Abstract
3′-Fluoro-3′-deoxythymidine (FLT) positron emission tomography (PET) has been proposed for imaging thymidylate synthase (TS) inhibition. Agents that target TS and shut down de novo synthesis of thymidine increase the uptake and retention of FLT in vitro and in vivo because of a compensating increase in the salvage pathway. Increases in both thymidine kinase-1 (TK1) and the equilibrative nucleoside transporter hENT1 have been reported to underlie this effect. We examined whether the effects of one TS inhibitor, 5-fluorouracil (5FU), on FLT uptake require proliferating cells and whether the effects are limited to increasing TK1 activity.
Methods
The effects of 5FU on FLT transport and metabolism, TK1 activity, and cell cycle progression were evaluated in the human tumor cell line, A549, maintained as either a proliferating or non-proliferating culture.
Results
There were dose-dependent increases in FLT uptake that peaked after a 10μM 5FU exposure and then declined to baseline levels or below at higher doses in both proliferating and non-proliferating cultures. The dose-dependence for FLT uptake was mirrored by changes in TK1 activity. S phase fraction did not correlate with FLT uptake in proliferating cultures. Chemical inhibition of hENT1 reduced overall levels of FLT uptake but did not affect the low dose increase in FLT uptake.
Conclusions
5FU only affects FLT uptake in proliferating A549 cells and increases in FLT uptake are directly related to increased TK1 activity. Our studies did not support a role for hENT1 in the increased uptake of FLT after exposure to 5FU. Our studies with A549 cells support the suggestion that FLT-PET could provide a measure of TS inhibition in vivo.
Keywords: FLT-PET Imaging, 5-Fluorouracil, ENT1, Nucleoside Metabolism
INTRODUCTION
The positron emission tomography (PET) tracer 3′-fluoro-3′-deoxythymidine (FLT) is considered to be a top candidate for measuring tumor proliferation changes in response to treatment [1-3]. FLT, a thymidine analog, marks DNA synthesis processes. FLT is not incorporated into DNA but is phosphorylated by thymidine kinase-1 (TK1), a cell cycle dependent enzyme. Phosphorylation of FLT traps the compound in cells. The uptake and retention of FLT is directly dependent on TK1 activity [4]. TK1 activity is low in G1. It starts to rise just before S phase through an S-phase-specific transcriptional activation of TK1. The protein is subsequently degraded in mitosis in part by ubiquitination-mediated processes [5, 6]. TK1 activity tracks closely with S phase in many cell lines that have been evaluated in vitro and in vivo [4, 7, 8]. Thus TK1 activity levels provide a marker of S phase activity and, by inference, cellular proliferation rate. As the uptake and retention of FLT is directly dependent on TK1 activity, FLT provides a measure of proliferation.
While TK1 is an absolute requirement for concentration of FLT in cells, there are multiple other activities that can modify FLT uptake and retention, and, in turn influence the relationship between proliferation and FLT uptake and retention. These include the equilibrative nucleoside transporter hENT1 [8-11], nucleotide efflux pumps [12, 13], and enzyme activities involved in nucleotide metabolism [12, 13].
The relationship between proliferation and FLT uptake and retention is influenced by exposure to agents that target thymidylate synthase (TS) such as 5-fluorouracil (5FU). TS is a critical enzyme in de novo nucleotide biosynthesis [14] and a key target for cancer control [14-20]. The cytotoxic effects of 5FU are due in part to inhibition of TS by the 5FU metabolite 5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP) [21]. To overcome the effects of TS inhibition, cells can up-regulate salvage nucleotide pathways [22]. The increased activity of the salvage pathway will lead to the increased uptake of thymidine and FLT [22-30]
5FU treatment produces increased levels of FLT uptake even after cytotoxic doses [26, 28, 29]. In in vitro studies, this increase in FLT uptake has been reported to reflect increased TK1 activity and/or higher levels of hENT1 [22, 29, 31]. In vivo, increased tumor retention of FLT and radiolabeled thymidine has also been reported following 5FU treatment [24, 30]. Other inhibitors of TS show similar responses for both FLT and thymidine [23-25].
It has been suggested that the identification of an increase in either FLT or thymidine uptake and retention following chemotherapy treatment would provide a positive measure of TS inhibition [23, 24]. However, not all studies report an increase in FLT/thymidine uptake, and there does not seem to be any consensus as to whether elevated TK1 and/or hENT1 are the underlying causes [26, 27]. For example, Direcks et al. [27] reported a 5FU-induced increase in TK1 activity, but in the absence of any increase in FLT uptake.
