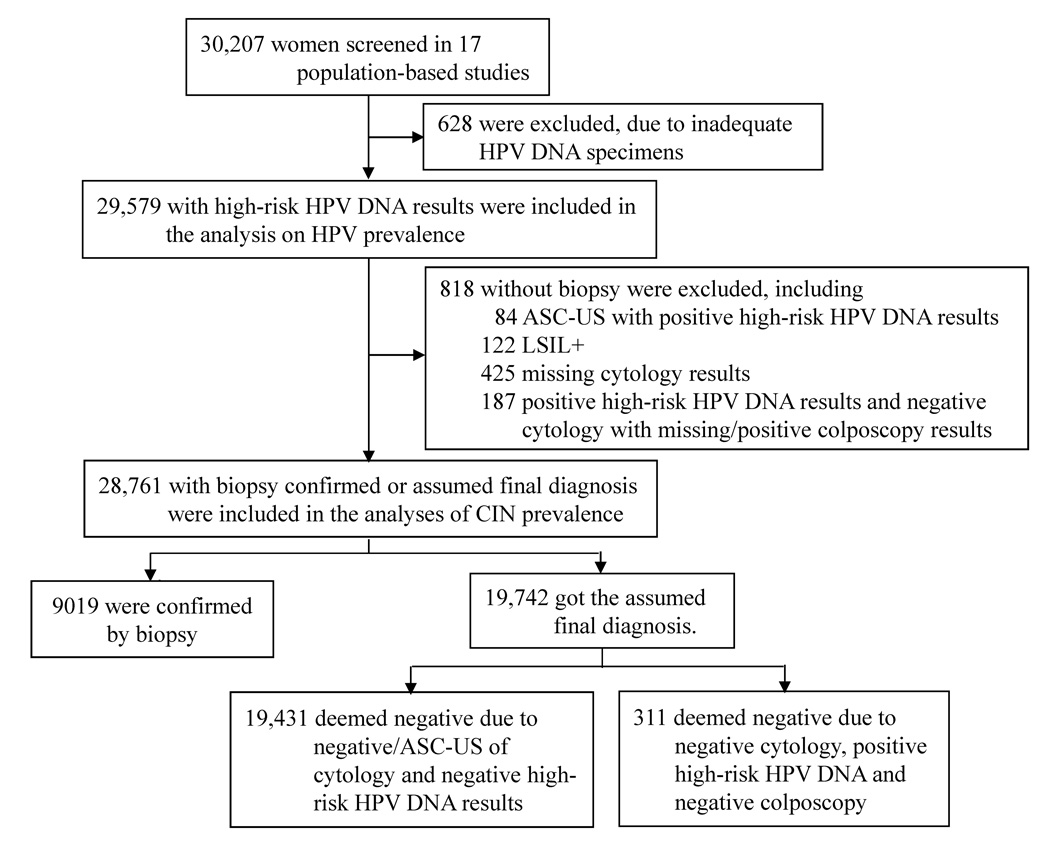
In total, 30,207 women were screened. Figure 1 describes the process by which we obtained our final sample of 29,579 women with HR-HPV testing results, of which 28,761 had biopsy confirmed (31.4%) or assumed (68.6%) final diagnosis. Most women (88.4%) in this study population were from rural areas. The average age of rural women was 40.2 years (SD: 6.2, range: 17–59 years): most rural women were aged 35–39 (31.3%), 40–44 (28.5%), or 45–49 years (18.8%), though rural women aged 17–24 (1.6%), 25–29 (1.7%), 30–34 (12.2%), and 50–59 years (5.9%) were also included. Among rural women in our study population, almost all were married (98.7%), with a median number of three pregnancies (range: 0–13; IQR:2–4) and two live births (range: 0–8; IRQ: 2–3); the mean age of first childbirth was 22.0 years (standard deviation: 2.81 years).
Figure 1.
Flow chart of inclusion and exclusion criteria of the study sample
The remainder of our study population (11.6%,) consisted of urban women. Their average age was 37.9 years (SD: 9.9, range: 17–59 years); 9.2% of urban women aged 17–24 years; 16.1% 25–29; 15.9% 30–34; 14.6% 35–39; 15.3% 40–44; 13.2% 45–49; and 15.7% aged 50 or older. The majority of urban women were married (89.7%), with a median of two pregnancies (range: 0–11, IQR:1–3) and one live birth (range: 0–5, IQR:1-1). The mean age at first childbirth among urban women was 26.0 years (standard deviation 3.28 years).
Sexual behavior differed by geographic location: rural women had a younger mean age of sexual debut (20.8 years, SD: 2.2 years) compared to urban women (23.7 years, SD: 3.1 years). Similarly, 22.1% of rural women reported having had two or more lifetime sexual partners compared to 15.7% of urban women. The mean age and sexual debut age of women excluded from the analysis was 39.1 (SD: 7.1) and 19.5 (SD: 2.6). The excluded population had the same median number of pregnancies and live births as included women.
