Abstract
Hepatocellular carcinoma (HCC) surveillance is underutilized among patients with cirrhosis. Understanding which steps in the surveillance process are not being performed is essential for designing effective interventions to improve surveillance rates. Our study's aim was to characterize reasons for failure in the HCC surveillance process among a cohort of cirrhotic patients with HCC. We conducted a retrospective cohort study of cirrhotic patients diagnosed with HCC at a large urban safety-net hospital between 2005–2011. Patients were characterized by receipt of HCC surveillance over a two-year period prior to HCC diagnosis. Among patients without HCC surveillance, we classified reasons for failure into four categories: failure to recognize liver disease, failure to recognize cirrhosis, failure to order surveillance, and failure to complete surveillance despite orders. Univariate and multivariate analyses were performed to identify predictors of failures. We identified 178 patients with HCC, of whom 20% had undergone surveillance. There were multiple points of failure- 20% had unrecognized liver disease, 19% had unrecognized cirrhosis, 38% lacked surveillance orders, and 3% failed to complete surveillance despite orders. Surveillance was more likely among patients seen by hepatologists (OR 6.11, 95%CI 2.5–14.8) and less likely in those with alcohol abuse (OR 0.14, 95%CI 0.03–0.65). Although a retrospective analysis in a safety-net hospital, our data suggest only one in five patients received surveillance prior to HCC diagnosis. There are multiple points of failure in the surveillance process, with the most common being failure to order surveillance in patients with known cirrhosis. Future interventions must target multiple failure points in the surveillance process to be highly effective.
Keywords: Screening, underutilization, quality of care, liver cancer, cirrhosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and has an increasing incidence in the United States due to the current epidemic of non-alcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV)(1). Prognosis for patients with HCC depends on tumor stage at diagnosis, with curative options only available for patients diagnosed at an early stage(2). Patients with early HCC achieve 5-year survival rates near 70% with resection and transplantation, whereas those with advanced HCC have a median survival of less than one year(3, 4).
Surveillance using ultrasound at six-month intervals is recommended in patients with cirrhosis to detect HCC at an early stage(5). Although surveillance is efficacious for detecting early HCC(6), its effectiveness in clinical practice is impacted by several factors, including low utilization rates(7, 8). A recent meta-analysis demonstrated that fewer than 20% of patients with cirrhosis undergo surveillance(9).
To date, no studies have provided in-depth analyses of correlates for HCC surveillance underutilization. Surveillance is a complex process in clinical practice, with multiple potential steps that are prone to failure(10). Providers must accurately identify high-risk patients and order appropriate surveillance testing, the healthcare system must schedule the tests, and patients must adhere with surveillance recommendations(11). A breakdown at any step results in surveillance failure. This challenge is particularly relevant in primary care settings, where providers face increasing time constraints and might be less knowledgeable about HCC guidelines(12). A better understanding of surveillance breakdowns is necessary to identify appropriate intervention targets. Our study's purpose was to characterize surveillance process failures among a cohort of cirrhotic patients with HCC.
METHODS
Study Population
We conducted a retrospective cohort study of cirrhotic patients diagnosed with HCC at Parkland Memorial Health and Hospital System, the safety-net system for Dallas County, between January 2005 and June 2011. With eleven primary care clinics in low-income neighborhoods, Parkland cares for approximately 50% of HCC patients in Dallas County. Given this integrated structure, patients admitted to Parkland often receive their continuity care through Parkland Hospital. Furthermore, Parkland is one of the few safety-net hospitals with an integrated electronic medical record for the hospital and clinics, including primary care clinics. Patients were initially identified by ICD-9 codes for HCC (155.0 or 155.2), tumor conference presentation lists, and prior databases of patients who underwent surgical (resection or transplantation) or interventional (transarterial chemoembolization or local ablation) treatments for HCC. Patients were required to have their first patient encounter at Parkland more than one year prior to HCC diagnosis, so surveillance failure rates could be accurately determined. Patients without primary care or hepatology clinic visits within two years of HCC diagnosis were excluded, as we could not determine whether they were receiving care at another institution (Supplemental Figure).
Two authors (A.S. and A.Y.) adjudicated HCC cases to confirm they met diagnostic criteria, based on American Association for the Study of Liver Disease (AASLD) guidelines(13). For tumors larger than 1 cm, diagnosis was made by a typical vascular pattern on dynamic imaging (arterial enhancement and delayed washout) or histology. This study was approved by the Institutional Review Board of UT Southwestern Medical Center.
