Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers owing to a number of characteristics including difficulty in establishing early diagnosis and the absence of effective therapeutic regimens. A large number of genetic alterations have been ascribed to PDAC with mutations in the KRAS2 proto-oncogene thought to be an early event in the progression of disease. Recent lineage-tracing studies have shown that acinar cells expressing mutant KrasG12D are induced to transdifferentiate, generating duct-like cells through a process known as acinar-ductal metaplasia (ADM). ADM lesions then convert to precancerous pancreatic intraepithelial neoplasia (PanIN) that progresses to PDAC over time. Thus, understanding the earliest events involved in ADM/PanIN formation would provide much needed information on the molecular pathways that are instrumental in initiating this disease. Since studying the transition of acinar cells to metaplastic ductal cells in vivo is complicated by analysis of the entire organ, an in vitro 3D culture system was employed to model ADM outside the animal. KrasG12D-expressing acinar cells rapidly underwent ADM in 3D culture, forming ductal cysts that silenced acinar genes and activated duct genes, characteristics associated with in vivo ADM/PanIN lesions. Analysis of downstream KRAS signaling events established a critical importance for the Raf/MEK/ERK pathway in ADM induction. Additionally, forced expression of the acinar-restricted transcription factor Mist1, which is critical to acinar cell organization, significantly attenuated KrasG12D-induced ADM/PanIN formation. These results suggest that maintaining MIST1 activity in KrasG12D-expressing acinar cells can partially mitigate the transformation activity of oncogenic KRAS. Future therapeutics that target both the MAPK pathway and Mist1 transcriptional networks may show promising efficacy in combating this deadly disease.
Keywords: Mist1, pancreatic cancer, lineage-tracing, signaling pathways, 3D tissue culture
INTRODUCTION
With a 5-year survival rate of <5% for patients with pancreatic ductal adenocarcinoma (PDAC), the disease remains one of the most lethal malignancies primarily due to late diagnosis and ineffective chemo and radiation therapies (1). Recent identification of three distinct PDAC precursors - pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm and intraductal papillary mucinous neoplasm - provides new hope for early detection and a much better understanding of the underlying mechanisms that are instrumental in the progression of the disease (2). Among these precursor lesions, PanINs are the best characterized (3). Indeed, a number of genetic mutations associated with PDAC have been detected in PanINs at various stages including mutations in the KRAS2, TP53 and SMAD4 genes which also are hallmarks of advanced PDAC, consistent with a PanIN → PDAC step-wise progression model (2). These observations are further supported by genetically engineered mouse models where PanIN/PDAC progression is initiated by single point mutations in the Kras gene and modified by additional genetic alterations in other oncogenes or tumor suppressors (4-10). However, the molecular mechanisms that govern each step of PanIN/PDAC progression remain elusive.
More controversial is the cellular origin of PDAC. PDAC has long been considered a disease of pancreatic ducts. Early efforts to model the disease by expressing Kras in pancreatic duct cells did not yield discenable pathology (11), although a recent study by Ray et al. (12) has shown that activation of endogenous KrasG12D in large pancreatic ducts leads to rare early PanIN lesions. Despite this example, increasing evidence supports the idea that PanIN/PDAC can originate from differentiated acinar cells, which represent the major cellular component of the pancreas parenchyma (10, 13-15). The development of duct-like PanIN lesions from acinar cells necessitates massive remodeling of these cells, both morphologically and with respect to gene expression profiles. The transition from acinar to ductal cell properties has been termed acinar-ductal metaplasia (ADM) and lineage-tracing studies have confirmed that this process results from direct transdifferentiation of adult acinar cells that convert to a duct cell phenotype upon KrasG12D expression (10, 13, 16). The relevance of ADM to PDAC is also supported by the observation that ADM is frequently associated with human PanIN lesions from PDAC patients (17, 18). Additionally, ADM development has been shown to precede PanIN formation in mouse KrasG12D models (17), suggesting that ADM represents the initial stage of PDAC development.
Although it is clear that KrasG12D expression in adult acinar cells can generate ADM lesions that progress to PanINs and PDAC, the molecular signaling pathways that are instrumental in these conversion events remain poorly defined. Indeed, KrasG12D expression alone does not guarantee ADM/PanIN development because in mouse PDAC models where ~95% of acinar cells express KrasG12D only a small cohort of cells generate detectable ADM/PanINs after a significant latent period (months), demonstrating that differentiated acinar cells are not universally responsive to KRAS signaling. This observation is compounded by the complexity of following ADM/PanIN development within the context of the intact organ. In an effort to develop an in vitro system to study the very earliest events of ADM formation, we focused on the initial transition of acinar cells converting to ductal cells upon KrasG12D expression in a 3D culture model. We found that KrasG12D-expressing acinar cells in 3D culture rapidly converted to ductal cells that mimicked properties associated with in vivo ADM and PanIN lesions, including silencing of acinar genes and expression of duct genes. Analysis of RAS signaling components demonstrated that activation of the Raf/MEK/ERK pathway was essential for KrasG12D-induced ADM. Furthermore, the ability of acinar cells to undergo a transdifferentiation process to ductal cells was significantly attenuated by forced expression of the acinar-restricted transcription factor Mist1, suggesting that maintaining acinar cell identity mitigates the strong oncogenic potential of KrasG12D expression. These results provide possible new therapeutic approaches to treat early ADM/PanIN cases by simultaneously targeting the MAPK pathway and Mist1 transcriptional networks.