High risk (HR) HPV DNA Prevalence by Geographic Location
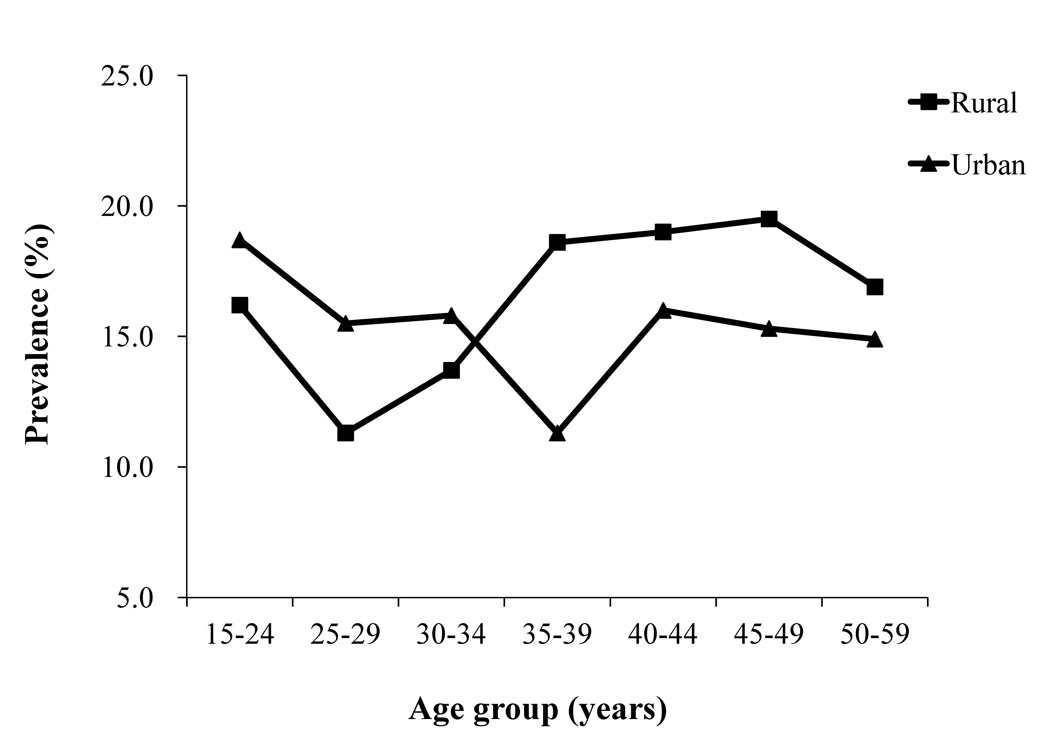
Overall crude and age-standardized HR-HPV prevalence in our study population were 17.7% and 16.8%, respectively (Table 2). When stratified by geographic location, crude and age-standardized HR-HPV prevalence was 18.0% and 16.3% (95%CI: 16.0%–16.6%) in rural women, respectively, and 15.2% and 16.0% (95%CI: 15.7%–16.3%) in urban women, respectively (Table 2). When stratified by age, crude HR-HPV prevalence in rural women declined from 16.2% in 15–24 year-old women to 11.3% in 25–29 year-old women before increasing in women aged 35–39 years (18.6%) and then remaining stable (Ztrend=5.079, Ptrend<0.0001) (Figure 2). Among urban women, the crude HR-HPV prevalence declined from 18.7% in 15–24 year-old women to 11.3% in 35–39 year-old women before increasing again to approximately 16.0% in women aged 40 or older (Ztrend=−0.986, Ptrend=0.324) (Figure 2). Logistic regression analysis also showed that, compared with the women aged 15–24 years, those aged 25–29 (OR=0.769, OR 95%CI: 0.593–0.999) and 30–34 years (OR=0.752, OR 95%CI: 0.609–0.929) had lower HPV prevalence, while women older than 35 years had a higher HPV prevalence comparable to that of women aged 15–24 years. This analysis also showed HPV prevalence was lower in urban areas than in rural areas (OR=0.870, OR 95%CI=0.785–0.965).
Table 2.