Data Collection
Patient demographics, clinical history, laboratory data and imaging results were obtained through review of computerized and paper medical records. Two authors (E.O. and A.Y.) independently extracted information using standardized forms, with a third investigator (A.S.) available to resolve discrepancies. Age, gender, race/ethnicity, and lifetime alcohol and smoking history were recorded, with active alcohol abuse defined as drinking more than 40 grams/day. Dates of liver disease diagnosis, cirrhosis diagnosis, HCC surveillance testing, and HCC diagnosis were abstracted. Date of first medical encounter and number of primary care and hepatology clinic visits were documented. Data regarding liver disease included underlying etiology and presence of decompensation (ascites or encephalopathy). We classified patients according to etiology of liver disease, including HCV, HBV, alcohol-related liver disease, NAFLD, and other. NAFLD was often associated with components of the metabolic syndrome (obesity, diabetes, dyslipidemia) but was a diagnosis of exclusion, only made in the absence of other causes of liver disease including viral hepatitis and alcohol abuse. Laboratory data of interest included platelet count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, international normalized ratio (INR), and alpha fetoprotein (AFP). Tumor characteristics were determined by imaging studies (4-phase CT or MRI), interpreted by radiologists at our institution.
Statistical Analysis
We characterized patients based on receipt of HCC surveillance, which was our primary outcome of interest. Inconsistent surveillance was defined as one abdominal ultrasound, for screening purposes, over the two-year period prior to HCC diagnosis. We also calculated rates of consistent surveillance (“repeat surveillance”) for patients with at least two years of medical care at Parkland prior to HCC diagnosis. Consistent surveillance was defined as at least one abdominal ultrasound study, for screening purposes, every 12 months over the two-year period prior to HCC diagnosis, as recommended by guidelines during the study period(13). Imaging was determined to be for surveillance purposes through chart review of imaging reports and clinical notes.
Among patients without surveillance, we classified reasons for failure to complete surveillance into four mutually exclusive categories: failure to recognize liver disease, failure to recognize cirrhosis, failure to order surveillance, or failure to complete surveillance despite orders (Supplemental Table). Failure to recognize liver disease was defined as lack of any specific testing (e.g. viral hepatitis serologies) or mention of liver disease in clinical notes. Patients with known liver disease were classified as failure to recognize cirrhosis if they did not have histology or abdominal imaging documenting cirrhosis prior to HCC diagnosis. Failure to order surveillance was defined as lack of abdominal imaging orders, for purposes of HCC surveillance, among patients with known cirrhosis. Finally, patients were categorized as failure to complete surveillance if surveillance orders were present, but an ultrasound was not performed.
Fisher exact and Mann Whitney rank-sum tests were performed to identify patient- and system-factors associated with process failures at each step. Therefore, dependent variables included the presence of any surveillance, failure to recognize liver disease, failure to recognize cirrhosis, failure to order surveillance, and failure to complete surveillance despite orders. We assessed patient socio-demographic and clinical characteristics, including age, gender, race/ethnicity, language, alcohol abuse, insurance, performance status, number of primary care visits, receipt of hepatology care, etiology of liver disease, platelet count, bilirubin, and Child-Pugh class as independent variables. Multivariate logistic regression was performed using factors significant on univariate analysis. Statistical significance was defined as a p-value <0.05 on univariate and multivariate analyses. All data analysis was performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
Between January 2005 and June 2011, 397 patients with cirrhosis were diagnosed with HCC. We excluded 165 patients with less than one year of care prior to HCC diagnosis and 54 due to lack of primary care or hepatology clinic visits within two years of HCC diagnosis (Supplemental Figure). Table 1 shows baseline characteristics of the remaining 178. The median age of patients was 57 years (range 34–89), and more than 75% were men. Our population was racially diverse, with 40% African Americans, 23% non-Hispanic Caucasians, and 28% Hispanic Caucasians. Nearly 49% of patients were uninsured, and only 7% had private health insurance. The most common etiologies of cirrhosis were HCV (72.5%), alcohol-induced liver disease (11.2%), and NAFLD (6.7%). The median Child-Pugh score at diagnosis was 7 (range 5–15), with 39% of patients having Child-Pugh A cirrhosis.
Table I.