RESULTS
KrasG12D expression converts pancreatic acini to ductal cysts in vitro
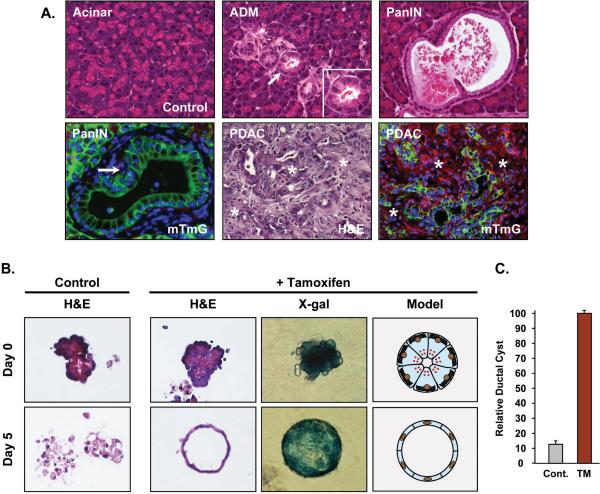
Kras mutations are virtually universal in human PDAC, although identification of the initial adult cell (duct, acinar, centroacinar) that acquires the mutation and initiates the transformation cascade remains unknown. Recent studies examining pancreatic cancer in mouse models have shown that Kras oncogene expression in adult acinar cells leads to PDAC that fully mimics the human disease (10, 13, 15, 16). Lineage-tracing of Elastase-CreER; LSL-KrasG12D/+; R26RmTmG mice confirmed that activation of KrasG12D in adult acinar cells induces acinar-ductal metaplasia (ADM), which can progress to pancreatic intraepithelial neoplasia (PanIN) and on to PDAC (Figure 1A).
Figure 1.
Acinar-specific KrasG12D expression leads to ADM → PanIN → PDAC. (A) Adult Elastase-CreER; LSL-KrasG12D/+; R26RmTmG and Elastase-CreER; LSL-KrasG12D/+; LSL-Trp53R172H; R26RmTmG mice were given corn oil (control) or TM and pancreas sections were processed for standard histology after 1-5 months. The earliest lesions to appear are ADM (inset) followed by PanINs and then PDAC. R26mTmG lineage tracing confirms that ADM, PanINs and PDAC are derived from KrasG12D-expressing acinar cells (green). Red signal represents unrecombined R26mTmG stromal cells. arrows - ADM and PanIN lesions; asterisks - adjacent stromal tissue. (B) Mist1CreER/+; LSL-KrasG12D/+; R26RLacZ acinar cells were isolated from 7 day post-treated mice and individual acini were cultured for 5 days in a 3D collagen matrix. The majority of control acinar cells undergo extensive cell death by 5 days, whereas KrasG12D-expressing acinar cells rapidly transdifferentiate into ductal cysts (H&E sections) that retain β-gal expression (whole mount X-gal staining). Identical results were obtained with Elastase-CreER; LSL-KrasG12D/+; R26RmTmG mice. (C) Whereas corn oil treated control Mist1CreER/+; LSL-KrasG12D/+; R26RLacZ acinar cells generate very few ductal cysts, TM-treated acinar cells generate a large number of ductal cysts in 3D collagen matrix.
Despite this fundamental process of initiating a transformation pathway, it has been difficult to study the intricacies of ADM → PanIN → PDAC in the intact animal. In an effort to dissect the initial KrasG12D-dependent pathways in vitro, we asked if ADM could be modeled in tissue culture. Previous studies showed that wildtype acinar cells treated with TGFα and maintained in a 3D collagen matrix converted to duct-like cysts that activated EGFR downstream signaling pathways, including Ras (4, 19). To determine if constitutive KRAS activity could similarly lead to ductal cyst formation, Mist1CreER/+; LSL-KrasG12D/+; R26RLacZ or Elastase-CreER; LSL-KrasG12D/+ mice were treated with corn oil (control) or tamoxifen (TM) to activate acinar-specific KrasG12D expression. Pancreata were then harvested 7 days post-treatment, digested with collagenase and individual acini clusters placed in a 3D collagen matrix. As expected, control and KrasG12D-expressing acinar cells initially retained their normal apical-basal polarity with the rER accumulating at the basal aspect and zymogen granules clustered at the apical pole (Figure 1B). However, by 5 days in 3D culture there was a dramatic difference in cell viability between the KrasG12D-expressing and non-expressing cell populations. Control acinar cells rapidly became disorganized and underwent massive cell death. In contrast, a high proportion (~70-90%) of KrasG12D-expressing acinar cells survived and converted to ductal cyst structures that were comprised of a single layer of epithelial cells surrounding an empty luminal space, resembling the metaplastic ADM/PanIN lesions observed in vivo (Figure 1A,B). Interestingly, these metaplastic cysts were virtually identical to structures formed by primary pancreatic duct cells (20). Lineage tracing using the R26RLacZ reporter allele confirmed that the ductal cysts were derived via transdifferentiation of mature KrasG12D-expressing acinar cells (Figure 1B). In agreement with a recent study by Heid et al. (21), ductal cyst conversion was dependent on KrasG12D activity as the vast majority of control acinar cells (-TM) failed to convert to ductal cysts over the duration of the experiment (Figure 1C).