Crude and age-standardized prevalence of HPV infection and CINs by areas among all women and HPV positive women
| Population | Area | HPV positive | Normal | CIN 1 | CIN 2 | CIN 3+* | |
|---|---|---|---|---|---|---|---|
| All population | Rural | ||||||
| Crude prevalence (%) | 4700 (18.0) | 23647 (93.0) | 918 (3.6) | 414 (1.6) | 452 (1.8) | ||
| Age-standardized (95%CI) | 16.3 (16.0–16.6) | 93.9 (93.7–94.1) | 3.4 (3.2–3.5) | 1.5 (1.4–1.6) | 1.2 (1.2–1.3) | ||
| Urban | |||||||
| Crude prevalence (%) | 535 (15.2) | 3217 (96.6) | 66 (2.0) | 27 (0.8) | 20 (0.6) | ||
| Age-standardized (95%CI) | 16.0 (15.7–16.3) | 96.6 (96.4–96.7) | 2.1 (2.0–2.2) | 0.7 (0.7–0.8) | 0.6 (0.5–0.7) | ||
| Total | |||||||
| Crude prevalence (%) | 5235 (17.7) | 26864 (93.4) | 984 (3.4) | 441 (1.5) | 472 (1.6) | ||
| Age-standardized (95%CI) | 16.8 (16.5–17.1) | 94.5 (94.3–94.7) | 3.1 (2.9–3.2) | 1.3 (1.2–1.3) | 1.2 (1.1–1.2) | ||
| HPV positive women | Rural | ||||||
| Crude prevalence (%) | 100.0 | 2935 (65.2) | 732 (16.3) | 393 (8.7) | 440 (9.8) | ||
| Age-standardized (95%CI) | —— | 64.3 (63.9–64.7) | 19.1 (18.8–19.4) | 8.9 (8.7–9.1) | 7.6 (7.4–7.9) | ||
| Urban | |||||||
| Crude prevalence (%) | 100.0 | 258 (72.3) | 52 (14.6) | 27 (7.6) | 20 (5.6) | ||
| Age-standardized (95%CI) | —— | 73.0 (72.6–73.3) | 14.7 (14.4–15.0) | 7.2 (7.0–7.4) | 5.2 (5.0–5.3) | ||
| Total | |||||||
| Crude prevalence (%) | 100.0 | 3193 (65.7) | 784 (16.1) | 420 (8.6) | 460 (9.5) | ||
| Age-standardized (95%CI) | —— | 67.4 (67.0–67.8) | 17.4 (17.1–17.8) | 7.8 (7.6–8.1) | 7.3 (7.1–7.5) | ||
There were 48 cervical cancers among rural areas, and only one cervical cancer case among urban women..
Figure 2.
Prevalence of high-risk HPV DNA stratified by residence
CIN1,CIN2 and CIN3+ Prevalence
Overall crude and age-standardized prevalence was 3.4% and 3.1% (95%CI: 2.9%–3.2%), respectively, for CIN1, 1.5% and 1.3% (95%CI: 1.2%–1.3%), respectively, for CIN2, and 1.6% and 1.2% (95%CI: 1.1%–1.2%), respectively, for CIN3+ (Table 2). Forty-nine women (48 rural, one urban) were diagnosed with cervical cancer (Table 2). Among rural women age-standardized prevalence in rural women was 3.4% (95%CI: 3.2%–3.5%) for CIN1, 1.5% (95%CI: 1.4%–1.6%) for CIN2, and 1.2% (95%CI:1.2%–1.3%) for CIN 3+. Lower age-standardized prevalence rates of CIN1 (2.1%; 95% CI: 2.0%–2.2%), CIN2 (0.7%; 95% CI: 0.7%–0.8%) and CIN3+ (0.6%; 95% CI: 0.5%–0.7%) were found in urban women (Table 2).
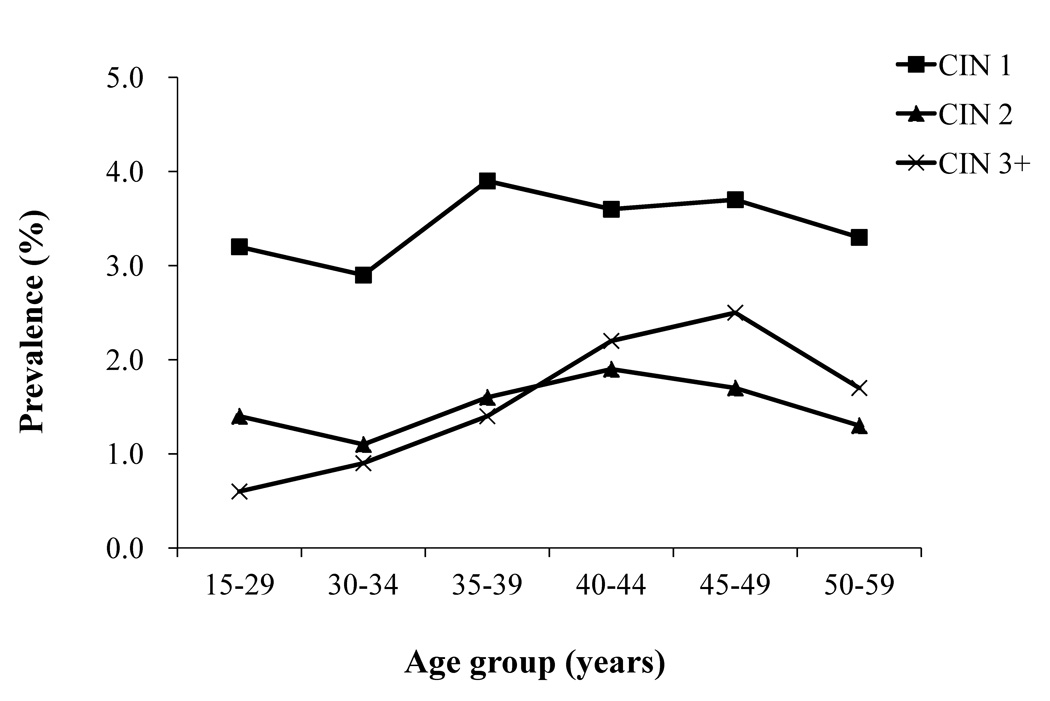
Age-stratified prevalence of CIN1, 2, and 3+ in rural women is shown in Figure 3. CIN1 prevalence was lowest in women younger than age 35 but increased in 35–39 year-old women (3.9%) and remained constant afterwards (Ztrend=0.710, Ptrend=0.478) (Figure 3). CIN2 prevalence in rural women was rather constant, with the highest level (1.9%) in 40–44 year-old women (Ztrend=1.350, Ptrend=0.177). CIN3+ prevalence steadily increased up to 2.5% in 45–49 year-old women before slightly diminishing to 1.7% in women aged 50–59 years (Ztrend=5.803, Ptrend<0.0001) (Figure 3).