Given the lack of consensus as to the mechanism for increased FLT or thymidine uptake and retention following 5FU treatment, more information about the response and its relationship with TS inhibition is needed. A better understanding of the underlying mechanism for the increased intracellular levels of FLT/thymidine metabolites following 5FU treatment would improve interpretation of a FLT-PET-based assay for TS inhibition. In the present study we examined the effect of 5FU on FLT transport and metabolism in more detail. A549 cells, which have previously been reported to show increased uptake and retention of FLT following 5FU exposure [31], were exposed to different doses of 5FU under both exponential growth conditions and growth-inhibited, plateau-phase culture conditions. We observed that increases in TK1 activity were sufficient to explain the increased uptake of FLT in 5FU-treated cells.
MATERIALS AND METHODS
Materials
[methyl-3H]-3′-Deoxy-3′-fluorothymidine ([3H]-FLT; 351.5GBq/mmol) and [methyl-3H]-thymidine ([3H]-TdR; 185GBq/mmol) were purchased from Moravek Biochemicals (Brea, CA). High-purity nitrobenzylmercaptopurine ribonucleoside (NBMPR) and other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture media and supplements were purchased from Invitrogen (Carlsbad, CA). Antibodies were purchased from Invitrogen (Grand Island, NY).
Cell Culture Conditions
The human lung adenocarcinoma cell line A549 was used for all studies. A549 cells are relatively stable and will arrest under plateau-phase culture conditions [4]. Cells culture conditions were previously described [32]. For experiments on non-cycling cells, cultures were grown to 100% confluence and maintained for 8-10 days by changing the media every other day, including a media change the day prior to experiments. Studies on asynchronously cycling cells were carried out on cultures maintained at sub-confluent levels. Cultures were incubated continuously for 24h in 0-500μM doses of 5FU before tracer studies were initiated.
3H-labeled Tracer Influx Studies
Tracer influx was carried out in sodium-containing HEPES-buffered Ringer’s solution containing 135 mM NaCl, 5 mM KCl, 3.33 mM NaH2PO4, 0.83 mM Na2HPO4, 1.0 mM CaCl2, 1.0 mM MgCl2, 10 mM D-glucose, and 5 mM HEPES, pH 7.4. Single cell suspensions were prepared and cells were washed twice with buffer at room temperature. The pre-incubation buffer was replaced with buffer containing 74kBq/mL (2μCi/mL) of 3H-labeled FLT. Parallel samples had FLT-containing buffer supplemented with the hENT inhibitor NBMPR (100μM) to examine the role of equilibrative nucleoside transport. Cells were incubated with 210 pmol of FLT for 60 min before being pelleted and the tracer-containing buffer rapidly aspirated. Cells were washed twice in excess of cold phosphate buffered saline. Cells were incubated overnight in 5% Triton X-100 at room temperature and radioactivity was measured in a Tri-Carb 1900 Liquid Scintillation Counter using Ultima Gold scintillation cocktail (Perkin Elmer). Cell numbers and average cell volume were determined by Coulter counting. Experiments were performed in triplicate. Calculations of intracellular tracer concentration relative to tracer levels in buffer were determined as previously described [4].
Cell Cycle Determination
Parallel cultures of cells were incubated for 30 min in media containing 100μM 5-bromo-2′-deoxyuridine (BrdU). The media was removed and cells were rapidly washed with ice-cold phosphate-buffered saline (PBS) containing 20mM TdR. Cells were fixed in cold 70% ethanol and stored at −20°C for up to 2 weeks prior to analysis. BrdU label was detected by FITC-conjugated mouse anti-BrdU antibody. Cells were counter-stainded in propidium iodide and then analyzed on a Becton-Dickinson LSR2 cytometer using FACSDiva software (version 6.0). 20,000 events were collected for each sample, and data were analyzed with FlowJo software (version 9.0, Tree Star Inc., Ashland, OR, USA).
TK1 Activity Determination
Cells were removed from culture media, washed once in ice-cold PBS, then incubated for 30min in ice-cold lysis buffer containing 10mM Tris (pH 7.5), 250mM sucrose, 160mM KCl, 1.5mM MgCl2, 3mM β-mercaptoethanol, 0.5% NP-40, and protease inhibitors. Cells were vortexed, then centrifuged for 15min at 13,000 x g. Supernatants were snap-frozen in liquid nitrogen and stored at −80°C for up to 2 weeks prior to analysis. Protein content in lysates was determined with a bicinchoninic acid (BCA) assay. Reactions were initiated by adding reaction mix to lysates in a ratio of 1:1.7, and incubating at 37°C. The reaction mix contained 270mM NaH2PO4, 2.2mM MgAc, 2.7mM ATP, and 10 MBq/mL (270μCi/mL) [methyl-3H]-thymidine. Reactions were terminated at 0, 5, 10, and 15 minutes by removing aliquots of this mixture to tubes containing 40μL of ice-cold 6% trichloroacetic acid (TCA). Samples were then vortexed for 20s, centrifuged at 13,000 x g for 10minutes, and supernatants were removed to new tubes. After neutralizing supernatants with 10μL of saturated potassium bicarbonate, relative levels of thymidine and thymidine-monophosphate were determined by HPLC analysis as described below.