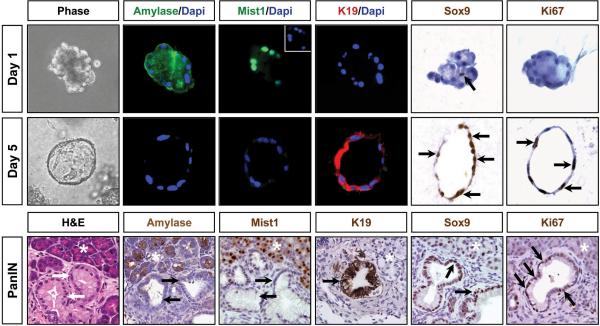
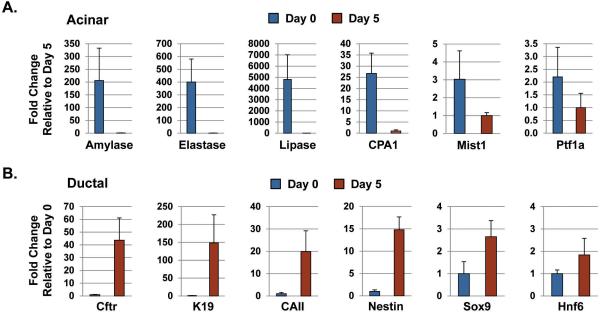
The profound morphological changes in the 3D cultures were reminiscent of the in vivo changes that take place in the whole organ upon KrasG12D activation. Indeed, a dramatic switch from acinar to ductal gene expression profiles occurred during the 5 day culture period. At day 0, individual acini expressed high levels of amylase, elastase, carboxypeptidase and lipase, with virtually no detectable expression of duct gene products including keratin 19 (K19), cystic fibrosis transmembrane conductance regulator (CFTR) and carbonic anhydrase II (CAII) (Figure 2; 3), confirming the purity of the starting acinar cell preparation. As expected, these cells also expressed high levels of the acinar-specific transcription factors Ptf1a and Mist1 (Figure 2; 3), with Mist1 being essential to maintaining an adult acinar cell phenotype (22). However, by day 5 KrasG12D-induced ductal cysts exhibited a dramatic shift in their gene expression profiles that mirrored the expression pattern of PanIN lesions. Ductal cysts and PanINs uniformly silenced acinar genes and activated expression of duct-specific genes, including CFTR, K19, CAII and the duct-restricted transcription factors Sox9 and HNF6 (Figure 2; 3; S1; S2). These cells also expressed Nestin (Figure 3; S2), a marker of ductal cells in early PanINs (23). As predicted, PanINs in Mist1CreER/+; LSL-KrasG12D/+ pancreata were highly proliferative and a similar proliferation index was observed with the KrasG12D-induced ductal cysts (Figure 2; S3). The parallel between ductal cyst formation in vitro and ADM/PanIN genesis in vivo suggests that the 3D acinar culture faithfully mimics the intracellular pathways that regulate acinar-ductal metaplasia.
Figure 2. KrasG12D-expressing acinar cells undergo conversion to a ductal phenotype when maintained in 3D collagen matrix.
Fluorescence and light microscopy reveals that KrasG12D acinar cells express acinar gene products at day 1 but rapidly convert to a ductal cell phenotype with activation of K19 and Sox9 expression. Inset for day 1 Mist1 panel shows the Dapi stained nuclei for this acinus. Note that a single centroacinar cell (arrow) in an isolated acinus is SOX9 positive. Pancreas serial sections from experimental littermates confirm the absence of AMYLASE and MIST1 and the expression of SOX9 and K19 in acinar-derived PanINs (arrows). Both PanINs and ductal cysts are also Ki67 positive (arrows). asterisks - adjacent acinar tissue.
Figure 3. KrasG12D-induced ductal cyst conversion leads to repression of the acinar gene program and activation of the ductal gene program.
(A,B) RT-qPCR of day 0 and day 5 cultures confirms that KrasG12D acinar cells express high levels of acinar gene products at day 0 but rapidly down-regulate acinar genes and up-regulate duct genes by day 5, a time that corresponds to maximum ductal cyst formation.
MAPK activity is elevated in PanINs and is essential for ductal cyst formation
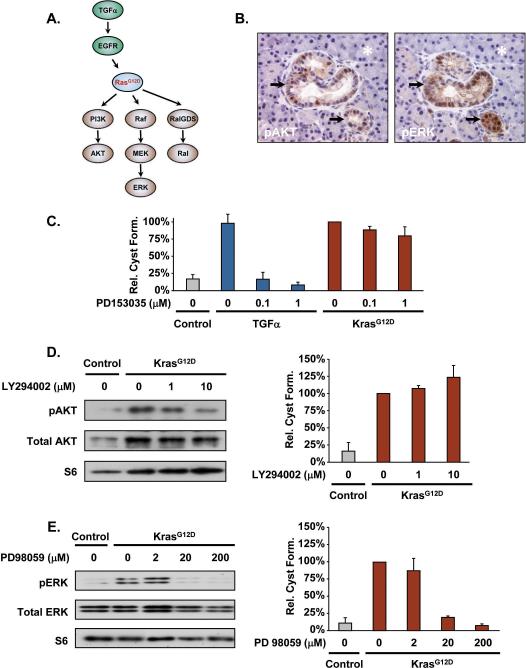
It is well established that KrasG12D expression induces cellular transformation by activating downstream signaling events, including the Raf/MEK/ERK, PI3K/AKT and RalGDS/Ral pathways (Figure 4A). Although these pathways have been the subject of potential therapeutic benefit against pancreatic tumors (24, 25), little is known about the role they have in the earliest transformation events of ADM and PanIN formation. In an effort to dissect the importance of the PI3K/AKT and Raf/MEK/ERK pathways prior to tumor formation, we examined Mist1CreER/+; LSL-KrasG12D/+ (HET/Kras) pancreata 3 months post-TM to ascertain if ADM/PanIN regions exhibited elevated KRAS signaling. Interestingly, despite the fact that ≥90% of adult acinar cells express KrasG12D, only regions of ADM/PanIN exhibited sufficiently high pAKT and pERK levels that could be detected by immunohistochemistry (Figure 4B). Thus, expression of KrasG12D in acinar cells (for up to 3 months) is not sufficient to uniformly activate the PI3K/AKT and Raf/MEK/ERK arms of the KRAS pathway in all cells.
