Abstract
There is considerable interest in the identification of natural agents capable of affording protection to skin from the adverse effects of solar ultraviolet B (UVB) radiation. Pomegranate (Punica granatum L) fruit possess strong anti-oxidant, anti-inflammatory and anti-proliferative properties. Recently, we have shown that oral feeding of pomegranate fruit extract (PFE) to mice afforded substantial protection from the adverse effects of single UVB radiation via modulation in early biomarkers of photocarcinogenesis. This study was designed to investigate the photochemopreventive effects of PFE (0.2%, w/v) after multiple UVB irradiations (180 mJ/cm2; on alternative day; for a total of seven treatments) to the skin of SKH-1 hairless mice. Oral feeding of PFE to SKH-1 mice inhibited UVB-induced epidermal hyperplasia, infiltration of leukocytes, protein oxidation and lipid peroxidation. Immunoblot analysis demonstrated that oral feeding of PFE to mice inhibited UVB-induced (i) nuclear translocation and phosphorylation of NF-κB/p65, (ii) phosphorylation and degradation of IκBα, (iii) activation of IKKα/IKKβ, and (iv) phosphorylation of MAPK proteins and c-Jun. PFE consumption also inhibited UVB-induced protein expression of (i) COX-2 and iNOS, (ii) PCNA and cyclin D1, and (iii) matrix metalloproteinases-2,-3 and -9 in mouse skin. Taken together, these data show that PFE consumption afforded protection to mouse skin against the adverse effects of UVB radiation by modulating UVB-induced signaling pathways.
Introduction
The nonmelanoma skin cancers (NMSC), comprising of basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), are the most frequently diagnosed cutaneous malignancy in the United States (1,2). Despite the fact that these skin cancers are readily treatable and most preventable, their high prevalence has become a major human health concern (3,4). Exposure of the skin to solar ultraviolet (UV) radiation, particularly its UVB component (280–320 nm), is thought to be the main cause of skin cancers (5,6). UV-induced responses result in inflammation, oxidative stress, dysregulation of apoptosis leading to abnormal proliferation of keratinocytes containing DNA damage, acquisition of p53 mutations, alterations in signal transduction pathways and immunosuppression, all of which contribute to the onset of skin cancers (7–10).
Considerable body of evidence suggests that exposure of the skin to UV radiation results in the generation of reactive oxygen species (ROS) that are capable of oxidizing proteins, lipids and DNA leading to oxidative stress (11–12). In addition, persistent damage of these critical cellular molecules results in the activation of mitogen activated protein kinases (MAPK) and transcription factors such as nuclear factor-kappa B (NF-κB) (11,12). Studies have shown that UV-induced activation of MAPK and NF-κB are important mediators of intracellular oxidative stress, inflammation, cellular proliferation, and photocarcinogenesis (13–15). Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteolytic endopeptidases with extracellular martix remodeling and degrading properties (16). MMPs have been associated with growth, migration and invasion because of their ability to degrade type IV collagen in extracellular matrices (17). In particular, MMPs such as MMP-2 (gelatinase A), MMP-3 (stromelysin-1), and MMP-9 (gelatinase B) have emerged as regulators of development, angiogenesis, and tumor progression (18–21). Studies have also shown that inflammatory cells expressing MMP-9 may trigger neoplastic transformation during squamous epithelial carcinogenesis (22).
The use of dietary polyphenols that possesses antioxidant, anti-inflammatory, anti-proliferative, and DNA repair capability by modulating cellular signaling pathways has gained considerable attention as photoprotective and/or photochemopreventive agents (2–12). Pomegranate (Punica granatum L.) is an edible fruit cultivated in Mediterranean countries and some part of the United States. Pomegranate is consumed fresh and in beverage as juice or wine worldwide and also has been extensively used as a folk medicine by many cultures. We have shown that pomegranate fruit extract (PFE) is a rich source of anthocyanins (such as pelargonidin 3-glucoside, cyanidin 3-glucoside, delphinidin 3-glucoside, pelargonidin 3,5-diglucoside, cyanidin 3,5-diglucoside, and delphinidin 3,5-diglucoside), ellagitannins and hydrolysable tannins (such as punicalin, pedunculagin, punicalagin, gallagic and ellagic acid esters of glucose) (23). Studies have demonstrated that the antioxidant activity of PFE is higher than that of green and red wine (24). PFE can reduce prostaglandin and leukotriene formation by inhibition of lipoxygenase activities (25). In addition, PFE possesses strong anti-inflammatory, anti-proliferative, anti-atherosclerotic and anti-tumorgenic properties in cell culture and in mouse models (23,26,27). Most recently, we have shown that oral feeding of PFE to mice enhanced repair of UVB-induced formation of cyclobutane pyrimidine dimers and 8-oxo-7,8-dihydro-2′-deoxyguanosine (28). In addition, PFE treatment further enhanced UVB-mediated increase in protein expression of cyclin-dependent kinase inhibitor p21 (28). In the present study, we investigated the effect of oral feeding of PFE against multiple UVB (180 mJ/cm2; on alternative day; for a total of seven exposures) irradiations caused adverse effects in SKH-1 hairless mice. We selected to use seven exposures as an intermediate point between chronic and acute exposure that may possibly reflect the persistent damage in mouse skin. The effect of PFE against multiple UVB exposure-induced: (i) hyperplasia and infiltration of leukocytes, (ii) oxidative stress such as lipid peroxidation and protein oxidation, (iii) activation of NF-κB and MAPK, (iv) inflammation, and (v) proliferation in mouse skin was determined. We found that oral feeding of PFE to mice can inhibit the adverse effects of multiple UVB exposure.
Materials and Methods
Materials
The phosphorylated monoclonal and polyclonal antibodies against ERK1/2 (Thr202/Tyr204), JNK1/2 (Thr183/Tyr185), p38 (Thr180/Tyr204), IκBα and NF–κB/p65, and nonphosphorylated antibodies against IκBα, ERK1/2 and NF-κB/p65 were obtained from Cell Signaling Technology (Beverly, MA). JNK1/2, p38, IKKα, IKKβ, Cyclin D1, PCNA, COX-2, iNOS, MMP-2, MMP-3, MMP-9 and phospho c-Jun antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-mouse and anti-rabbit secondary antibodies horseradish peroxidase (HRP) conjugate were procured from Amersham Life Science, Inc. (Arlington Heights, IL). Novex precast Tris-glycine gels were obtained from Invitrogen (Carlsbad, CA). The DC BioRad Protein assay kit was purchased from BioRad Laboratories (Herculus, CA).