HPLC Analysis of Tracer Metabolites
For metabolism studies, cells were exposed to 3H-labeled tracers for 60min and then washed with buffer as described above for the influx studies. After washing cells, ice-cold 6% trichloroacetic acid (TCA) was added to cell pellets. Cells were vortexed for 20s, incubated on ice for 10min, vortexed again, and centrifuged at 14,000 x g for 10 min. The resulting supernatants were stored at −80°C for up to 2 weeks and neutralized with saturated potassium bicarbonate prior to analysis. Samples were analyzed using a Hewlett-Packard HP1050 HPLC system as previously described [12].
RESULTS
FLT Uptake in Exponentially Growing Cultures
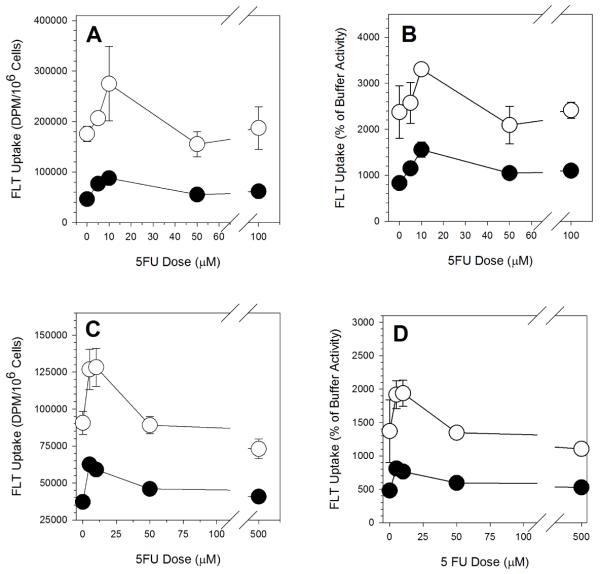
As has been reported by others [22, 26, 28, 29, 31], there was a low-dose 5FU-induced increase in FLT uptake in exponentially growing A549 cells (Figure 1A). Under the treatment conditions used here, FLT uptake peaked after a 10μM exposure. There was a 57% increase over control after a 10μM exposure. Exposures to 50μM and above yielded FLT uptake levels similar to control levels. Inhibition of hENT1 with NBMPR reduced FLT uptake by more than two-thirds, but there was still a 10μM 5FU-induced increase in FLT uptake (Figure 1A). This observation suggests that the dose-dependent FLT increase in exponentially growing A549 cells was not due to any measurable effect on hENT1 levels.
Figure 1.
5FU Dose-Dependent FLT Uptake in A549 Cells. (A) Intracellular levels of tracer (dpm/106 cells) in exponentially growing cells incubated in tracer-containing buffer. (B) Intracellular levels of tracer (dpm/106 cells) in plateau-phase cells incubated in tracer-containing buffer. (C) Intracellular levels of tracer (% of activity in cell-equivalent volume of buffer) in exponentially growing cells incubated in tracer-containing buffer. (D) Intracellular levels of tracer (% of activity in cell-equivalent volume of buffer) in plateau-phase cells incubated in tracer-containing buffer. Open symbols: incubation with FLT in buffer. Filled symbols: incubation with FLT in NBMPR-containing buffer. Results are the mean ± SD of at least three replicates.
The average diameter of exponential A549 cell populations went from 15.54 ± 0.38 μm in untreated cells to 16.28 ± 0.22 μm in 10μM treated samples. Average cell diameters following higher dose exposures were similar to the 10μM treatment values (16.24 ± 0.13 μm). The increase is likely a reflection of changes in the cell cycle phase distribution (see below). Calculation of FLT uptake as a percentage of buffer FLT levels controlled for any treatment-induced changes in cell volume. The shapes for the dose response curve using this value (percentage of buffer FLT levels; Figure 1B) was similar to that observed in Figure 1A, were suggesting that variations in cell volume cannot explain the increased FLT signal. Untreated A549 cells concentrated FLT to levels of 23-fold higher than buffer activity (Figure 1B). This value increased to about 33-fold after a 10μM 5FU exposure before returning to untreated levels (21- to 24-fold) between 50μM and 100μM exposures. The corresponding values for the NBMPR-treated samples were 8-fold concentration in untreated samples, 15.5-fold following a 10μM 5FU exposure, and 10- to 11-fold after 5FU doses between 50μM and 100μM.