Figure 4. The Raf/MEK/ERK pathway is essential to early ADM/PanIN formation in vitro and in vivo.
(A) Canonical upstream and downstream pathways associated with Kras. (B) ADM/PanIN lesions (arrows) in the HET/Kras pancreas exhibit elevated levels of pAKT and pERK. Note that the majority of acinar cells (asterisks) in HET/Kras pancreata are KrasG12D positive but pAKT/pERK negative. (C) TGFα treatment of control wildtype acinar cells leads to ductal cyst formation in 3D culture. As expected, inhibition of EGFR activity (PD153035 treatment) blocks TGFα-dependent ductal cyst formation but has no effect on the downstream KrasG12D pathway of HET/Kras acinar cells. (D) Inhibition of PI3K by LY294002 blocks pAKT activity but does not inhibit ductal cyst formation. (E) The MEK inhibitor PD98059 efficiently blocks pERK activity in HET/Kras acinar 3D cultures. Inhibition of MEK also leads to complete inhibition of KrasG12D-induced ductal cyst formation.
To examine the importance of the pAKT and pERK pathways in converting acinar cells to ADM, we investigated these KRAS downstream pathways in the 3D culture model. As an initial test, we examined the importance of the upstream EGFR pathway with respect to KrasG12D-induced ductal cyst formation. As previously reported (4, 19, 21), wildtype acinar cells placed in 3D culture exhibited only background levels of ductal cysts. However, when cells were provided TGFα, rapid ductal cyst formation occurred (Figure 4C). As expected, inhibition of EGFR by the EGFR tyrosine kinase inhibitor PD153035 completely blocked ductal cyst formation of wildtype acinar cells but had no effect on HET/Kras ductal cyst formation, confirming that KRAS functions downstream of EGFR (Figure 4A,C).
We next examined the importance of the PI3K/AKT and Raf/MEK/ERK pathways for ductal cyst formation in Het/Kras mice. As predicted, elevated pAKT was observed in KrasG12D-isolated acinar cells when compared to control cells isolated from corn oil treated littermates (Figure 4D). Treatment of the cultures with the PI3K inhibitor LY294002 led to a ~70% reduction in pAKT levels but had no significant effect on ductal cyst formation in the 3D culture model. Likewise, 90% reduction of pAKT levels by the PI3K inhibitor VIII produced only modest decreases in ADM conversion (Figure S4). Together, these results suggest that the PI3K/AKT pathway is not required for, nor essential to, acinar-ductal metaplasia.
A similar analysis was performed on the Raf/MEK/ERK pathway. Whereas control cells exhibited low levels of pERK, Het/Kras acinar cells had elevated pERK levels (Figure 4E). Treatment of cells with the MEK inhibitor PD98059 efficiently blocked accumulation of pERK at concentrations as low as 20 μM. Interestingly, inhibition of MEK activity perfectly correlated with decreased ductal cyst formation (Figure 4E). We propose that activation of the Raf/MEK/ERK pathway in a subset of KrasG12D-expressing acinar cells is required to initiate an ADM response in mouse models of PDAC.
Sustained Mist1 expression suppresses ductal cyst formation in vitro and ADM/PanIN formation in vivo
KrasG12D-expressing acinar cells rapidly lose their acinar characteristics as they acquire a duct-like phenotype (13, 17). An early event of this switch is silencing the Mist1 locus, which encodes a basic helix-loop-helix transcription factor critical to acinar cell differentiation (4, 17). Mist1 is highly expressed in pancreatic acinar cells and its expression correlates with cellular differentiation and the establishment of the secretory machinery (22, 26). Previous studies have shown that human acinar cells lose Mist1 expression upon ADM/PanIN formation (4, 17), and in mouse models devoid of the Mist1 gene, KrasG12D-expressing acinar cells rapidly undergo ADM and develop extensive PanIN lesions through a pathway that involves elevated pERK activity (4, 27). Therefore, we hypothesized that MIST1 promotes acinar cell differentiation and suppresses the oncogenic activity of Kras by keeping pERK levels low. Given that ADM is dependent on MAPK activity (Figure 4E), we examined if sustained Mist1 expression could restrict the conversion of KrasG12D-expressing acinar cells to ductal cysts.