Preparation of pomegranate fruit extract
Fresh fruit of pomegranate was peeled, and its edible portion (seed coat and juice) was squeezed in 70% acetone-30% distilled water (1:20, by w/v). The red extract was filtered through filter paper (Whatman No. 1). The filtrate was condensed, freeze-dried and stored at 4°C as described earlier (23).
Animals and treatment
Female SKH-1 hairless mice (6-weeks-old) obtained from Charles River Laboratories (Wilmington, MA) were used in this study. After their arrival in the animal facility, the animals were allowed to acclimatize for one week before the start of the experiments. The animals were fed Purina Chow diet and water ad libitum. The mice were maintained at standard conditions: temperature of 24 ± 2°C, relative humidity of 50% ± 10%, and 12 h room light/12 h dark cycle. For UVB irradiation, the mice were housed in specially designed cages where they were held in dividers separated by Plexiglas. Forty eight female SKH-1 hairless mice, maintained as described, were divided into four groups of twelve animals each. The mice in the first group received drinking water and served as a control, and those in the second group received PFE (0.2%, wt/vol) dissolved in drinking water. The mice in the third group were exposed to UVB (180 mJ/cm2) every alternate day for a total of seven exposures with a custom designed Research Irradiation Unit (Daavlin, Bryan, OH). The mice in the fourth group received PFE (0.2%, wt/vol) for 14 days before multiple UVB (180 mJ/cm2; on alternative day; for a total of seven exposures) irradiations and PFE treatment continued till the termination of the experiment. The UV lamps emit UVB (280–320 nm; ~80% of total energy) and UVA (320–375 nm; ~20% of total energy), with UVC emission being insignificant. In this system dose units can be entered in MilliJoules/cm2 for UVB; variations in energy output are automatically compensated for the delivery of the desired dose. Using this system, the mice were exposed to accurate dosimetery of UVB radiation. The mice were sacrificed 24 h after the last UVB exposure and skin biopsies were harvested for biochemical analysis.
Hyperplasia
To assess the inhibitory effect of oral feeding of PFE on multiple UVB-induced hyperplasia mice were sacrificed 24 h after last UVB (180 mJ/cm2) exposure. Skin was removed, fixed in 10% formalin, and embedded in paraffin. Vertical sections (5 μm) were cut, mounted on a glass slide, and stained with hematoxylin and eosin.
Determination of protein oxidation
For determination of protein oxidation, protein carbonyl immunoblot kit was used (Cell Biolabs, Inc., San Diego, CA). Briefly gel proteins were transfered to the PVDF membrane. Following the electroblotting step, PVDF membrane was immersed in 100% methanol for 15 sec, and dried at room temperature for 5 min, then equilibrated in TBS containing 20% methanol for 5 min. Membrane was washed in 2N HCl for 5 min and incubated in dinitrophenylhydrazine (DNPH) solution for 5 min. Following derivitization with DNPH membrane was washed three times with 2N HCl, and then five times with 100% methanol, 5 min each. The blot containing the derivitized protein was blocked in blocking buffer and incubated with primary antibody against DNPH for 3 h at room temperature followed by incubation in HRP labeled secondary antibody for 2 h and detected by enhanced chemiluminescence and autoradiography.
Lipid peroxidation (LPO) assay
After treatment, skin samples were harvested and washed with PBS, and microsomal fraction was prepared as described earlier (28). Briefly, cells were homogenized with a Polytron homogenizer in PBS buffer containing potassium chloride (1.19%, w/v) and centrifuged at 18,000g for 15 min at 4°C to prepare microsomal fraction. The LPO assay was performed in microsomal fraction obtained from the different treatment groups. The generation of malondialdehyde (MDA) was employed as a marker of LPO and estimated by the method as described earlier (28). Briefly, microsomal fraction (2.0 mg protein) was incubated for 1 h at 37 °C in the presence of ferric ions (1 mM) and ADP (5mM) in Ca2+-free phosphate buffer (0.1 M; pH: 7.4) containing MgCl2 (0.1mM). The reaction was terminated by addition of 0.6 ml of 10% (w/v) trichloroacetic acid followed by 1.2 ml of 0.5% (w/v) 2-thiobarbituric acid. The mixture was heated for 20 min at 90 °C in a water bath. After cooling, the MDA levels were measured in the clear supernatant by recording absorbance at 532 nm. The final concentration of MDA generated during the reaction was calculated using a molar extinction coefficient of 1.56×105/M/cm.
Preparation of epidermal skin lysate and nuclear lysate
Epidermis from the whole skin was separated as described earlier (23) and was homogenized in ice-cold lysis buffer [50mM Tris-HCl, 150mM NaCl, 1mM EGTA, 1mM EDTA, 20mM NaF, 100mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1mM PMSF (pH 7.4)] with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA). The homgenate was then centrifuged at 14,000 × g for 25 min at 4° C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80° C. For the preperation of nuclear lysate, 0.2 g of the epidermis was homogenized into 1.0 ml of ice-cold phosphate-buffered saline (pH 7.6) and centrifuged at 12,000 × g for 5 min at 4°C. The pellet was resuspended in 1 ml of cold buffer containing 10 mM HEPES (pH 7.9), 2 mM MgCl2, 10 mM KCI, 1 mM dithiothreitol, 0.1 mM EDTA and 0.1 mM PMSF. After homogenization in a tight-fitting Dounce homogenizer, the homogenates were left on ice for 10 min then were centrifuged at 25,000 × g for 10 min. The nuclear pellet was resuspended in 0.1 ml of the buffer containing 10 mM HEPES (pH 7.9), 300 mM NaCI, 50 mM KCI, 0.1 mM EDTA, 1 mM dithiothreitol, 0.1 mM PMSF, and 10% glycerol with freshly added protease inhibitor cocktail. The suspension was gently shaken for 20 min at 4°C. After centrifugation at 25000 × g for 10 min, the nuclear extracts (supernatants) were collected and quickly frozen at −80°C. The protein content in the lysates was measured by DC BioRad assay as per the manufacturer’s protocol.