Patient Characteristics
| Patient Characteristics | Included patients (n=178) |
|---|---|
| Age | 56.9 (33.6 – 89.2) |
|
| |
| Gender (% Male) | 138 (77.5%) |
|
| |
| Race | |
| Caucasian | 41 (23.0%) |
| Black | 72 (40.5%) |
| Hispanic | 50 (28.1%) |
| Asian | 15 (8.4%) |
|
| |
| Etiology | |
| Hepatitis C | 129 (72.5%) |
| Hepatitis B | 13 (7.3%) |
| Alcohol | 20 (11.2%) |
| NAFLD | 12 (6.7%) |
| Other | 4 (2.3%) |
|
| |
| Insurance status | |
| Medicare | 52 (29.2%) |
| Medicaid | 26 (14.6%) |
| Private Insurance | 13 (7.3%) |
| None | 87 (48.9%) |
|
| |
| Alcohol (% active) | 42 (23.6%) |
|
| |
| Presence of ascites | 79 (44.4%) |
|
| |
| Presence of hepatic encephalopathy | 37 (20.8%) |
|
| |
| Presence of any hepatic decompensation | 85 (47.8%) |
|
| |
| Platelet count * 1000/mm3 | 124.5 (6 – 542) |
|
| |
| AST (U/L) | 88 (17 – 968) |
|
| |
| ALT (U/L) | 48 (9 – 283) |
|
| |
| Bilirubin (mg/dL) | 1.4 (0.2 – 22.4) |
|
| |
| Albumin (g/dL) | 3.2 (1.6 – 4.5) |
|
| |
| INR | 1.2 (.9 – 3.3) |
|
| |
| Number of primary care clinic visits prior to HCC diagnosis | 6 (0 – 39) |
|
| |
| Receipt of hepatology care prior to HCC diagnosis | 66 (37.1%) |
|
| |
| Functional status (% ECOG 0–1) | 156 (87.6%) |
|
| |
| Child-Pugh score | 7 (5 – 15) |
|
| |
| Child-Pugh classification | |
| Child A | 70 (39.3%) |
| Child B | 64 (36.0%) |
| Child C | 44 (24.7%) |
All data are expressed as median (range) unless otherwise specified
ALT – alanine aminotransferase; AST – aspartate aminotransferase; ECOG – Eastern Cooperative Oncology Group; HCC – hepatocellular carcinoma; INR – international normalized ratio; NAFLD – nonalcoholic fatty liver disease
Surveillance Utilization
Patients had been followed at Parkland for a median of 4.7 years (range 1.0–11.6) prior to HCC diagnosis. Twenty-nine patients had been followed for 1–2 years, 23 for 2–3 years and 126 for more than 3 years. The median number of primary care visits in the two years preceding HCC diagnosis was 6 (range 0–39), with 66 (37.1%) having at least one hepatology clinic visit.
Overall, inconsistent surveillance had been performed in 36 (20.2%) patients, with 142 (79.8%) not receiving any surveillance over the two years. Of 149 patients followed for at least two years, consistent surveillance had been performed in 9 (6.0%) patients. Patients with consistent surveillance had a higher proportion of early stage tumors, but this did not reach statistical significance (66.7% vs. 37.1%, p=0.09).
Process of Care Failure Rates
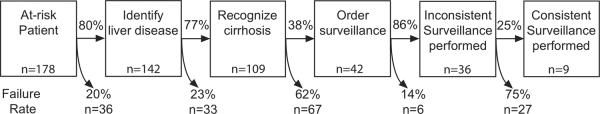
There were multiple points of failure in the surveillance process (Figure 1). Lack of surveillance was attributed to failure of recognizing liver disease in 36 (20.2%) patients. Of 142 patients with known liver disease, 33 (23.2%) had a failure to recognize cirrhosis. The most common point of failure in the surveillance process was lack of HCC surveillance orders, with 67 (61.5%) of 109 patients with known cirrhosis having failure at this step. Failure to complete surveillance despite orders was the least common reason for failure, occurring in only 6 (14.3%) of 42 patients.
Figure 1.