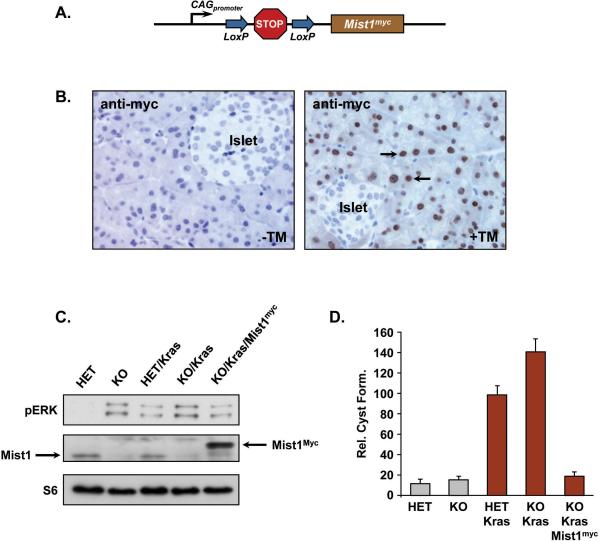
To test this concept, a transgenic mouse line (LSL-Mist1myc) that produces constitutive Mist1 expression upon Cre-mediated recombination was generated (Figure 5A). Transgene expression of myc-tagged Mist1 was driven by a CMV early enhancer/chicken β-actin hybrid promoter, whose activity is independent of the differentiation status of cells (28). When Mist1CreER/CreER; LSL-KrasG12D/+; LSL-Mist1myc (KO/Kras/Mist1myc) mice were treated with TM, Mist1myc expression was observed in >90% acinar cells with no expression detected in duct cells or in corn oil treated (-TM) control mice (Figure 5B). Next, we examined MAPK activity in pancreata +/- KrasG12D and Mist1 expression. As shown in Figure 5C, loss of Mist1 (KO) led to elevated pERK levels, even in the absence of KrasG12D expression. However, sustained Mist1myc expression in the KO/Kras/Mist1myc pancreata generated lower pERK levels when compared to KO/Kras littermates (Figure 5C). We then examined how constitutive Mist1myc expression influenced ductal cyst conversion in 3D cultures. As expected, Mist1+/- (HET) and Mist1-/- (KO) acini generated few ductal cysts when placed in collagen matrix for 5 days (Figure 5D). In contrast, TM-treated Mist1CreER/+; LSL-KrasG12D/+ (HET/Kras) acini rapidly converted to ductal cysts and the percentage of conversion increased when Mist1CreER/CreER; LSL-KrasG12D (KO/Kras) acinar cells were tested (Figure 5D; S5). Importantly, when KO/Kras/Mist1myc acini were examined, ductal cyst formation was reduced to levels approaching those observed in KrasG12D-negative (Mist1+/-, Mist1-/-) cells (Figure 5D; S5). Similar results were obtained with the TGFα model where Mist1myc expression blocked TGFα-induced ductal cyst formation of Mist1-/- acinar cells (Figure S6). These results demonstrate that sustained Mist1 expression lowers pERK levels and protects acinar cells from TGFα- or KrasG12D-induced ADM formation in vitro.
Figure 5. Sustained Mist1 expression suppresses KrasG12D-induced ductal cysts.
(A) Schematic diagram of the LSL-Mist1myc transgene. (B) Anti-myc immunohistochemistry of KO/Kras/Mist1myc pancreata reveals extensive acinar-specific expression of LSL-Mist1myc in tamoxifen-treated mice (arrows). (C) Immunoblot of pERK activity in HET vs. KO and KO/Kras vs. KO/Kras/Mist1myc pancreata. The absence of Mist1 leads to increased pERK activity while sustained Mist1myc expression reduces pERK levels in KO/Kras/Mist1myc pancreata. (D) Quantification of ductal cyst formation in control and KrasG12D-expressing cells. Mice were treated with TM and acinar cells placed in 3D culture as described in Materials and Methods. Sustained Mist1myc expression dramatically inhibits KrasG12D-induced ductal cyst formation.
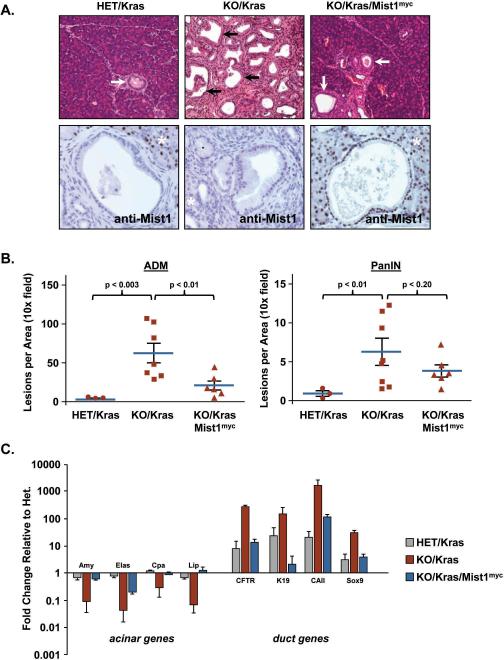
Finally, to determine if sustained Mist1 expression could similarly deter ADM/PanIN development in vivo, adult KO/Kras/Mist1myc mice and their littermate controls were treated with TM and pancreata were examined three months post-TM for the presence of ADM and PanIN lesions. HET/Kras mice developed rare focal areas of ADM and PanIN lesions at 3 months and in all cases the Mist1 locus was transcriptionally silenced in acinar-derived PanINs and in advanced ADM lesions (Figure 6A,B). As expected, KO/Kras pancreata developed organ-wide extensive ADM/PanINs owing to the absence of Mist1 prior to KrasG12D activation (Figure 6A,B). In contrast, sustained Mist1myc expression greatly attenuated ADM/PanIN development in the KO/Kras/Mist1myc mice as the majority of the organ consisted of normal appearing acinar tissue with only isolated areas of ADM/PanINs (Figure 6A,B). Indeed, the number of PanINs that developed in KO/Kras/Mist1myc mice and reported in Figure 6B actually represent an upper limit because not all PanINs (~18%) were MIST1myc positive, presumably due to selected Cre-mediated recombination of the LSL-KrasG12D locus and not the LSL-Mist1myc transgene. Thus, a subset of KrasG12D-expressing acinar cells did not have an opportunity to respond to sustained MIST1 protein. However, despite the clear protective effect of Mist1myc expression, MIST1myc protein per se did not fully inhibit KrasG12D-induced PanINs as MIST1myc+ PanINs were still generated, albeit at a greatly reduced level (Figure 6A,B). The MIST1myc-dependent reduction in ADM/PanIN formation was also reflected in the expression profiles of key acinar vs. ductal genes where MIST1myc significantly reduced ductal gene activity while maintaining acinar gene expression to levels approaching, or exceeding, those obtained with HET/Kras mice (Figure 6C). These results are consistent with MIST1 exerting an anti-ADM/PanIN activity in the intact pancreas and support the concept that constitutive Mist1 expression mitigates the activity of oncogenic KrasG12D in acinar cells, leading to a reduction in ADM/PanIN formation.