Western blot analysis
For western analysis, 25–50 μg of the protein was resolved over 8–12% Tris-glycine gels and transferred to a nitrocellulose membrane. The blot containing the transferred protein was blocked in blocking buffer (5% nonfat dry milk, 1% Tween 20; in 20 mM TBS, pH 7.6) for 1h at room temperature followed by incubation with appropriate monoclonal primary antibody in blocking buffer for 2 h to overnight at 4 °C. This was followed by incubation with anti-mouse or anti-rabbit secondary antibody horse-radish peroxidase for 2 h and then washed several times and detected by chemiluminescence and autoradiography. Densitometric measurements of the band in Western blot analysis were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
Immunostaining of cyclin D1 and proliferating cell nuclear antigen (PCNA)
Skin samples were collected 24 h after last UVB irradiation and were fixed in 10% neutralized formalin and embedded in paraffin. 5μm sections were cut, deparaffinized in xylol and rehydrated, through graded ethanol to 70% and washed in PBS. For antigen retrieval, sections were heated at 95°C for 30 min in citrate buffer (pH 6.0) and then cooled for 20 min and washed in PBS. Endogenous peroxidase was quenched by incubation in 0.3% hydrogen peroxide, for 20 min and washed in washing buffer (PBS + Tween). Nonspecific binding sites were blocked by incubating the sections with goat serum blocking solution for 1 h. Sections were incubated with primary antibody against cyclin D1 and PCNA overnight at 4°C followed by incubation with specific HRP-labeled secondary antibody for 1 h at room temperature. After washing in wash buffer, the sections were incubated with DAB peroxidase substrate solution for 2 min at room temperature, rinsed with distilled water followed by counterstaining with Mayers Hematoxylin solution. Sections were rinsed in tap water, dehydrated through 70–100 % graded alcohol cleared in xylene and finally mounted in permanent mounting medium.
Statistical analysis
The results are expressed as the mean ± SE. Statistical analysis of all the data between groups receiving UVB exposure alone and that with PFE treatment plus UVB exposure was performed by Student’s t-test. The P value <0.05 was considered statistically significant.
Results
Oral feeding of PFE inhibits multiple UVB-exposure-induced hyperplasia, infiltration of leukocytes, protein oxidation and LPO in SKH-1 hairless mice
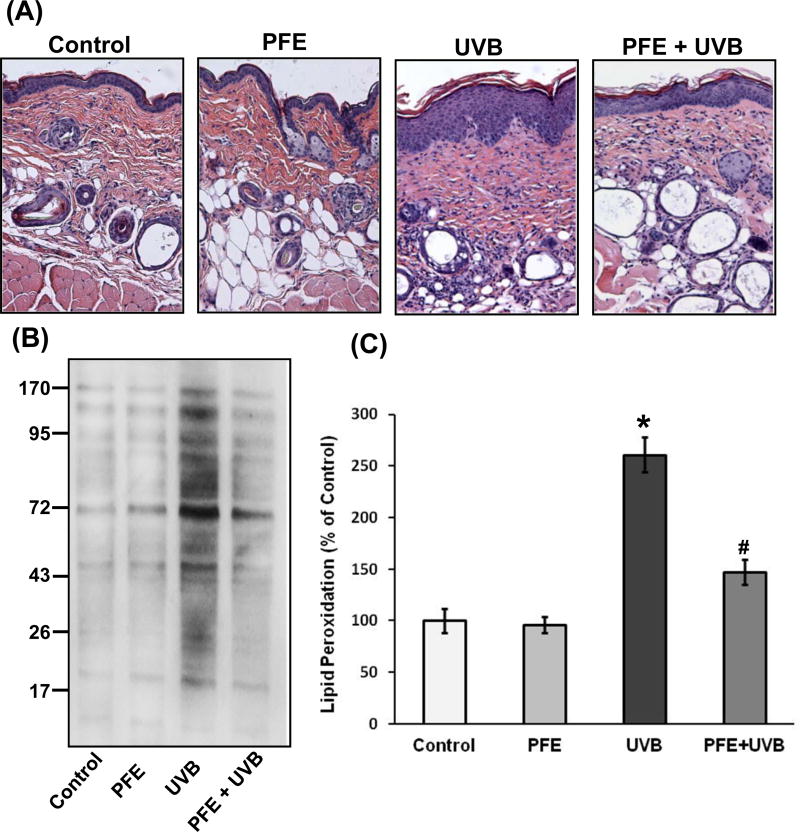
Earlier, we have shown that oral feeding of PFE (0.2%, wt/vol) inhibits single UVB-exposure-induced short-term biomarkers of photocarcinogenesis in SKH-1 hairless mouse skin (28). Further, studies were designed to investigate the effect of oral feeding of PFE on multiple UVB (180 mJ/cm2; on alternative day; for a total of seven exposures)-irradiations-induced cellular biomarkers of carcinogenesis in SKH-1 hairless mouse epidermis. PFE was provided in drinking water to SKH-1 hairless mice for 14 days before they were exposed to UVB and continued till the termination of the experiment. The animals were sacrificed 24 h after the last UVB exposure. UVB-induced inflammatory responses are characterized by infiltrating leukocytes that are considered to be a major source of inflammatory reactions and oxidative stress (29,30). Therefore, we assessed the effect of oral feeding of PFE on multiple UVB-induced hyperplasia and infiltration of leukocytes. Microscopic analysis of hematoxylin and eosin stained skin sections revealed that multiple UVB exposure resulted in an increase in hyperplasia (epidermal thickness) and infiltration of inflammatory leukocytes (activated monocytes/macrophages and neutrophils), compared to control- and PFE-treated groups (Figure 1A). However, oral feeding of PFE reduced UVB-mediated increase in epidermal hyperplasia and infiltration of leukocytes (Figure 1A). Oral feeding of PFE alone did not have any effect in skin histology when compared with control animals.