Figure 3.
Prevalence of CIN1, CIN2 and CIN3+ stratified by age group among rural women
After adjusting for age and geographic location, logistic regression analysis showed CIN2+ prevalence was lower in studies that had histological slides reviewed by international experts (2.8%, 437/15697) compared to those that had no international re-interpretation (3.6%, 476/13064) (OR=0.758, OR 95%CI: 0.663–0.867).
CIN1, CIN2, and CIN3+ Prevalence in HR-HPV positive women
Overall, 4,500 rural women and 357 urban women were positive for HR-HPV (Table 2). Within the subset of rural women, overall crude prevalence was 16.3% for CIN1, 8.7% for CIN2, and 9.8% for CIN3+, while the age-standardized prevalence was 19.1% (95%CI: 18.8%–19.4%) for CIN1, 8.9% (95%CI: 8.7%–9.1%) for CIN2, and 7.6% (95%CI: 7.4%–7.9%) for CIN3+ (Table 2). Comparatively, HR-HPV positive urban women had lower crude and age-standardized CIN1, CIN2, and CIN3+ prevalence (Table 2).
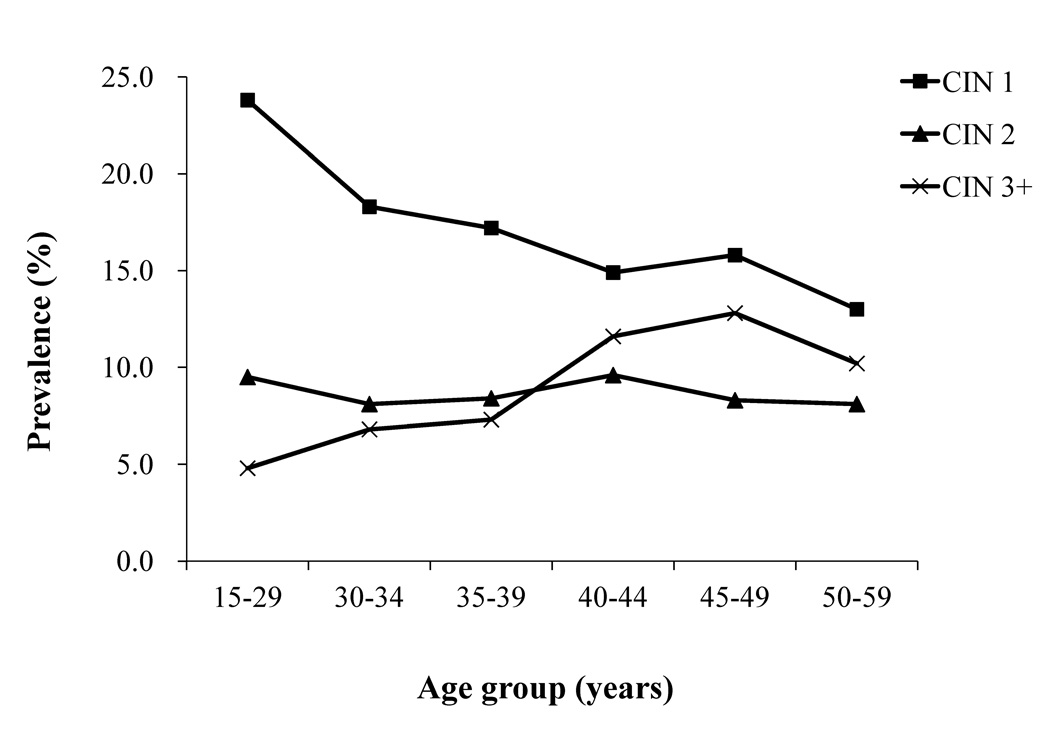
Age-stratified prevalence of CIN1, 2, and 3+ among HR-HPV positive women was, as before, restricted to the subpopulation of rural women (Figure 4). CIN1 prevalence among HR-HPV positive rural women declined with age from 23.8% in 15–29 year-old women to 13.0% in 50–59 year-old women (Ztrend=−2.713, Ptrend=0.007). CIN2 prevalence was approximately constant with age and ranged between 8.1% in women aged 30–34 years and 50–59 years and 9.6% in 40–44 year-old women (Ztrend=0.046, Ptrend=0.964). CIN3+ prevalence in HR-HPV positive rural women steadily increased with age up to 12.8% in 45–49 year-old women and then slightly declined (Ztrend=4.719, P<0.0001) (Figure 4).