Figure 6. Sustained Mist1myc expression inhibits ADM and PanIN formation in vivo.
(A) PanIN formation (arrows) is greatly accelerated in KO/Kras pancreata but suppressed by sustained Mist1myc expression in KO/Kras/Mist1myc mice. Acinar-derived PanINs are Mist1 negative in HET/Kras mice. Interestingly, induced Mist1myc expression alone is not sufficient to inhibit all PanIN formation since some MIST1myc+ PanINs still develop in KO/Kras/Mist1myc pancreata. asterisks - adjacent acinar tissue. (B) Quantification of ADM and PanIN lesions in HET/Kras, KO/Kras and KO/Kras/Mist1myc samples. Note that constitutive Mist1myc leads to significant reductions in ADM/PanIN formation. (C) Analysis of gene expression profiles by RT-qPCR confirms the reduced ADM/PanIN formation in KO/Kras/Mist1myc samples when compared to KO/Kras littermates. All values were normalized to the control HET samples, which were set to 1.0.
DISCUSSION
The role of acinar cells in pancreatic tumorigenesis has long been suspected, but definitive evidence had been lacking until lineage tracing studies established that PanINs and PDAC can arise from adult acinar cells (10, 13, 16). These studies also implicated the process of acinar-ductal metaplasia as a bridge between normal acinar cells and duct-like PanINs. Consistent with this hypothesis, ADM occurrence precedes PanIN development in PDAC mouse models (17). Despite the central role for KrasG12D in inducing ADM, Kras mutations are not a prerequisite. Mutant Kras is often detected in ADM and low-grade PanIN lesions in human diseased organs but these mutations are not universally present (2, 29), suggesting that additional underlying events can influence ADM formation. Indeed, exposure to EGFR growth factors leads to metaplastic lesions in vitro and in vivo in the absence of active Kras (30, 31). Similarly, EGFR activity is a hallmark of Kras-induced ADM/PanIN lesions (17), implying a central role for this pathway in Kras-dependent and Kras-independent ADM. ADM is also commonly associated with acute and chronic pancreatitis in both humans and rodents (18, 32, 33), suggesting that inflammation and/or cell damage can simultaneously generate an ADM response without Kras activity. Although a number of studies have shown that metaplastic cells in pancreatitis settings eventually resume an acinar cell phenotype (33, 34), the presence of Kras mutations efficiently redirects metaplastic cells toward PanIN development (15, 35). These results, together with the observation that chronic pancreatitis increases the risk for PDA (36), underscore the relevance of ADM in pancreatic tumorigenesis and the need to elucidate the molecular mechanisms that regulate the fate of metaplastic cells.
In this study, we adopted a collagen matrix 3D culture assay to model the conversion of mature acinar cells to ductal metaplastic cells within a compressed timeframe (5 days vs. months). As expected, expression of the KrasG12D oncogene promoted formation of duct-like cysts, with an efficacy similar to treatment of wildtype acinar cells with exogenous TGFα (4, 19, 21). Further analysis confirmed that the starting cell preparation was highly enriched for amylase-expressing acinar cells, which converted to K19-expressing ductal cells in the presence of KrasG12D. Using inhibitors that specifically blocked individual signaling components, we also demonstrated that Raf/MEK/ERK represent a critical downstream effector pathway through which KRAS operates to induce ADM. Interestingly, BrafV599E and KrasG12D, the predominant raf and ras gene mutations, respectively, in pancreatic cancer operate in a mutually exclusive pattern in pancreatic tumors, suggesting that upstream (KrasG12D) or downstream (BrafV599E) effectors of the Raf/MEK/ERK pathway exhibit interchangeable functions in converting acinar cells to ADM lesions (37). Recent lineage-tracing studies of primary human acinar cells also revealed that surviving acinar cells undergo an ADM response that is dependent on MAPK activity (38). Likewise, we have found that TGFα-induced ADM is also dependent on the Raf/MEK/ERK pathway (unpublished results), revealing that these downstream effectors represent a common regulator of ADM in mouse and human Kras-dependent and Kras-independent settings. Future studies will test if individual dominant-negative mutants of the MAPK pathway similarly block ADM in models of tumorigenesis and pancreatitis.
While these studies were in progress, Heid et al. (21) reported that acinar cells isolated from Ptf1aCre/+; LSL-KrasG12D mice were also capable of producing ductal cysts in 3D culture. Additionally, conditional deletion of Rac1 significantly blocked ductal cyst formation in vitro and PanIN development in vivo. This was a surprising finding as Rac1 is often thought to be activated by the PI3K pathway which we showed by selective inhibition to be nonessential for ADM formation. However, a number of studies have shown that Rac can also be activated in a PI3K-independent fashion via the Raf-specific guanine exchange factor Tiam1 (39, 40). Importantly, Tiam1 null mice are resistant to Ras-induced tumor formation (41), confirming the importance of the Tiam1-Rac1 axis for the Ras signaling pathway. Thus, our study and the work by Heid et al. (21) strongly suggest that there are two key pathways (MAPK and Rac1) that are instrumental in Kras-induced ADM and that both pathways are essential to converting normal acinar cells into ADM and PanIN lesions. Future PDAC therapeutic strategies that target both pathways are likely to have higher efficacy than approaches targeting only a single effector.