Figure 1. Effect of oral feeding of pomegranate fruit extract on multiple UVB-exposure-induced hyperplasia, infiltration of leukocytes, protein oxidation and LPO in SKH-1 hairless mice.
The mice (12 per group) were either unexposed (control), received PFE in drinking water (0.2%, wt/vol), exposed to UVB radiation (180 mJ/cm2) every alternate day for a total of seven treatments, received PFE in drinking water (0.2%, wt/vol) for 14 days before multiple UVB (180 mJ/cm2) irradiation and PFE treatment continued till the termination of the experiment. The animals were sacrificed 24 hours after the last UVB exposure and skin samples were processed. [A] Hematoxylin and eosin staining. Representative pictures are shown. [B] Protein oxidation. The epidermis was separated and epidermal protein lysates prepared and western blot analysis was performed after derivitization of transferred protein PVDF membrane with DNPH. The western blot shown here are representative of three independent experiments with similar results. [C] Lipid peroxidation. Epidermal microsomal fractions were prepared and generation of MDA was measured. The data represents the mean ± SE of 12 mice (*p < 0.001 vs control; #p <0.001 vs UVB).
Oxidation of certain amino acids (such as lysine, arginine and proline) by UVB radiation results in the formation of carbonyl derivatives that affects the function of proteins in biological systems leading to oxidative stress (31). Therefore, we analyzed the effect of oral feeding of PFE on multiple UVB-induced protein carbonyl formation as a measure of oxidative damage in mouse skin. Formation of carbonyl groups on proteins was analyzed by western blot analysis in the mouse skin lysates using a specific antibody against DNPH. As shown in Figure 1B, multiple UVB radiation resulted in increased protein oxidation as evident by more intense carbonyl groups bands when compared to control- and PFE-treated mouse skin. Oral feeding of PFE significantly reduced UVB-induced oxidation of proteins in the form of carbonyl groups when compared to UVB alone group. In addition, PFE alone did not induce carbonyl formation or oxidation of protein molecules. LPO-induced by ROS is considered one of the major manifestations of oxidative stress. We next assessed the effect of oral feeding of PFE on multiple UV exposure to mouse skin by measuring the concentration of the short-chain aldehyde, MDA, which is by-product of LPO. PFE treatment inhibited multiple UVB-mediated increase in the levels of epidermal MDA formation compared with UVB alone group (Figure 1C).
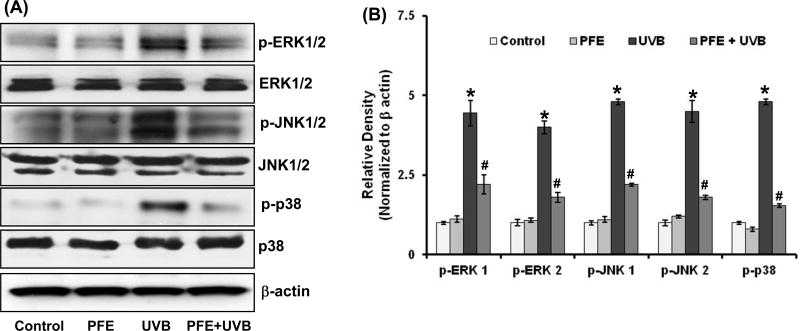
Oral feeding of PFE inhibits multiple UVB-exposure-induced phosphorylation of MAPK in SKH-1 hairless mice
Previous studies have shown that UVB exposure results in the activation of MAPK, which have been associated to play an important role in the promotion of photocarcinogenesis (30,32). Therefore, we investigated the photoprotective effect of oral feeding of PFE to SKH-1 hairless mice on multiple UVB-induced activation of MAPK (such as ERK1/2, JNK1/2 and p38) by employing western blot analysis. We found that multiple UVB exposure to mouse skin resulted in increased phosphorylation of all the three MAPK. As shown in Figure 2, western blot analysis and relative density of the bands normalized to β-actin revealed that PFE significantly reduced UVB-mediated phosphorylation of ERK1/2 (Thr202/Tyr204), JNK1/2 (Thr183/Tyr185) and p38 (Thr180/Tyr204) proteins as compared to UVB alone group.
Figure 2. Effect of oral feeding of pomegranate fruit extract on multiple UVB-exposure-induced phosphorylation of MAPKs proteins in SKH-1 hairless mice.
Animals in each group were treated as described in Figure 1. Twenty four hours after last UVB irradiation, the animals were sacrificed, skin samples were collected and the epidermis was separated and epidermal protein lysates were prepared and [A] Western blot analysis for protein expression, and [B] Relative density was performed. The western blot blots shown here are representative of three independent experiments with similar results. Equal loading was confirmed by stripping the western blot and reprobing it for β-actin. The relative density of the bands was measured from three individual western blots and normalized to β-actin. The data represents the mean ± SE of 12 mice (*p < 0.001 vs control; #p <0.001 vs UVB).