Figure 4.
Prevalence of CIN1, CIN2 and CIN3+ in rural women with high-risk HPV stratified by age group
The CIN2+ prevalence among HR-HPV positive women was slightly higher in studies in which histological slides were reviewed by international experts (18.8%, 420/2240) compared to those that did not have international experts re-interpret histology (17.6%, 460/2617), but statistical difference was not shown (OR=1.073, OR 95%CI: 0.922–1.248).
Discussion
This pooled analysis is the largest study ever conducted on the prevalence of HR-HPV infection and CIN in China, with seventeen study sites in nine Chinese provinces represented. Data were collected in several communities throughout the country and our study population was composed predominantly of rural women, in agreement with the general female population in the country.5
Compared to the prevalence of HPV (9.9%) in Beijing,22 which is one of the seven cancer registries in China included in Cancer Incidence in Five Continents, the age-standardized HR-HPV prevalence in our study population (16.8%) was much higher, but similar to that in Southern India23 and higher than that in many other low- and intermediate resource countries in Asia (including Thailand, Nepal, Vietnam, and Pakistan).24–26 Only in Mongolia27 was substantially higher HR-HPV prevalence found. When stratified by age and geographic location, age-specific HR-HPV prevalence curves showed a transient decline in rural women aged 25–29 years and 35–39 years in urban women. Bimodal distribution of HPV prevalence is consistent with prior studies from Latin America28 and Asia29 but different from the flat age-specific HPV prevalence curves observed in other studies from Asia.23 Our findings are also at variance with those from recent meta-analyses which showed that the HR-HPV curve in Asia decreased steadily with increasing women’s age. 24,30
The difference in age-specific HR-HPV prevalence by area of living may depend on age-related variations in sexual and reproductive behavior between urban and rural Chinese women. The period of childbearing in China and elsewhere tends be accompanied by stronger family ties and fewer extra-marital sexual affairs. Earlier dip in HR-HPV prevalence in rural women may therefore be explained by the fact that age at first intercourse and first birth were three and four years earlier, respectively, for rural women compared to urban women. Furthermore, more rural women reported having two or more lifetime sexual partners (22%) than urban women (16%). Although this study did not include information on changes husband’s sexual behavior after attaining final family size, new husband’s extramarital sexual relationships may also contribute to a second rise of HR-HPV prevalence in married women.
In agreement with the relatively elevated prevalence of HR-HPV infection, our study, showed also high prevalence of every grade of CIN among Chinese women. Nearly 2% of women in our study harbored CIN3+, an elevated prevalence that is most probably explained by the inadequacy of past and current cervical cancer screening practices in China, particularly among rural women. Of note, the assessment of age-stratified CIN prevalence in our study had to be restricted to rural women not only due to the relatively low number of urban women in individual age groups but also due to comparability problems between studies. In fact, studies in rural areas tended to have more comprehensive histological assessment for the presence of CIN than did those from urban areas.
The prevalence of CIN among HR-HPV positive women is of special interest as these lesions may eventually progress to cervical cancer. Age-stratified CIN1 prevalence among HR-HPV positive rural women steadily declined until age 40–44 years, indicating that CIN1 is an early correlate of virus acquisition, particularly among younger women.31 Conversely, similar to previous research,32 in our inadequately screened population, the prevalence of CIN3+ increased with age up until age 45–49 years. In addition to the fact that the lack of lesion removal through screening may contribute to this pattern, a biological interpretation for the increasing CIN3+ prevalence is the natural effect of aging. More specifically, recent research33 has shown a strong menopause-related effect on cervical cancer risk, with an increasing risk of cancer following incident infection but decreasing overall prevalence of new CIN3+ since CIN3 cases (though not cervical cancer) arise less frequently among older women compared to younger women. As expected, the prevalence of CIN2 among HR-HPV positive women shows an age-related pattern intermediate between those of CIN 1 and CIN3+ supporting prior evidence of the heterogeneity of CIN2 and the concomitant difficulty of distinguishing CIN 2 lesions from lower or higher grades of CIN.34
Combined prevalence of CIN2 and CIN3+ in Chinese women (approximately 3%) was higher than the corresponding prevalence in other Asian countries, where it ranged between 0.5% in Thailand25 and 1.6% in Mongolia.27 In addition, the maximal prevalence of combined CIN 2 and CIN3+ was observed Chinese women older than 40–44 years, which was later than the maximal prevalence of CIN2+ in a recent meta-analysis of Asian women31 and a previous study in Hong Kong.29