Process of Care Failure Rates for Hepatocellular Carcinoma Surveillance
Predictors for Receipt of Surveillance
In univariate analysis, inconsistent surveillance was positively associated with hepatic decompensation (p=0.005), thrombocytopenia (p=0.04), higher bilirubin levels (continuous) (p=0.04), and hepatology care (p<0.001) and was inversely associated with active alcohol abuse (p=0.004). Inconsistent surveillance was not associated with gender (p=0.26), race (p=0.19), performance status (p=0.36), or Child Pugh class (p=0.06). Although insurance status was not associated with inconsistent surveillance (p=0.23), this may relate to Parkland's sliding fee scale program, which provides a subsidy for medical care, such as receipt of HCC surveillance. In multivariate analysis, alcohol abuse remained associated with lower rates of HCC surveillance (OR 0.14, 95%CI 0.03–0.65) and hepatology care was associated with higher rates of surveillance (OR 6.11, 95%CI 2.52–14.81) (Table 2). Patients with alcohol abuse had surveillance performed in 4.8% (2/42) of patients, compared to 25.0% (34/136) in patients who were not currently drinking alcohol. Surveillance had been performed at least once in 40.9% (27/66) of patients followed in hepatology clinic, compared to 8.0% (9/112) of patients without hepatology care.
Table II.
Predictors of Inconsistent Hepatocellular Carcinoma Surveillance (n=178*)
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Active alcohol abuse | 0.15 | 0.03 – 0.65 | 0.14 | 0.03 – 0.65 |
| Hepatology subspecialty care | 7.92 | 3.42 – 18.34 | 6.11 | 2.52 – 14.81 |
| Hepatic decompensation | 3.10 | 1.42 – 6.80 | 2.21 | 0.89 – 5.47 |
| Platelet count < 150 * 1000/mm3 | 2.40 | 1.02 – 5.65 | 1.72 | 0.65 – 4.53 |
| Bilirubin level (continuous) | 1.04 | 0.96 – 1.13 | 1.01 | 0.90 – 1.14 |
142 patients with no surveillance vs. 36 patients with inconsistent surveillance
The only factor associated with consistent surveillance was receipt of hepatology care (OR 7.39, 95%CI 1.48–37.0). Although rates were low in both groups, patients being followed in hepatology clinic were significantly more likely to have consistent surveillance (13.5% vs. 2.1%, p=0.009).
Predictors for Failures in the Surveillance Process
Recognition of liver disease was significantly associated, in univariate analysis, with younger age (p=0.002), thrombocytopenia (p=0.01), higher bilirubin (p=0.03), hepatic decompensation (p=0.006), lack of NAFLD (p<0.001), and presence of viral hepatitis (p<0.001). Age was collinear with other covariates (variance inflation factor 10.3) and was removed from multivariate analysis. Recognition of liver disease was driven by liver disease etiology in multivariate analysis, with the highest rates among patients with viral hepatitis (OR 3.60, 95%CI 1.31–9.93) and lowest rates among patients with NAFLD (OR 0.12, 95%CI 0.02–0.74) (Table 3). Nine (81.8%) of eleven patients with NAFLD had unrecognized liver disease, compared to 12.8% (18/141) of patients with viral hepatitis.
Table III.
Predictors for Successful Recognition of Liver Disease (n=178*)
| Variable ** | Univariate Analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Viral etiology | 6.47 | 2.87 – 14.59 | 3.60 | 1.31 – 9.94 |
| NAFLD etiology | 0.04 | 0.01 – 0.21 | 0.12 | 0.02 – 0.74 |
| Hepatic decompensation | 2.91 | 1.31 – 6.48 | 2.23 | 0.88 – 5.61 |
| Bilirubin level (continuous) | 1.09 | 0.95 – 1.24 | 1.05 | 0.91 – 1.21 |
| Platelet count (continuous) | 1.00 | 0.99 – 1.00 | 1.00 | 0.99 – 1.00 |
142 patients with known liver disease vs. 36 patients with failure to recognize liver disease
Age was significant on univariate analysis (p=0.002) but was found to be collinear with other included variables (VIF 10.3) and therefore removed from the multivariate model.
Predictors of recognizing cirrhosis in univariate analysis included thrombocytopenia (p<0.001), higher bilirubin (p=0.020), hepatic decompensation (p=0.006), and hepatology care (p=0.001). In multivariate analysis, factors that remained associated with recognition of cirrhosis were platelet count <150 (OR 5.80, 95%CI 2.35–14.33) and hepatology care (OR 2.86, 95%CI 1.01–8.10) (Table 4). More patients with thrombocytopenia had their cirrhosis recognized compared to those with higher platelet counts (46.0% vs. 11.1% respectively). Cirrhosis was recognized in 89.8% (53/59) of patients followed in hepatology clinic, compared to 67.5% (56/83) of patients without hepatology care.