FLT Uptake in Plateau-Phase Cultures
While overall levels of FLT uptake were lower in plateau-phase cultures than in exponentially growing culture, the same FLT dose-response curve shape was seen when these cells were exposed to 5FU (Figure 1C). FLT uptake peaked between 5μM and 10μM exposures, about 50% higher than control levels. At higher dose exposures, FLT uptake decreased to below control levels. Inhibition of hENT1 with NBMPR reduced FLT uptake by more than half, but the shape of the dose response curve for NBMPR-treated samples mirrored that for the non-NBMPR samples, suggesting that the increase in FLT uptake was not due to any effect on hENT1 levels.
The average diameter of plateau-phase cultures of A549 cell populations did not change significantly (P=0.36) after treatment. Average diameter went from 14.43 ± 0.05 μm in untreated cells to 14.58 ± 0.23 μm in cells treated with 10μM and larger 5FU doses. The shapes for the concentration dose responses were similar to the activity measurements (Figure 1D). Control FLT concentration levels were 14-fold over buffer. This increased to 19-fold following a 10μM 5FU exposure, and then declined to about 12-fold after higher dose exposures. The corresponding values for the NBMPR-exposed samples were 4.8-fold for untreated, 7.6-fold for 10μM treated, and 5.6-fold for larger 5FU concentrations.
TK1 Activity
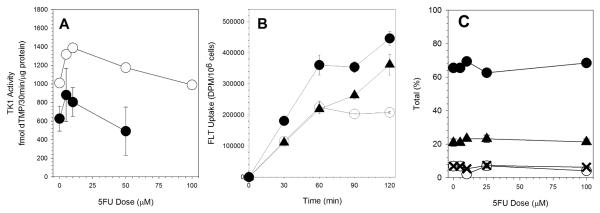
As reported by others [22, 31], 5FU exposure increased TK1 activity in exponentially-growing cultures of A549 cells (Figure 2A). There was about a 40% increase in TK1 activity after a 10μM 5FU exposure as compared to control cultures. TK1 activity decreased after higher doses of 5FU reaching control levels following a 100μM exposure. As we previously reported [8], plateau-phase cultures of A549 cells have lower levels of TK1 activity. The TK1 activity in plateau-phase cultures was about 40% less than in exponential cultures. However, 5FU exposure did increase TK1 activity in plateau-phase cultures reaching a peak that was about 50% higher than control after a 5μM 5FU exposure. A 50μM exposure reduced activity to just below control levels.
Figure 2.
5FU Dose-Dependent Changes in (A) TK Activity (○, exponential cultures; •, plateau phase cultures), (B) FLT uptake kinetics (○, control cultures; •, 10μM 5FU treated cultures; ▲, 100μM 5FU treated cultures), and (C) FLT metabolism (○, FLT; •, FLT-MP; X, FLT-DP; ▲, FLT-TP).
FLT Uptake Kinetics and Metabolism
The time course for FLT uptake was determined for control cultures and cultures exposed to either 10μM or 100μM 5FU for 24h. The rate of uptake after the 100μM exposure was similar to the initial rate of uptake for control cultures (Figure 2B). Consistent with the higher TK1 levels, cultures exposed to 10μM had faster rates of FLT accumulation.
In control cultures, FLT uptake in A549 cells increased and then reached a plateau after 60min incubation (Figure 2B). In contrast to the control cultures, cells treated with 5FU showed continued increase in FLT uptake up to 120min with no obvious change in slope. The results are not easily explained simply by the increased TK1 activity in these 5FU-treated cultures. by that there were additional factors .
There was a small increase in FLT-monophosphate (FLTMP) in cells following treatment with 10μM 5FU (Figure 2C). This was associated with a small decrease in FLT and FLT-diphosphate (FLTDP) and a small increase in FLT-triphosphate (FLTTP). Beyond the 10μM 5FU dose, the relative proportions of the different FLT metabolites were the same as untreated samples. The ratios for FLTMP:FLTTP ranged from 2.70 to 3.14. The mean was 3.04 ± 0.08.