The high prevalence of CIN2+ in middle age Chinese women should inform cervical cancer screening policies in China and other low- and intermediate-resource countries. Current cervical cancer screening recommendations are that women between the ages of 35 and 40 years be screened at least once with cervical cytology, HPV DNA testing, or visual inspection with acetic acid (in low-resource settings).35 However, our findings show that Chinese women aged 40 years or older should also be screened as they harbor a substantial proportion of precancerous cervical lesions that accumulated over time and may still be the target of life-saving treatments. Indeed, a recent population-based study from rural Nigeria showed that the positive predictive value of an HR-HPV positive test was greater in women aged 50 years or older than in women younger than age 30 and not significantly lower than in 30–49 year-old women.36
About 40% of women with undiagnosed CIN3 have been shown to develop cervical cancer.37 Our present findings indirectly raise, therefore, the possibility that cervical cancer incidence in China may be higher than currently estimated (9.6 per 100,000 women1). Chinese cancer registries on which nationwide cancer incidence are estimated include only 2% of Chinese population and are located in five urban settings.1,3 Residents of these five cities have annual incomes that range from 1.3 to 3 times higher than the national Chinese average,4,5 suggesting that the population composition of Chinese cancer registries may not be representative of the whole country. Furthermore, some comprehensive data on cervical cancer mortality4 showed that in rural China cervical cancer mortality rates were higher than those for any other cancer in women.38 Although our present study population is more representative of the Chinese population than are the national cancer registry areas, and we carefully evaluated a majority of participating women for HR-HPV and CIN prevalence, residual underestimation CIN3+ prevalence is likely to be present also in our study for different reasons. Firstly, a not negligible fraction of HR-HPV positive women who did not receive a cervical biopsy were excluded from our present analysis. Secondly, under-ascertainment of CIN2+ may have occurred in our study sites (mainly urban sites) where 4-quadrant biopsies were not performed.39
Our present pooled analysis has several strengths. It includes 17 population-based studies all conducted by CICAMS that utilized similarly trained staff and similar screening protocols. Moreover, cytology results and histology results in all studies were verified by senior cytopathologists at CICAMS and/or international experts. Nearly half of final diagnoses derived from biopsy results, and four-quadrant punch biopsies were used in selected sites when no suspicious lesion was observed in colposcopy.18 Finally, reliance on three negative screening tests to classify a woman as negative should have allowed good sensitivity level.40 Because the CIN prevalence in our study was relatively higher than that of other Asian countries, we evaluated the potential effect of international reviewing of histology to verify whether over-diagnosis of CIN2+ from Chinese pathologists existed. This analysis showed that CIN2+ prevalence was lower in studies that had international review (2.8%, 437/15697) compared to those that had not (3.6%, 476/13064) (OR=0.758, OR 95%CI: 0.663–0.867). However, once the analysis was limited to HPV-positive women, no statistical difference was shown between CIN2+ prevalence in internationally reviewed studies (18.8%, 420/2240) and that of non-internationally reviewed studies (17.6%, 460/2617) (OR=1.073, OR 95%CI: 0.922–1.248). Considering that 96.4% of CIN2+ lesions (880/913) occurred in HPV-positive women, the statistical difference revealed by logistic regression modeling in CIN2+ prevalence of all women was probably secondary to the large sample size. Moreover, 10 of 17 studies had been reviewed by international experts, so the over-diagnosis was very limited. A limitation to our study is the relatively small size of urban women that limited our possibility to compare CIN prevalence by area of living in individual age groups.
In conclusion, our findings on high prevalence of HR-HPV infection and CIN2+ suggest that the cervical cancer burden in China is heavy and comprehensive screening and HPV immunization efforts are warranted.
Novelty/Impact.
This largest population-based study in China revealed a remarkably high prevalence of HR-HPV infection and every grade of CIN among Chinese women, indicating China could face a significantly increased cervical cancer burden if comprehensive vaccination and screening programs are not implemented in the vast population in time. The bimodal distribution of HPV prevalence by age will have significant programmatic implications for cervical cancer screening policies in China as well as other similar countries.
Acknowledgements
YLQ and FHZ had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. YLQ, JLB1 and SF organized the original individual studies concept and design. FC, LYL, QMZ, RFW, CQL, LHW, ADX, WHZ, QJP and XZ acquired the raw data. FHZ, AKL and SYH analyzed and interpreted the data. FHZ and AKL drafted the manuscript. SF, JLB, JWS and JSS revised the manuscript. SYH did statistical analyses. This work was supported by the Fogarty International Center at the National Institutes of Health through the Fogarty International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988) (AKL, SYH); the Academic Capacity Development Program of Beijing Municipal Commission of Education Grant (XK100230447) (FHZ); and MOH Special Research Grant (200902002) (YLQ and FHZ). The authors wish to thank the local doctors and the women who participated in our study.