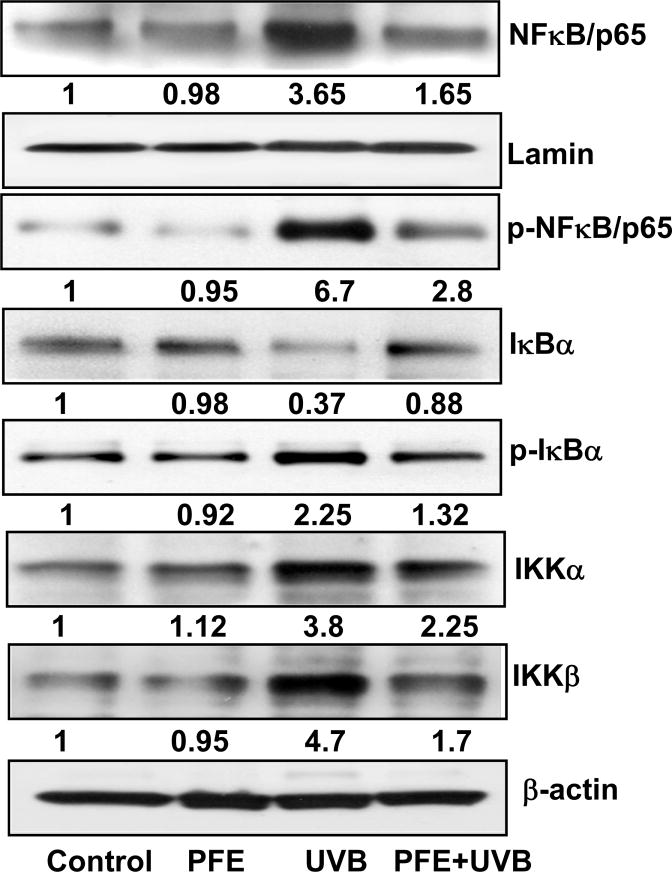
Oral feeding of PFE inhibits multiple UVB-exposure-induced activation of NF-κB pathway in SKH-1 hairless mice
Because of the role of NFκB in inflammatory responses, cell growth and proliferation (2), we next determined whether oral feeding of PFE could inhibit multiple UVB-exposure-mediated activation of NF-κB, which is a downstream target of the MAPK pathway. As shown in Figure 3, our data clearly demonstrated that multiple UVB exposure resulted in nuclear translocation and phosphorylation of NF-κB at Ser536, which were inhibited by oral feeding of PFE. One of the critical events in NF-κB activation and nuclear translocation is its dissociation from its inhibitory protein IκBα. To examine whether the inhibitory effect of PFE on UVB-induced activation and nuclear translocation is due to restoration of IκBα, we determined the cytoplasmic level of IκBα protein expression. Employing western blot analysis we found that oral feeding of PFE inhibited UVB-mediated degradation and phosphorylation of IκBα (Figure 3). IKK phosphorylates serine residues in IκBα and plays an important role in its degradation. Since these phosphorylation and proteolytic degradation of IκBα results in activation and nuclear translocation of NF-κB, we therefore measured IKKα/β protein level. We found that oral feeding of PFE reduced UVB-mediated activation of IKKα/β (Figure 3).
Figure 3. Effect of oral feeding of pomegranate fruit extract on multiple UVB-exposure-induced activation of NF-κB signaling pathway in SKH-1 hairless mice.
Animals in each group were treated as described in Figure 1. Twenty four hours after last UVB irradiation, the animals were sacrificed, skin samples were collected and the epidermis was separated and epidermal cytosolic and nuclear protein lysates were prepared for western blot analysis. Equal loading was confirmed by stripping the western blot and reprobing it for β-actin (cytoplasmic) and lamin (nuclear). The values below the figures represent relative density of the bands normalized to β-actin or lamin. The western blot shown here are representative of three independent experiments with similar results.
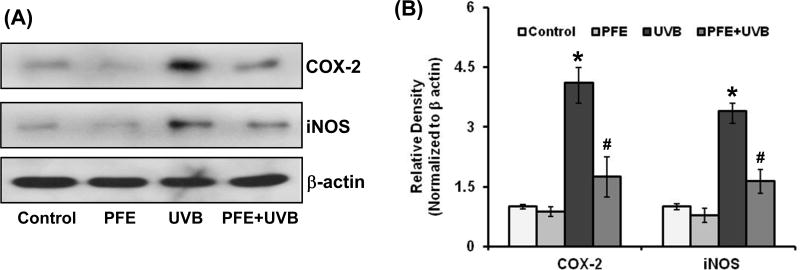
Oral feeding of PFE inhibits multiple UVB-exposure-induced cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) protein expression in SKH-1 hairless mice
Activated NF-κB regulates the transcription of various genes that encode for inflammatory regulators including COX-2 and iNOS (13). Since COX-2 and iNOS play an important role in cutaneous inflammation, cell proliferation and skin tumor promotion, we next determined the effect of oral feeding of PFE on multiple UVB-mediated increase in epidermal COX-2 and iNOS expression. Multiple UVB exposure resulted in increased COX-2 and iNOS epidermal protein expression when compared to control- and PFE-treated groups (Figure 4). However, oral feeding of PFE significantly reduced the protein expression of COX-2 and iNOS when compared to UVB alone group as evident from western blot analysis and densitometric analysis of the bands normalized by β-actin. Oral feeding of PFE alone did not produce any change in epidermal COX-2 and iNOS proteins expression when compared to control animals (Figure 4).
Figure 4. Effect of pomegranate fruit extract on multiple UVB-exposure-induced protein expression of COX-2 and iNOS in SKH-1 hairless mice.
Animals in each group were treated as described in Figure 1. Twenty four hours after last UVB irradiation, the animals were sacrificed, skin samples were collected and the epidermis was separated and epidermal protein lysates were prepared and [A] Western blot analysis for protein expression, and [B] Relative density was performed. The western blots shown here are representative of three independent experiments with similar results. Equal loading was confirmed by stripping the western blot and reprobing it for β-actin. The relative density of bands were measured from three individual western blots and normalized to β-actin. The data represents the mean ± SE of 12 mice (*p < 0.001 vs control; #p <0.001 vs UVB).