Cell Cycle Progression Effects
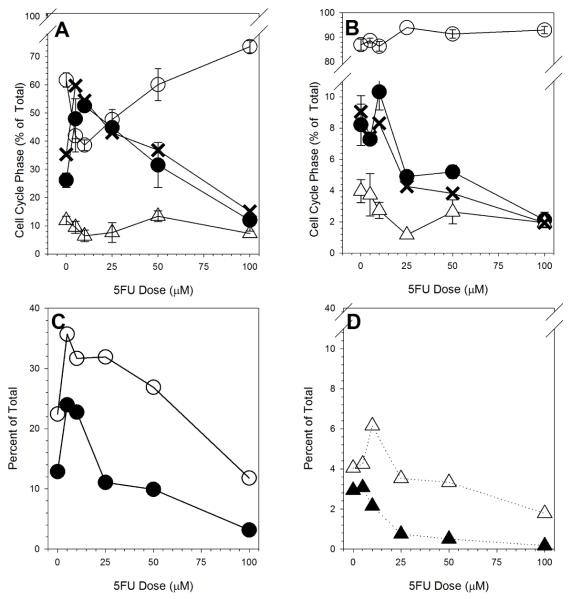
All cell cycle phase measurements were made immediately after the 24h 5FU treatment. There were dose-dependent changes in cell cycle progression in the 5FU-exposed exponentially-growing cultures (Figure 3A). Low dose 5FU treatment induced an increase in S phase cells with a corresponding decrease in G1 cells. There was a smaller decrease in G2-phase cells. After higher 5FU dose exposures, the S phase fraction decreased to well below control levels following a 100μM 5FU exposure. Over the same dose levels, the G1 component of the population increased. There was little difference in S phase fraction measured by either DNA content or BrdU-incorporation.
Figure 3.
5FU Dose-Dependent Changes in (A) Cell cycle phase in exponentially growing cultures (○, G1 phase cells; •, BrdU-labeled S phase cells; X BrdU-unlabeled S phase cells; Δ, G2/M phase cells), (B) Cell cycle phase in plateau-phase cultures (○, G1 phase cells; •, BrdU-labeled S phase cells; X BrdU-unlabeled S phase cells; Δ, G2/M phase cells), (C) Fraction of cells in early S phase (○) or late S phase (•) in 5FU-treated exponentially growing cultures, and (D) Fraction of cells in early S phase (Δ) or late S phase (▲) in 5FU-treated plateau-phase cultures.
Plateau-phase cultures contained mostly cells in G1 (Figure 3B). Only 8% of plateau-phase cells were in S phase by either DNA content or BrdU labeling. Low dose 5FU treatment increased BrdU-labeled S-phase levels to about 8%. Higher dose exposures reduced the S phase percentage to 2%, well below control levels. Little or no changes in the proportion of cells in other cell cycle phases were observed.
In exponential cultures, the distribution of cells within S phase, as measured by DNA content, also changed following 5FU treatment. Dittmann et al. [28] reported a preferential increase in early S phase (smaller DNA content) cells 24h following 5FU exposure. We compared early and late replicating populations by arbitrarily dividing the BrdU-labeled population in half based on cell size. There were increases in the frequencies of cells in both early and late S phase after low dose 5FU exposure (Figure 3C). After higher dose 5FU exposures, there were sharp decreases in frequency for both with the dose-dependent decreases in late S phase proportions being more pronounced.
Plateau-phase cultures showed a much different response (Figure 3D). For the early S phase population, there is an increase in BrdU-labeled cells that decreased sharply at 5FU doses greater than 10μM. For the late S phase cells, there was a sharp decline in number that began above the 5μM dose. It is important to emphasize that the fraction of S phase cells in the plateau-phase cultures was quite small.
Comparisons between Different Endpoints
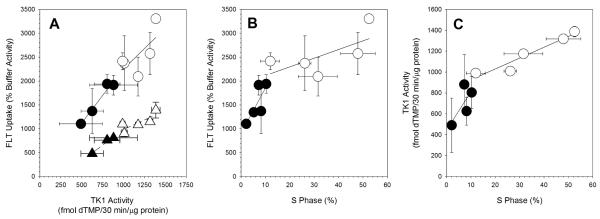
There was a highly significant (P=0.0006) linear relationship (r2=0.81) between TK1 activity and 1h FLT uptake (Figure 4A). There was more scatter in the points for the exponential cultures than for the plateau culture. Based on the slopes of the regression lines, the relationship between TK1 activity and 1h FLT uptake appeared to be similar for both exponentially-growing cultures (1.71 ± 1.09) and plateau-phase cultures (1.75 ± 0.46) .
Figure 4.
Comparisons between endpoints. (A) FLT Uptake versus TK activity (○, exponentially growing cultures; •, plateau-phase cultures; Δ, exponentially growing cultures incubated in NBMPR-containing buffer; ▲ plateau-phase cultures incubated in NBMPR-containing buffer). (B) (A) FLT Uptake versus S phase percentage (○, exponentially growing cultures; •, plateau-phase cultures; Δ, exponentially growing cultures incubated in NBMPR-containing buffer; ▲ plateau-phase cultures incubated in NBMPR-containing buffer). (C) TK activity versus S phase percentage (○, exponentially growing cultures; •, plateau-phase cultures). Regression lines are shown.
Treatment with NBMPR reduced overall levels of FLT uptake (Figure 4A) but still produced a highly significant linear relationship (P=0.003, r2=0.90) between TK1 activity and 1h FLT uptake. As for cultures not treated with NBMPR, there was more scatter in the data points for the exponential cultures than for the plateau-phase cultures. The slope for NBMPR-treated cultures (1.03 ± 0.31) was less than that observed for the cultures not treated with NBMPR (1.86 ± 0.31).