Table IV.
Predictors for Successful Recognition of Cirrhosis (n=142*)
| Variable | Univariate Analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Platelet count < 150 * 1000/mm3 | 6.81 | 2.88 – 16.12 | 5.80 | 2.35 – 14.33 |
| Hepatology subspecialty care | 4.26 | 1.63 – 11.13 | 2.86 | 1.01 – 8.10 |
| Hepatic decompensation | 3.40 | 1.47 – 7.83 | 2.48 | 0.98 – 6.24 |
| Bilirubin level (continuous) | 1.04 | 0.93 – 1.17 | 1.03 | 0.92 – 1.15 |
109 patients with known cirrhosis vs. 33 patients with failure to recognize cirrhosis
Failure to order surveillance among patients with recognized cirrhosis was associated with alcohol abuse (p=0.006), lack of hepatology care (p=0.003), and fewer primary care visits (p=0.01) in univariate and multivariate analysis (Table 5). Patients with more primary care visits (entered as continuous variable) (OR 1.08, 95%CI 1.01–1.16) and those receiving hepatology care (OR 3.15, 95%CI 1.32–7.48) were more likely to have HCC surveillance orders, whereas patients with alcohol abuse (OR 0.29, 95%CI 0.09–0.97) were less likely to have orders. More patients with alcohol abuse had failure of surveillance orders than patients who were not drinking alcohol (84.6% vs. 54.2%, respectively). Failure of surveillance orders was the reason for lack for HCC surveillance in 47.2% (25/53) of patients followed in hepatology clinic, compared to 75.0% (42/56) among those followed in primary care clinics alone. Patients with surveillance orders had a median number of 7.5 primary care visits, compared to 4 visits among those without surveillance orders.
Table V.
Predictors for the Presence of Surveillance Orders (n=109*)
| Variable | Univariate Analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Active alcohol abuse | 0.22 | 0.07 – 0.68 | 0.29 | 0.09 – 0.97 |
| Hepatology subspecialty care | 3.36 | 1.49 – 7.56 | 3.15 | 1.32 – 7.48 |
| Number of primary care clinic visits (continuous) | 1.08 | 1.01 – 1.16 | 1.08 | 1.01 – 1.16 |
42 patients with presence of orders for HCC surveillance vs. 67 patients with failure to order HCC surveillance despite known cirrhosis
DISCUSSION
Our study is the first to provide in-depth analysis of reasons for HCC surveillance process failures and report why surveillance is underutilized (7, 9, 14, 15). We found that only 20% of patients received HCC surveillance over a two-year period. Although failure to order surveillance was the most common reason, we found multiple failure points in the surveillance process, including nearly 40% of patients having unrecognized liver disease and/or cirrhosis. Therefore, interventions only aimed at increasing surveillance orders, such as reminder systems, would likely have limited effectiveness. Future interventions should help primary care providers identify patients with liver disease and cirrhosis as well as promote ordering of HCC surveillance among at-risk patients.
Our study demonstrates that under-recognition of liver disease and cirrhosis substantially contributes to HCC surveillance failure. This issue is consistent with a study by Stravitz and colleagues, in which 21.9% of patients presented with HCC without known cirrhosis(16). Our higher rates of unrecognized cirrhosis may be due to methodologic differences; we determined if cirrhosis was known 1–2 years prior to HCC diagnosis, when surveillance should have been performed, whereas Stravitz assessed if cirrhosis was known at HCC diagnosis. We found the subset of patients with NAFLD were at highest risk of having unrecognized liver disease, with lack of surveillance being attributed to unrecognized liver disease in over 80% of cases. Given that NAFLD is a diagnosis of exclusion, with no serologic markers, providers must rely on high clinical suspicion in at-risk patients. With the prevalence of NAFLD increasing in the United States(17), this issue may become more problematic in the future.
Consistent with prior studies demonstrating high levels of patient acceptance for HCC surveillance(15), our study suggests that patient-level factors, such as adherence, and system-level factors, including scheduling capacity, are not currently major barriers to HCC surveillance. However, careful process evaluation during intervention implementation will be crucial. Although failure to complete surveillance despite orders was only documented in 3% of patients, this could increase if interventions increased surveillance orders and created a larger burden on the radiology scheduling system.