Are there other ways to block ADM? We can find some leads in studies of pancreatitis, where most ADM spontaneously heals and re-differentiates in the absence of KrasG12D signaling. For example, β-catenin activation following acute pancreatitis is required for acinar cell regeneration, while stabilized β-catenin protects acinar cells against Kras--induced PanIN formation (16). Similarly, Mist1 exhibits a protective role not only in KrasG12D-induced ADM but also in ADM associated with acute pancreatitis, where Mist1 null pancreata endure more severe damage following caerulein treatment (42). At this time, it is unclear how Mist1 influences ADM decisions. One possibility is that MIST1 protects acinar cells from metabolic stress associated with pancreatitis or KrasG12D expression by regulating aspects of the MAPK pathway. Indeed, Mist1 is silenced in pancreatitis-associated acinar cells (unpublished results) and Mist1KO acinar cells exhibit increased MEK and ERK phosphorylation (4). Given the central role of the MAPK pathway in ADM and the observation that pancreatitis enhances KrasG12D-induced tumorigenesis (15, 35, 43), it is likely that Mist1 inhibits ADM by maintaining basal levels of these MAPK effectors. Since Mist1 is influential in converting embryonic stem cells to an acinar cell fate (44), sustained Mist1 expression also likely keeps key acinar characteristics intact in KrasG12D cells. Further investigation into the mechanisms of this regulatory network and identification of MIST1 target genes should lead to better therapeutic interventions and improved clinical outcomes for PDAC patients.
Materials and Methods
Mouse strains and genotyping
Mist1CreER/+, LSL-KrasG12D/+, R26RLacZ and R26RmTmG reporter mouse lines have been described previously (4, 13, 45). Elastasepr-CreER mice were generated according to standard protocols using a previously reported Elastasepr-Mist1MB construct where the Mist1MB coding region was replaced by a CreERT2 coding region to drive acinar-specific expression of CreERT2 (46). LSL-Mist1myc transgenic mice were generated using the pCAG-LoxP-CAT-LoxP-LacZ construct in which the LacZ gene was replaced by a myc-tagged Mist1 coding sequence (47). Induction of CreERT2 activity was accomplished by providing adult mice (6-9 wk) tamoxifen (TM, 4 mg/mouse/day) for 2-3 consecutive days. Genotyping primer sets are listed in Supplemental Table I. All animal studies were conducted in compliance with NIH and the Purdue University IACUC guidelines.
3D acinar cell culture
Mice were treated with TM 7 days prior to harvesting the pancreas. Immediately after sacrificing, pancreata were rinsed in cold 1X HBSS (Invitrogen, Carlsbad, CA) and cut into small pieces with scissors. Primary acini were released by collagenase P (Roche Applied Science, Mannheim, Germany) digestion (200 μg/ml in 1x HBSS) for 10-20 minutes at 37°C. Isolated acini were washed 3 times in cold 5% FBS, 1x HBSS and then filtered sequentially through 500 μm and 105 μm nylon meshes (Spectrum Laboratories, Rancho Dominguez, CA). Cell suspensions were carefully layered on top of 30% FBS, 1x HBSS and acini were collected by centrifugation (1000 rpm, 2 min at 4°C) and then resuspended in 8 ml of 3D culture base medium (RPMI, 10% fetal bovine serum, 0.1 mg/ml soybean trypsin inhibitor, 1 μg/ml dexamethasone and antibiotics). 24-well tissue culture plates were coated with a 250 μl/well collagen layer (100 μl 10x RPMI, 900 μl 3mg/ml collagen, neutralized with either 4.2% NaHCO3 or 0.34N NaOH) at least one hr prior to acini isolation. The cell suspension was then mixed with collagen 1:1 and plated (0.5 ml per well). The cell-collagen mix was allowed to solidify for 1 hr at 37°C before adding 1 ml 3D culture media. Media was changed on days 1 and 3. On day 5 the percentage of acini that converted to ductal cysts was calculated by counting individual clusters in all wells. Collagen matrix was then digested for 10-15 min at 37°C with 200 μg/ml collagenase P in 1x HBSS to release cells. RNA was prepared using the E.Z.N.A. total RNA kit (Omega Bio-Tek, Norcross, GA). Alternatively, collagen discs were fixed in 4% formaldehyde, embedded in paraffin and 5 μm sections were prepared for standard immunohistochemistry as described below.
Histology and immunohistochemistry
Mouse pancreata were fixed in 10% neutral buffered formalin, paraffin embedded, sectioned to 5 μm and stained using conventional hematoxylin and eosin. Sections were deparaffinized and retrieved using the 2100-Retriever (PickCell Laboratories, Amsterdam, The Netherlands) and antigen unmasking solution (Vector Laboratories, Burlingame, CA). Samples were blocked using the MOM blocking reagent (Vector Laboratories) and incubation of primary antibodies was conducted for 1 hr at room temperature or overnight at 4°C. Biotinylated secondary antibodies were applied for 10 min at 25°C. Visualization was accomplished via 3,3'-Diaminobenzidine peroxidase staining or tertiary avidin-conjugated fluorescent antibodies. Primary antibodies and conditions are provided in Supplemental Table II.
Protein immunoblots
Twenty μg of whole cell protein extracts were separated on 12% acrylamide gels, transferred to PVDF membranes and incubated with primary antibodies (antibody conditions are provided in Supplemental Table III). Immunoblots were developed using an ECL kit (Thermo Scientific, Waltham, MA).
RT-qPCR gene expression analysis
Pancreas RNA or RNA from 3D cultures was isolated using the E.Z.N.A. total RNA kit and reverse transcribed using the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). Equal amounts of cDNA reactions were amplified with FastStart Universal SYBR Green Master (Roche Applied Science, Indianapolis, IN) using the primer sets listed in Supplemental Table IV. Target sequences were amplified with 95°C/30 sec, 59°C/60 sec, 72°C/30 sec conditions. Fold changes in gene expression between TM-treated and control animals or between day 0 and day 5 3D cultures were calculated using the comparative 2-ΔΔCt method. Error bars represent the S.E.M.