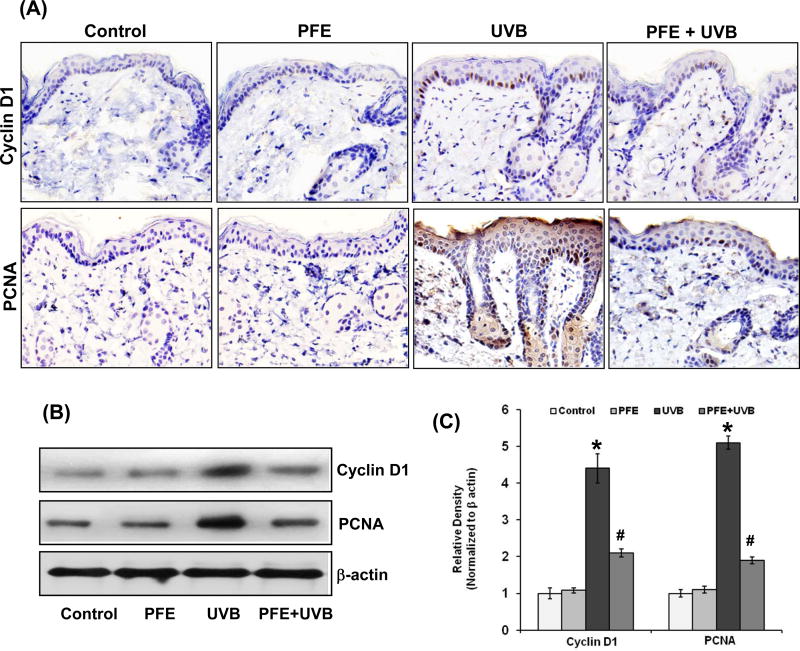
Oral feeding of PFE inhibits multiple UVB-exposure-induced cyclin D1 and PCNA protein expression in SKH-1 hairless mice
Because persistent inflammation results in epidermal hyperproliferation and enhanced proliferation rate is a hallmark of tumorigenesis, we assessed the anti-proliferative effect of PFE on UVB-mediated induction of proliferation markers namely cyclin D1 and PCNA. Immunohistochemical data analysis revealed that multiple UVB exposure resulted in enhanced proliferation of epidermal keratinocytes as revealed by staining pattern of PCNA and cyclin D1 when compared to control- and PFE-treated mouse skin. However, oral feeding of PFE significantly reduced the staining pattern of cyclin D1 and PCNA in the mouse epidermis (Figure 5A). The levels of these proteins were also verified by western blot analysis and densitometric analysis of the bands normalized to β-actin, which further confirmed that oral feeding of PFE significantly reduced the protein expression of cyclin D1 and PCNA induced by UVB (Figure 5B,C).
Figure 5. Effect of pomegranate fruit extract on multiple UVB-exposure-induced protein expression of cyclin D1 and PCNA in SKH-1 hairless mice.
Animals in each group were treated as described in Figure 1. Twenty four hours after last UVB irradiation the animals were sacrificed and skin samples were collected for [A] Immunostaining for cyclin D1 and PCNA. A representative picture from three independent immunostaining is shown; [B] Western blot analysis for protein expression, and [C] Relative density was performed. Equal loading was confirmed by stripping the western blot and reprobing it for β-actin. The relative density of the bands was measured from three individual western blots and normalized to β-actin. The data represents the mean ± SE of 12 mice (*p < 0.001 vs control; #p <0.001 vs UVB).
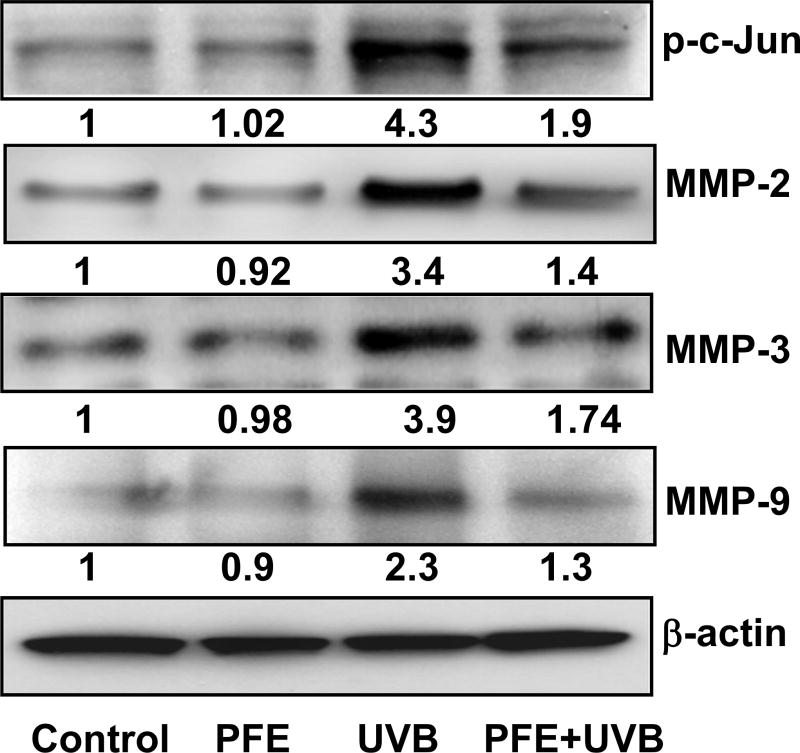
Oral feeding of PFE inhibits multiple UVB-exposure-induced phosphorylation of c-Jun and protein expression of MMPs in SKH-1 hairless mice
Studies have shown that activation of JNK1/2 lead to c-Jun phosphorylation and activate target genes that are involved in cellular proliferation and apoptosis (33). Since MAPK activation induces phosphorylation of c-Jun, a member of the activator protein (AP)-1 family; we next assessed the effect of oral feeding of PFE on multiple UVB-mediated phosphorylation of c-Jun. We found PFE inhibited multiple UVB-induced phosphorylation of c-Jun when compared to control- and PFE-treated groups (Figure 6). NFκB, MAPK and c-Jun are upstream pathways of MMPs that contribute to degradation of connective tissue and thus, in addition to wrinkle formation also promote invasion and metastasis of skin cancers (34,35). We therefore, investigated the effect of PFE on protein expression of MMPs, and observed that oral feeding of PFE inhibited UVB-induced gelatinase (MMP-2 & -9), and stromelysin (MMP-3) (Figure 6).
Figure 6. Effect of pomegranate fruit extract on multiple UVB-exposure-induced protein expression of MMPs and phosphorylation of c-Jun in SKH-1 hairless mice.
Animals in each group were treated as described in Figure 1. Twenty four hours after last UVB irradiation, the animals were sacrificed, skin samples were collected and the epidermis was separated and protein lysates were prepared for western blot analysis. Equal loading was confirmed by stripping the western blot and reprobing it for β-actin. The values below the figures represent relative density of the bands normalized to β-actin. The western blots shown here are representative of three independent experiments with similar results.