Abbreviations
- ADC
Adenocarcinoma
- AGC
atypical glandular cells of uncertain significance
- AIS
adenocarcinoma in situ
- ASC-H
atypical squamous cells- cannot exclude high-grade squamous intraepithelial lesion
- ASC-US
atypical squamous cells of undetermined significance
- >ASC-US
worse than atypical squamous cells of undetermined significance
- CI
Confidence interval
- CICAMS
Cancer Institute/Hospital of the Chinese Academy of Medical Sciences
- CIN
cervical intraepithelial neoplasia
- CIN1
cervical intraepithelial neoplasia grade 1
- CIN2
cervical intraepithelial neoplasia grade 2
- CIN2+
cervical intraepithelial neoplasia grade 2 or worse
- CIN3
cervical intraepithelial neoplasia grade 3
- CIN3+
cervical intraepithelial neoplasia grade 3 or worse
- ECC
endocervical curettage
- HC2
digene HPV HC2 DNA test
- HR-HPV
high-risk human papillomavirus
- HSIL
high-grade squamous intraepithelial lesion
- IARC
International Agency for Research on Cancer
- LBC
liquid-based cytology
- LSIL
low-grade squamous intraepithelial lesion
- LSIL+
low-grade squamous intraepithelial lesion or worse
- PATH
Program for Appropriate Technology in Health
- SCC
squamous cell carcinoma
- SPOCCS
Shanxi Province Cervical Cancer Screening Study
- START
Screening Technologies to Advance Rapid Testing Project
- VIA
visual inspection with acetic acid
- VILI
visual inspection with Lugol’s iodine
Footnotes
JLB has received support in kind (reagents, testing, funds for direct support and research), under the auspices of Preventive Oncology international Inc., from Hologic Inc., Qiagen, Gen-Probe, Merck Inc., and BGI Shenzhen. JSS has received grants and consultancy fees or honorarium from Gen-Probe, Hologic, GSK, Qiagen and Merk in the last four years.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) W. IARC Handbooks of Cancer Prevention: Cervix Cancer Screening. Lyon: IARC Press; 2005. Chapter 2. [Google Scholar]
- 3.Curado MP, Edwards B, Shin HR, Storm H, Frelay J, Heanue M, Boyle P. Cancer incidence in five continents. Vol IX. Lyon, IARC: IARC Scientific Publications No. 160; 2007. [Google Scholar]
- 4.Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: synthesis of the evidence. Int J Cancer. 2012;130(3):641–652. doi: 10.1002/ijc.26042. [DOI] [PubMed] [Google Scholar]
- 5.Xie FZ. Beijing: China Statistics Press; 2008. [accessed on 20-2-2010]. China Statistical Yearbook – 2008. Available at http://www.stats.gov.cn/tjsj/ndsj/2008/indexch.htm. [Google Scholar]
- 6.Wen C. China’s plans to curb cervical cancer. Lancet Oncol. 2005;6:139–141. doi: 10.1016/S1470-2045(05)01761-4. [DOI] [PubMed] [Google Scholar]
- 7.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, Rush BB, Glass AG, Schiffman M. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 8.Peto J, Gilbaro C, Deacon J, Taylor C, Evans C, Binns W, Haywood M, Elanko N, Coleman D, Yule R, Desai M. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer. 2004;91:942–953. doi: 10.1038/sj.bjc.6602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maucort-Boulch D, Franceschi S, Plummer M IARC HPV Prevalence Surveys Study Group. International correlation between human papillomavirus prevalence and cervical cancer incidence. Cancer Epidemiol Biomarkers Prev. 2008;17:717–720. doi: 10.1158/1055-9965.EPI-07-2691. [DOI] [PubMed] [Google Scholar]
- 10.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 11.Chen XS, Gong XD, Liang GJ, Zhang GC. Epidemiologic trends of sexually transmitted diseases in China. Sex Transm Dis. 2000;27:138–142. doi: 10.1097/00007435-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, Pan QJ, Fischer C, Lorincz A, Zahniser D. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–444. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 13.Belinson JL, Qiao YL, Pretorius RG, Zhang WH, Rong SD, Huang MN, Zhao FH, Wu LY, Ren SD, Huang RD, Washington MF, Pan QJ, et al. Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer. 2003;13:819–826. doi: 10.1111/j.1525-1438.2003.13611.x. [DOI] [PubMed] [Google Scholar]
- 14.Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, Huang RD, Sun LX, Meijer CJ, Qiao YL, Franceschi S. Human papillomavirus infection in Shanxi Province, People's Republic of China: a population-based study. Br J Cancer. 2006;95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belinson JL, Hu S, Niyazi M, Pretorius RG, Wang H, Wen C, Smith JS, Li J, Taddeo FJ, Burchette RJ, Qiao YL. Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: Implications for vaginal self-collection. Int J Cancer. 2010;127:1151–1157. doi: 10.1002/ijc.25144. [DOI] [PubMed] [Google Scholar]
- 16.Belinson JL, Pretorius RG, Zhang WH, Wu LY, Qiao YL, Elson P. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. 2001;98:441–444. doi: 10.1016/s0029-7844(01)01454-5. [DOI] [PubMed] [Google Scholar]
- 17.Pretorius RG, Kim RJ, Belinson JL, Elson P, Qiao YL. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. J Low Genit Tract Dis. 2006;10:5–9. doi: 10.1097/01.lgt.0000192694.85549.3d. [DOI] [PubMed] [Google Scholar]