Comparing FLT uptake to % S phase (Figure 4B) yielded a significant linear relationship for the combined exponential and plateau-phase cultures (P=0.002, r2=0.69). This was driven mostly by the differences between the two groups, exponential and plateau-phase culture conditions. Viewed separately, there was a marginally significant linear relationship for the plateau phase cultures (P=0.11, r2=0.50), but not for exponential cultures (P=0.23, r2=0.24). The slopes for the two culture conditions were very different with the slope for the exponential culture not significantly different from zero.
The relationship between TK1 activity and %S phase (Figure 5C) was linear and significant for the combined exponential and plateau-phase cultures (P=0.0004, r2=0.83). It was also significant for exponential cultures alone (P=0.01, r2=0.90). The relationship between TK1 activity and %S phase was not significant for plateau-phase cultures (P=0.28, r2=0.28).
DISCUSSION
In exponentially-growing A549 cells, increased TK1 activity was the primary cause of the flare in FLT uptake after low dose exposure to 5FU. 5FU treatment induced a dose-dependent FLT uptake response (Figure 1A,B) that mirrored the observed changes in TK1 activity (Figure 2A). There was no evidence for any role of hENT1 in this 5FU-induced increase in FLT uptake; While overall FLT uptake levels were lower in the NBMPR-treated group, the shape of the curve was the same as seen without NBMPR (Figure 1A,B). Similar observations were observed for the mostly non-proliferating cultures of cells (Figure 1C,D; Figure 2A). Overall, the relationship between FLT uptake and TK1 activity with or without hENT1 inhibition was highly significant and linear (Figure 5A). The slope for FLT uptake versus TK1 activity in plateau-phase cultures was similar to that for the exponential cultures (Figure 5A).
The effect of 5FU on FLT uptake in A549 cells was proliferation dependent. While the plateau-phase cultures also have a low-dose 5FU-induced increase in FLT uptake, this could be explained by the small fraction of S phase cells in these cultures (Figure 3B). There was a small increase in early S phase cells (Figure 3D) following low dose exposure to 5FU and that corresponded to increased FLT uptake (Figure 1C,D). At doses greater than 10μM, the fraction of S phase cells declined to about half of what was observed in untreated cultures (Figure 3B,D), with corresponding reductions in FLT uptake. The relationships between S phase and FLT uptake (Figure 4B) and TK1 activity (Figure 4C) in plateau-phase cultures suggest that the non-cycling portion of cells under these conditions retained some TK1 activity that was not affected by 5FU exposure.
The relationship between FLT uptake and cell cycle phase in 5FU-exposed exponential cultures was more complex. Low dose exposure of exponential A549 cultures to 5FU led to an accumulation of cells in S phase (Figure 3A). In these low-dose exposed exponential cultures, there was no preferential buildup of cells in early S phase as others have noted [28]. Increases in both early and late S phase cells were observed following a 10μM 5FU exposure (Figure 3C), consistent with a previously reported slowing in the overall rate of DNA synthesis [33] and activation of Chk1 [34]following TS inhibition. After high dose exposures, the proportion of cells in S phase declined to below control values. In contrast, FLT uptake values did not decline below control values. The simplest explanation for these observations is that cells arresting in G1 following high dose 5FU exposures maintain elevated TK1 activity.
In control cultures FLT uptake increased and then reached a plateau after 60min incubation (Figure 2B). This is similar to what we have reported for non-cycling, plateau-phase cultures of A549 cells [8]. In a previous analysis of FLT uptake in exponentially-growing A549 cells, we observed a slower rate of FLT uptake beyond 60min incubation rather than maintenance of a stable level of FLT [13]. In that study cells were cultured with FLT at 37° C which allows for continued progression in the cell cycle. In the present study all incubations were at room temperature which impedes cells cycle progression. FLT uptake and retention would be expected to increase in growing cell populations as additional cells enter S phase. Once FLTTP pools were filled, thermodynamic control of the relationship between the different FLT metabolites would help maintain a steady state of FLT in the cells.
In contrast to control cultures, cells treated with 5FU showed continued increase in FLT accumulation up to 120min with no obvious change in slope. This was true for both the 10μM-exposed cultures, which had a higher level of TK1 activity as compared to controls, and the 100μM-exposed cultures, which had the same TK1 activity levels as controls. The continuing increase in FLT uptake and retention likely reflects dTTP depletion caused by prolonged TS inhibition [35]. The time course is also consistent with observed increased thymidine retention following TS inhibition in vivo [24].