We found that hepatology care was associated with receipt of HCC surveillance. A similar benefit was seen among patients from the SEER-Medicare database, in which 27.3% of patients receiving subspecialty care underwent surveillance compared to 10.7% of those only seen by primary care physicians(7). Given limited availability of subspecialty care in some areas, referring every cirrhotic patient to subspecialists is not a viable option. Currently, primary care physicians follow most cirrhotic patients, with only 20–40% being followed by gastroenterologists/hepatologists(7). Although we were unable to determine reasons for this disparity in surveillance rates, it may relate to differences in provider knowledge regarding the benefits of surveillance. Accordingly, educating primary care physicians about the importance of HCC surveillance in patients with cirrhosis is critical.
The other factor associated with HCC surveillance was alcohol abuse, with current drinkers being significantly less likely to undergo HCC surveillance. This association may be related to multiple factors, including clinic time constraints if these patients had more active issues, provider beliefs regarding benefits of surveillance in this subgroup, or provider beliefs regarding likelihood of adherence. The association between number of primary care visits and presence of surveillance orders suggests physicians are more likely to order surveillance with repeated opportunities and clinic time constraints may play a role. Further studies are necessary to better characterize effects of provider factors, such as attitudes and knowledge, on surveillance utilization.
Our study has several limitations. Our conclusions reflect a retrospective analysis of patients with HCC seen at a large urban safety-net hospital, and therefore may not be generalized to other practice settings. Further studies, with larger sample sizes, are necessary to identify other potential predictors of surveillance failure and to determine if our results are generalizable. Given its retrospective nature, our study was also limited by possible unmeasured confounders and missing data. Although some patients may have received surveillance at outside institutions, we believe this is unlikely given that Parkland, as the safety-net health system for Dallas County, is the only option for most indigent patients. To minimize this bias, we excluded patients with less than one year of care at Parkland prior to HCC diagnosis. The retrospective nature of our study could have also led to measurement bias, including inaccurate estimates of alcohol intake. Overall, we believe our study's limitations are outweighed by its strengths including its well-characterized cohort, its racially and socio-economically diverse population, and its large sample size followed over a two-year period. Most importantly, our study is the first to characterize surveillance process failures that explain why HCC surveillance is being underutilized- the first step to identifying appropriate intervention targets.
In conclusion, HCC surveillance is underutilized, with fewer than one in five patients receiving any surveillance and fewer than one in ten receiving consistent surveillance. Underutilization is related to multiple failure points in the HCC surveillance process including nearly 40% of patients having unrecognized liver disease and/or cirrhosis. However, the most common reason for a lack of HCC surveillance was failure to order HCC surveillance in patients with known cirrhosis. Future interventions will need to target multiple failure points in the HCC surveillance process to be highly effective.
Supplementary Material
Acknowledgements
None
Grant Support: This project was supported in part by grants KL2 RR024983-05 and the ACG Junior Faculty Development Award.
Financial Support: This project was supported in part by grants KL2 RR024983-04 and UL1 RR024982 and the ACG Junior Faculty Development Award
Footnotes
Conflicts of Interest: Dr. Singal is on the speaker's bureau for Onyx Pharmaceuticals
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2010 May;26(3):189–95. doi: 10.1097/MOG.0b013e3283383ca5. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, Rodes J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999 Jan;29(1):62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996 Mar 14;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010 doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009 Jul;30(1):37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010 Jul;52(1):132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, Conjeevaram HS, Fu S, Volk ML, Fontana RJ, Askari F, Su GL, Lok AS, Marrero JA. Surveillance ultrasound has a poor sensitivity for early stage HCC in American patients with cirrhosis. International Liver Cancer Association; Montreal, Canada: 2010. [Google Scholar]
- 9.Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of Hepatocellular Carcinoma Surveillance Among American Patients: A Systematic Review. 2011 doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003 Jan;12(1):4–13. [PubMed] [Google Scholar]
- 12.Holmes-Rovner M, Valade D, Orlowski C, Draus C, Nabozny-Valerio B, Keiser S. Implementing shared decision-making in routine practice: barriers and opportunities. Health Expect. 2000 Sep;3(3):182–91. doi: 10.1046/j.1369-6513.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005 Nov;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011 Jan 18;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Singal A, Volk M, Rakoski M, Fu S, Su G, McCurdy H, Marrero J. Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol. 2011;45(8):727–32. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 16.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008 Feb;121(2):119–26. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.