Discussion
Solar UV radiation-induced skin cancer is increasing at an alarming rate in the United States and other parts of the world, especially among Caucasian populations (1,5). Therefore, additional efforts are needed to define novel agents-approaches to prevent skin cancer and other cutaneous damage that result as a consequence of UVB exposure. For this reason, it is important to identify mechanism-based photo-protective agents. We have shown previously that PFE treatment to mice before single UVB exposure resulted in inhibition of UVB-induced early biomarkers of carcinogenesis (28). The present study was designed to investigate the photo-protective effects of PFE against multiple UVB exposure (cumulative UVB dose: 1260 mJ/cm2) on markers of inflammation, proliferation and oxidative stress. In this study, we used seven exposures as an intermediate point between chronic and acute exposure that may possibly reflect the early effect of persistent damage, and still photo-protective effect of PFE was observed in mouse skin. UVB radiation has both direct and indirect effects on the skin that includes protein oxidation, DNA damage, lipid peroxidation, generation of ROS, inflammation, proliferation, oxidative stress and immunosuppression (2,8,12). We determined whether oral feeding of PFE, after multiple UVB exposure to mice, inhibits epidermal hyperplasia and infiltration of leukocytes. Our data clearly demonstrate that oral feeding of PFE significantly reduced multiple UVB-induced epidermal hyperplasia and infiltration of leukocytes. Persistent damage to critical cellular molecules, such as, proteins, lipids and DNA can result in oxidative stress that may lead to development of skin cancers (2,12). The oxidation of amino acids leads to the formation of carbonyl groups in proteins and has become a widely accepted measure of oxidative damage of proteins. Since proteins have different and distinctive biological functions, oxidative alterations to proteins can lead to diverse functional consequences. The formation of lipid peroxides in biological membrane is a ROS–mediated process and their enhanced level is associated with disruption of cellular membrane (36,37). Multiple exposures of the mouse skin to UVB radiation enhanced the formation of protein carbonyls and MDA in comparison to non-UVB exposed mouse skin. Oral feeding of PFE significantly inhibited multiple UVB irradiation-induced protein oxidation and lipid peroxidation in mouse skin.
UVB radiation is known to known to promote tumor growth and development by activating multiple cellular signaling pathways (2,12). UVB-induced responses depend on dose, cell type, duration of activation of the pathways and crosstalk between pathways that determine cell survival, inflammation, proliferation and apoptosis (5,6). MAPK are serine/threonine kinases that regulate the activity of NF-κB and AP-1 in response to wide variety of extracellular stimuli including UVB radiation (13,38). The MAPK is divided into the extracellular signal-regulated kinases (ERK1/2), the c-Jun N-terminal kinases (JNK), and the p38 kinases. Earlier, we have shown that UVB exposure to NHEK results in an increased production of hydrogen peroxide that in turn activates the MAPK signaling pathway (39). In the present study, we observed that oral feeding of PFE significantly reduced multiple UVB-mediated phosphorlation of ERK1/2, JNK1/2 and p38. UVB radiation results in the activation of NF-κB by a series of upstream kinases, including MAPK. Studies have demonstrated that MAPK family proteins such as ERK1/2 and p38 regulate the activation of NF-κB via the IKK pathway (40). The transcription factor NF-κB is a critical mediator of the cellular response to inflammation, proliferation, survival and cellular stress (4,41,42). NF-κB proteins (p65/RelA, RelB c-Rel, p50 and p52) form homo- or heterodimers that can bind to consensus DNA sequences of target genes and regulate their transcription. In resting cells, NF-κB is sequestered in the cytoplasm because of its association with inhibitory proteins of the IκB family (43). Upon stimulation by UVB radiation, inflammatory cytokines, phorbol esters and lipopolysaccharide, the multisubunit of IκB kinase (IKK) phosphorylates IκB proteins at Ser32/36, leading to its polyubiquitination and proteasomal degradation, which allows nuclear translocation of NF-κB dimers and activate transcription of target genes (44). We found that oral feeding of PFE to mice resulted in inhibition of UVB-induced degradation and phosphorylation of IκBα, activation of IKKα/β, nuclear translocation and phosphorylation of NF-κB/p65 at Ser536. Overall, our studies suggest that PFE modulates UVB-mediated activation of these cellular signaling pathways in vivo situation leading to photoprotection of skin cells.
Cellular signaling pathways act independently or coordinately to regulate expression of target genes involved in inflammation and proliferation (2,12). The expression of COX-2 and iNOS is primarily regulated by MAPK and NF-κB leading to inflammation, proliferation, cell transformation and tumor promotion (45,46). COX-2 is a key enzyme required for prostaglandins synthesis and has been linked to the pathophysiology of inflammation and skin cancers (47). iNOS produces nitric oxide that is associated in the pathogenesis of various inflammatory skin diseases (48). Studies have shown that p38 MAPK and NF-κB are regulators of iNOS expression in response to UVB radiation in mouse skin (13,48). In addition, p38 MAPK pathway is a key regulator of COX-2 both at the transcriptional and translational levels (49). Studies have shown that UVB-induced inflammatory responses lead to cellular proliferation in skin cells (50). We found that oral feeding of PFE significantly inhibited mutiple UVB-induced inflammatory responses in terms of inhibition of COX-2 and iNOS expression and reduction in the levels of epidermal cellular proliferation viz PCNA and cyclin D1. These studies demonstrate that the photoprotective effects of PFE are associated with the inhibition of UVB-induced inflammation and proliferation and their associated signaling molecules that regulate these events.
UVB radiation activate AP-1 that results in a series of complex biologic interactions leading to skin cancer (5,6). In addition, UV radiation activates AP-l that stimulates transcription of MMP genes encoding MMP-l, MMP-3 and MMP-9 in skin cells. These changes apparently occur through induction of AP-1 that is downstream target of MAPK (51). Studies have shown that the expression of MMP-2 and MMP-9 are known to play an important role in the degradation of type IV collagen, which is a major constituent of the basement membrane during cancer invasion and metastasis (34). We found that PFE consumption inhibited UVB-induced phosphorylation of c-Jun and protein expression of MMPs. Our results demonstrate that inhibition of UVB-induced expression of MMPs in mouse skin may be atleast in part be associated due to inhibition in the phosphorylation of c-jun, a member of the AP-1 family.
Taken together, these data show that PFE consumption affords protection to mouse skin against the adverse effects of UVB radiation by modulating UVB-induced signaling pathways. This study suggests the potential efficacy of PFE as a photochemopreventive agent for skin cancer.