Supplementary Material
Acknowledgements
We thank Judy Hallett of the Purdue Center for Cancer Research Transgenic Mouse Core Facility for generating the transgenic mouse strains used in this study and David Hess and Anju Karki for critical reading of the manuscript. This work was supported by grants to SFK (NIH CA124586, NIH DK55489 and the Phi Beta Psi Sorority for Cancer Research) and to DD (CTSI Predoctoral Fellowship).
Abbreviations
- ADM
acinar-ductal metaplasia
- AMY
amylase
- β-gal
β-galactosidase
- CAII
carbonic anhydrase II
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPA
carboxypeptidase
- Cre-ER
Cre recombinase-estrogen receptor
- Elas
elastase
- Het
Mist1 heterozygous
- K19
keratin 19
- KO
Mist1 homozygous null
- MAPK
mitogen-activated protein kinase
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PI3K
phosphatidylinositol 3-OH kinase
- RT-qPCR
reverse transcriptase-quantitative polymerase chain reaction
- TM
tamoxifen
Footnotes
Author Contributions: GS, DD, CQ, DB and DM designed and performed experiments; SFK designed and analyzed experiments and GS and SFK wrote the manuscript.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005 Mar;12(2):81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia - A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001 May;25(5):579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009 Apr;136(4):1368–78. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4(2):111–20. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005 May;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Bardeesy N, Aguirre AJ, Chu GC, Cheng K-h, Lopez LV, Hezel AF, et al. Both p16Ink4a and the p19Arf-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proceedings of the National Academy of Sciences. 2006 Apr 11;103(15):5947–52. doi: 10.1073/pnas.0601273103. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardeesy N, Cheng K-h, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes & Development. 2006 Nov 15;20(22):3130–46. doi: 10.1101/gad.1478706. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant Pancreatic Activation of Kras(G12D) and Tgfa Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN. Cancer Cell. 2007 Sep;12(3):266–79. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.De La OJ-P, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proceedings of the National Academy of Sciences. 2008 Dec 2;105(48):18907–12. doi: 10.1073/pnas.0810111105. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, et al. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003 May 1;63(9):2005–9. [PubMed] [Google Scholar]
- 12.Ray KC, Bell KM, Yan J, Gu G, Chung CH, Washington MK, et al. Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One. 2011;6(2):e16786. doi: 10.1371/journal.pone.0016786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008 Dec 2;105(48):18913–8. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander SYG, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, et al. Context-Dependent Transformation of Adult Pancreatic Cells by Oncogenic K-Ras. Cancer Cell. 2009;16(5):379–89. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007 Mar;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Morris JP, Cano DA, Sekine S, Wang SC, Hebrok M. [beta]-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. The Journal of Clinical Investigation. 2010;120(2):508–20. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007 Jul;171(1):263–73. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. The American journal of surgical pathology. 2006 Sep;30(9):1067–76. [PMC free article] [PubMed] [Google Scholar]
- 19.Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr., et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005 Aug 15;132(16):3767–76. doi: 10.1242/dev.01925. 2005. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA, et al. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004 Jul;127(1):250–60. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 21.Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, et al. Early requirement of rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011 Aug;141(2):719–30. e7. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001 Nov 12;155(4):519–30. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4437–42. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & Development. 2006 May 15;20(10):1218–49. doi: 10.1101/gad.1415606. 2006. [DOI] [PubMed] [Google Scholar]
- 25.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007 Jan;134(1):211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 27.Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006 Jan 1;66(1):242–7. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 28.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochemical and biophysical research communications. 1997 Aug 18;237(2):318–24. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Hong SM, Lim P, Kamiyama H, Khan M, Anders RA, et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res. 2009 Feb;7(2):230–6. doi: 10.1158/1541-7786.MCR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–35. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 31.Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998 Nov;115(5):1254–62. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- 32.Detlefsen S, Sipos B, Feyerabend B, Kloppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Archiv : an international journal of pathology. 2005 Nov;447(5):800–5. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 33.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007 Dec;133(6):1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005 Mar;128(3):728–41. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochemical and biophysical research communications. 2009 Mar 16; doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002 Dec;51(6):849–52. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calhoun ES, Jones JB, Ashfaq R, Adsay V, Baker SJ, Valentine V, et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) Mutations in Distinct Subsets of Pancreatic Cancer: Potential Therapeutic Targets. The American Journal of Pathology. 2003;163(4):1255–60. doi: 10.1016/S0002-9440(10)63485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houbracken I, de Waele E, Lardon J, Ling Z, Heimberg H, Rooman I, et al. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011 Aug;141(2):731–41. e4. doi: 10.1053/j.gastro.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 39.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002 Aug;4(8):621–5. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 40.De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J. 2002 Dec 16;21(24):6781–90. doi: 10.1093/emboj/cdf680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002 Jun 20;417(6891):867–71. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 42.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1123–32. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 43.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis accelerates initiation and progression to pancreatic cancer in mice expressing oncogenic kras in the nestin cell lineage. PLoS One. 2011;6(11):e27725. doi: 10.1371/journal.pone.0027725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rovira M, Delaspre F, Massumi M, Serra SA, Valverde MA, Lloreta J, et al. Murine embryonic stem cell-derived pancreatic acinar cells recapitulate features of early pancreatic differentiation. Gastroenterology. 2008 Oct;135(4):1301–10. 10, e1–5. doi: 10.1053/j.gastro.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003 Dec;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004 Apr;24(7):2673–81. doi: 10.1128/MCB.24.7.2673-2681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araki K, Araki M, Miyazaki J, Vassalli P. Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):160–4. doi: 10.1073/pnas.92.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.