There was essentially no effect of 5FU on the relative proportions of the different FLT metabolites (Figure 2C). The proportions of FLTMP, FLTDP, and FLTTP were the same for control and all doses of 5FU, consistent with the relationship between the different nucleotides being under thermodynamic control and independent of enzymatic activity once FLTMP has been produced.
Conclusion
It has been suggested that the identification of an increase in either FLT or thymidine uptake and retention following chemotherapy treatment would provide a measure of TS inhibition [23, 24]. Our studies with A549 cells support that suggestion and point to multiple elements that underlie the increased uptake and retention of FLT in proliferating tumor cells.
ACKNOWLEDGEMENT
This work was supported by grant CA118130 from the National Institutes of Health. The authors thank Tiffany Li for her help with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31:1659–72. doi: 10.1007/s00259-004-1687-6. [DOI] [PubMed] [Google Scholar]
- [2].Larson SM, Schoder H. New PET tracers for evaluation of solid tumor response to therapy. Q J Nucl Med Mol Imaging. 2009;53:158–66. [PubMed] [Google Scholar]
- [3].Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006;8:141–50. doi: 10.1007/s11307-006-0039-2. [DOI] [PubMed] [Google Scholar]
- [4].Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7. [PubMed] [Google Scholar]
- [5].Ke PY, Kuo YY, Hu CM, Chang ZF. Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev. 2005;19:1920–33. doi: 10.1101/gad.1322905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ke PY, Yang CC, Tsai IC, Chang ZF. Degradation of human thymidine kinase is dependent on serine-13 phosphorylation: involvement of the SCF-mediated pathway. Biochem J. 2003;370:265–73. doi: 10.1042/BJ20021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Effect of p53 activation on cell growth, thymidine kinase-1 activity, and 3′-deoxy-3′fluorothymidine uptake. Nucl Med Biol. 2004;31:419–23. doi: 10.1016/j.nucmedbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [8].Plotnik DA, Emerick LE, Krohn KA, Unadkat JD, Schwartz JL. Different modes of transport for 3H-thymidine, 3H-FLT, and 3H-FMAU in proliferating and nonproliferating human tumor cells. J Nucl Med. 51:1464–71. doi: 10.2967/jnumed.110.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Paproski RJ, Ng AM, Yao SY, Graham K, Young JD, Cass CE. The role of human nucleoside transporters in uptake of 3′-deoxy-3′-fluorothymidine. Mol Pharmacol. 2008;74:1372–80. doi: 10.1124/mol.108.048900. [DOI] [PubMed] [Google Scholar]
- [10].Paproski RJ, Wuest M, Jans H-S, Graham K, Gati WP, McQuarrie S, et al. Biodistribution and Uptake of 3′-Deoxy-3′-Fluorothymidine in ENT1-Knockout Mice and in an ENT1-Knockdown Tumor Model. J Nucl Med. 51:1447–55. doi: 10.2967/jnumed.110.076356. [DOI] [PubMed] [Google Scholar]
- [11].Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3′-deoxy-3′-fluorothymidine. Biochem Pharmacol. 79:587–95. doi: 10.1016/j.bcp.2009.09.025. [DOI] [PubMed] [Google Scholar]
- [12].Plotnik DA, McLaughlin LJ, Chan J, Redmayne-Titley JN, Schwartz JL. The role of nucleoside/nucleotide transport and metabolism in the uptake and retention of 3′-fluoro-3′-deoxythymidine in human B-lymphoblast cells. Nucl Med Biol. 38:979–86. doi: 10.1016/j.nucmedbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3′-deoxy-3′-[F-18]fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nucl Med Biol. 2004;31:829–37. doi: 10.1016/j.nucmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [14].Danenberg PV, Malli H, Swenson S. Thymidylate synthase inhibitors. Semin Oncol. 1999;26:621–31. [PubMed] [Google Scholar]
- [15].Aschele C, Lonardi S, Monfardini S. Thymidylate Synthase expression as a predictor of clinical response to fluoropyrimidine-based chemotherapy in advanced colorectal cancer. Cancer Treat Rev. 2002;28:27–47. doi: 10.1053/ctrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- [16].Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta. 2002;1587:164–73. doi: 10.1016/s0925-4439(02)00079-0. [DOI] [PubMed] [Google Scholar]
- [17].Bavetsias V, Jackman AL, Marriott JH, Kimbell R, Gibson W, Boyle FT, et al. Folate-based inhibitors of thymidylate synthase: synthesis and antitumor activity of gamma-linked sterically hindered dipeptide analogues of 2-desamino-2-methyl-N10-propargyl-5,8-dideazafolic acid (ICI 198583) J Med Chem. 1997;40:1495–510. doi: 10.1021/jm960878u. [DOI] [PubMed] [Google Scholar]