- 18.Pretorius RG, Zhang WH, Belinson JL, Huang MN, Wu LY, Zhang X, Qiao YL. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191:430–434. doi: 10.1016/j.ajog.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Zhao FH, Lin MJ, Chen F, Hu SY, Zhang R, Belinson JL, Sellors JW, Franceschi S, Qiao YL, Castle PE. Cervical Cancer Screening Group in China. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies in China. Lancet Oncol. 2010;11:1160–1171. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembat P, Kulasingam S, Sasieni P, Iftner T. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 21.Doll R, Payne P, Waterhouse J, editors. Cancer Incidence in Five Continents: a Technical Report. Berlin: Springer-Verlag (for UICC); 1966. [Google Scholar]
- 22.Li C, Wu M, Wang J, Zhang S, Zhu L, Pan J, Zhang W. A population-based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2010;19:2655–2664. doi: 10.1158/1055-9965.EPI-10-0212. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S, Rajkumar R, Snijders PJ, Arslan A, Mahe C, Plummer M, Sankaranarayanan R, Cherian J, Meijer CJ, Weiderpass E. Papillomavirus infection in rural women in southern India. Br J Cancer. 2005;92:601–606. doi: 10.1038/sj.bjc.6602348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschi S, Herrero R, Clifford Gm, Snijders PJ, Arslan A, Anh PT, Bosch FX, Ferreccio C, Hieu NT, Lazcano-Ponce E, Matos E, Molano M, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–2684. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 25.Sukrivash S, Smith JS, Tunsakul S, Munoz N, Kesararat V, Opasation O, Chichareon S, Kaenploy V, Ashley R, Meijer CJ, Snijders PJ, Coursaget, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003;187:1246–1256. doi: 10.1086/373901. [DOI] [PubMed] [Google Scholar]
- 26.Pham TH, Nguyen TH, Herrero R, Vaccarella S, Smith JS, Nguyen Thuy TT, Nguyen HN, Nguyen BD, Ashley R, Snijders PJ, Meijer CJ, Munoz N, et al. Human papillomavirus infection among women in South and North Vietnam. Int J Cancer. 2003;104:213–220. doi: 10.1002/ijc.10936. [DOI] [PubMed] [Google Scholar]
- 27.Dondog B, Clifford GM, Vaccarella S, Waterboer T, Unurjargal D, Avirmed D, Enkhtuva S, Kommoss F, Wentzensen N, Snijders PJ, Meijer CJ, Franceschi S, et al. Human papillomavirus infection in Ulaanbaatar, Mongaola: a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:1731–1738. doi: 10.1158/1055-9965.EPI-07-2796. [DOI] [PubMed] [Google Scholar]
- 28.Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, Balmaceda I, Greenberg MD, Alfaro M, Burk RD, Wacholder S, Plummer M, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 29.Chan PK, Chang AR, Yu MY, Li WH, Chan MY, Yeung AC, Cheun TH, Yau TN, Wong SM, Tau CW, Ng HK. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126:297–301. doi: 10.1002/ijc.24731. [DOI] [PubMed] [Google Scholar]
- 30.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(S.1):S4–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Ting J, Kruzikas D, Smith JS. A Global Review of Age-specific and Overall Prevalence of Cervical Lesions. Int J of Gynec Cancer. 2010;20:1244–1249. doi: 10.1111/igc.0b013e3181f16c5f. [DOI] [PubMed] [Google Scholar]
- 32.Rodriquez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman M, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, et al. Longitudinal Study of Human Papillomavirus Persistence and Cervical Intraepithelial Neoplasia Grade 2/3: Critical Role of Duration of Infection. J Natl Cancer Inst. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer M, Peto J, Franceschi S on behalf of the International Collaboration of Epidemiological Studies of Cervical Cancer. Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113:18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low resource settings. JAMA. 2001;285:3107–3115. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- 36.Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Eklund C, Reilly M, Hutchinson M, Wacholder S, Harford J, Soliman AS, Burk RD, Schiffman M. The age-specific prevalence of human papillomavirus and risk of cytologic abnormalities in rural Nigeria: Implications for screen-and-treat strategies. Int J Cancer. 2012;130:2111–2117. doi: 10.1002/ijc.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peto J, Gilham C, Fletcher O, Matthews F. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 38.Shi JF, Qiao YL, Smith JS, Dondog B, Bao YP, Dai M, Clifford GM, Franceschi S. Epidemiology and prevention of human papillomavirus and cervical cancer in China and Mongolia. Vaccine. 2008;26(S.12):M53–M59. doi: 10.1016/j.vaccine.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Kjaer SK, van den Brule AJ, Paull G, Svare EI, Sherman ME, Thomsen BL, Suntum M, Bock JE, Poll PA, Meijer CJ. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pretorius RG, Bao YP, Belinson JL, Burchette RJ, Smith JS, Qiao YL. Inappropriate gold standard bias in cervical cancer screening studies. Int J Cancer. 2007;121:2218–2224. doi: 10.1002/ijc.22991. [DOI] [PubMed] [Google Scholar]