Acknowledgments
This work was supported by grant from USPHS R21 AT002429-02.
Footnotes
This paper is part of the Special Issue in Commemoration of the 70th birthday of Dr. David R. Bickers
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19:2–11. doi: 10.1053/sonu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 4.Hart KM, Demarco RF. Primary prevention of skin cancer in children and adolescents: a review of the literature. J Pediatr Oncol Nurs. 2008;25:67–78. doi: 10.1177/1043454208314499. [DOI] [PubMed] [Google Scholar]
- 5.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 6.Afaq F, V, Adhami M, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Halliday GM, Lyons JG. Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem Photobiol. 2008;84:272–283. doi: 10.1111/j.1751-1097.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 8.de Gruijl FR, Rebel H. Early events in UV carcinogenesis--DNA damage, target cells and mutant p53 foci. Photochem Photobiol. 2008;84:382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 9.Katiyar SK. Green tea prevents non-melanoma skin cancer by enhancing DNA repair. Arch Biochem Biophys. 2011;508:152–158. doi: 10.1016/j.abb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin CL, Ullrich SE, Kripke ML, Ananthaswamy HN. p53 tumor suppressor gene: a critical molecular target for UV induction and prevention of skin cancer. Photochem Photobiol. 2008;84:55–62. doi: 10.1111/j.1751-1097.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 11.Adhami VM, Syed DN, Khan N, Afaq F. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochem Photobiol. 2008;84:489–500. doi: 10.1111/j.1751-1097.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2009;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 15.Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 17.Hujanen ES, Vaisanen A, Zheng A, Tryggvason K, Turpeenniemi-Hujanen T. Modulation of M(r) 72,000 and M(r) 92,000 type-IV collagenase (gelatinase A and B) gene expression by interferons alpha and gamma in human melanoma. Int J Cancer. 1994;58:582–586. doi: 10.1002/ijc.2910580422. [DOI] [PubMed] [Google Scholar]
- 18.Woenne EC, Lederle W, Zwick S, Palmowski M, Krell H, Semmler W, Mueller MM, Kiessling F. MMP inhibition blocks fibroblast-dependent skin cancer invasion, reduces vascularization and alters VEGF-A and PDGF-BB expression. Anticancer Res. 2010;30:703–711. [PubMed] [Google Scholar]
- 19.Zhang G, Luo X, Sumithran E, Pua VS, Barnetson RS, Halliday GM, Khachigian LM. Squamous cell carcinoma growth in mice and in culture is regulated by c-Jun and its control of matrix metalloproteinase-2 and -9 expression. Oncogene. 2006;25:7260–7266. doi: 10.1038/sj.onc.1209726. [DOI] [PubMed] [Google Scholar]
- 20.O’Grady A, Dunne C, O’Kelly P, Murphy GM, Leader M, Kay E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007;51:793–804. doi: 10.1111/j.1365-2559.2007.02885.x. [DOI] [PubMed] [Google Scholar]
- 21.Kerkela E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 24.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kedar AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;10:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 25.Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethanopharm. 1999;66:11–17. doi: 10.1016/s0378-8741(98)00222-0. [DOI] [PubMed] [Google Scholar]
- 26.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis. 2007;195:313–321. doi: 10.1016/j.atherosclerosis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Afaq F, Khan N, Syed DN, Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol. 2010;86:1318–1326. doi: 10.1111/j.1751-1097.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 30.Afaq F, V, Adhami M, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 31.Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122:1480–1487. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 32.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 33.Einspahr JG, Bowden GT, Alberts DS, McKenzie N, Saboda K, Warneke J, Salasche S, Ranger-Moore J, Curiel-Lewandrowski C, Nagle RB, Nickoloff BJ, Brooks C, Dong Z, Stratton SP. Cross-validation of murine UV signal transduction pathways in human skin. Photochem Photobiol. 2008;84:463–476. doi: 10.1111/j.1751-1097.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 34.Ko HM, Kang JH, Jung B, Kim HA, Park SJ, Kim KJ, Kang YR, Lee HK, Im SY. Critical role for matrix metalloproteinase-9 in platelet-activating factor-induced experimental tumor metastasis. Int J Cancer. 2007;120:1277–1283. doi: 10.1002/ijc.22450. [DOI] [PubMed] [Google Scholar]
- 35.Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med. 2005;37:230–239. doi: 10.1038/emm.2005.31. [DOI] [PubMed] [Google Scholar]
- 36.Girotti AW, Kriska T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid Redox Signal. 2004;6:301–310. doi: 10.1089/152308604322899369. [DOI] [PubMed] [Google Scholar]
- 37.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson SE, Olson ER, Zhang J, Cooper SJ, Melton T, Criswell PJ, Casanova A, Dong Z, Hu C, Saboda K, Jacobs ET, Alberts DS, Bowden GT. p38 MAP kinase plays a functional role in UVB-induced mouse skin carcinogenesis. Mol Carcinog. 2011;50:469–478. doi: 10.1002/mc.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 40.Chen CC, Sun YT, Chen JJ, Chang YJ. Tumor necrosis factor-alpha-induced cyclooxygenase-2 expression via sequential activation of ceramide-dependent mitogen-activated protein kinases, and IkappaB kinase 1/2 in human alveolar epithelial cells. Mol Pharmacol. 2001;59:493–500. doi: 10.1124/mol.59.3.493. [DOI] [PubMed] [Google Scholar]
- 41.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 43.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kole L, Giri B, Manna SK, Pal B, Ghosh S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur J Pharmacol. 2011;653:8–15. doi: 10.1016/j.ejphar.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 45.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 47.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 48.Chang EJ, Kundu JK, Liu L, Shin JW, Surh YJ. Ultraviolet B radiation activates NF-κB and induces iNOS expression in HR-1 hairless mouse skin: role of IκB kinase-β. Mol Carcinog. 2011;50:310–317. doi: 10.1002/mc.20646. [DOI] [PubMed] [Google Scholar]
- 49.Vermeulen L, Vanden Berghe W, Beck IM, De Bosscher K, Haegeman G. The versatile role of MSKs in transcriptional regulation. Trends Biochem Sci. 2009;34:311–318. doi: 10.1016/j.tibs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]