- [18].Ceppi P, Papotti M, Scagliotti G. New strategies for targeting the therapy of NSCLC: the role of ERCC1 and TS. Adv Med Sci. 55:22–5. doi: 10.2478/v10039-010-0017-4. [DOI] [PubMed] [Google Scholar]
- [19].Gmeiner WH. Novel chemical strategies for thymidylate synthase inhibition. Curr Med Chem. 2005;12:191–202. doi: 10.2174/0929867053363432. [DOI] [PubMed] [Google Scholar]
- [20].Jackman AL. Inhibition of thymidylate synthase: biochemical pharmacology of drugs with activity in colorectal cancer. Tumori. 1997;83:S65–6. [PubMed] [Google Scholar]
- [21].Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–64. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- [22].Pressacco J, Mitrovski B, Erlichman C, Hedley DW. Effects of thymidylate synthase inhibition on thymidine kinase activity and nucleoside transporter expression. Cancer Res. 1995;55:1505–8. [PubMed] [Google Scholar]
- [23].Pillai RG, Forster M, Perumal M, Mitchell F, Leyton J, Aibgirhio FI, et al. Imaging pharmacodynamics of the alpha-folate receptor-targeted thymidylate synthase inhibitor BGC 945. Cancer Res. 2008;68:3827–34. doi: 10.1158/0008-5472.CAN-08-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wells P, Aboagye E, Gunn RN, Osman S, Boddy AV, Taylor GA, et al. 2-[11C]thymidine positron emission tomography as an indicator of thymidylate synthase inhibition in patients treated with AG337. J Natl Cancer Inst. 2003;95:675–82. doi: 10.1093/jnci/95.9.675. [DOI] [PubMed] [Google Scholar]
- [25].Yau K, Price P, Pillai RG, Aboagye E. Elevation of radiolabelled thymidine uptake in RIF-1 fibrosarcoma and HT29 colon adenocarcinoma cells after treatment with thymidylate synthase inhibitors. Eur J Nucl Med Mol Imaging. 2006;33:981–7. doi: 10.1007/s00259-005-0060-8. [DOI] [PubMed] [Google Scholar]
- [26].Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, et al. 3′-deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res. 2003;63:3791–8. [PubMed] [Google Scholar]
- [27].Direcks WG, Berndsen SC, Proost N, Peters GJ, Balzarini J, Spreeuwenberg MD, et al. [18F]FDG and [18F]FLT uptake in human breast cancer cells in relation to the effects of chemotherapy: an in vitro study. Br J Cancer. 2008;99:481–7. doi: 10.1038/sj.bjc.6604523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dittmann H, Dohmen BM, Kehlbach R, Bartusek G, Pritzkow M, Sarbia M, et al. Early changes in [18F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging. 2002;29:1462–9. doi: 10.1007/s00259-002-0925-z. [DOI] [PubMed] [Google Scholar]
- [29].Perumal M, Pillai RG, Barthel H, Leyton J, Latigo JR, Forster M, et al. Redistribution of nucleoside transporters to the cell membrane provides a novel approach for imaging thymidylate synthase inhibition by positron emission tomography. Cancer Res. 2006;66:8558–64. doi: 10.1158/0008-5472.CAN-06-0898. [DOI] [PubMed] [Google Scholar]
- [30].Kenny LM, Contractor KB, Stebbing J, Al-Nahhas A, Palmieri C, Shousha S, et al. Altered tissue 3′-deoxy-3′-[18F]fluorothymidine pharmacokinetics in human breast cancer following capecitabine treatment detected by positron emission tomography. Clin Cancer Res. 2009;15:6649–57. doi: 10.1158/1078-0432.CCR-09-1213. [DOI] [PubMed] [Google Scholar]
- [31].Lee SJ, Kim SY, Chung JH, Oh SJ, Ryu JS, Hong YS, et al. Induction of thymidine kinase 1 after 5-fluorouracil as a mechanism for 3′-deoxy-3′-[18F]fluorothymidine flare. Biochem Pharmacol. 80:1528–36. doi: 10.1016/j.bcp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [32].Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. J Nucl Med. 2003;44:2027–32. [PubMed] [Google Scholar]
- [33].Robinson HM, Jones R, Walker M, Zachos G, Brown R, Cassidy J, et al. Chk1-dependent slowing of S-phase progression protects DT40 B-lymphoma cells against killing by the nucleoside analogue 5-fluorouracil. Oncogene. 2006;25:5359–69. doi: 10.1038/sj.onc.1209532. [DOI] [PubMed] [Google Scholar]
- [34].Xiao Z, Xue J, Sowin TJ, Rosenberg SH, Zhang H. A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene. 2005;24:1403–11. doi: 10.1038/sj.onc.1208309. [DOI] [PubMed] [Google Scholar]
- [35].Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, et al. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J Biol Chem. 1987;262:8235–41. [PubMed] [Google Scholar]