Abstract
Though the comorbidity between borderline personality disorder (BPD) and substance abuse is well-established, there are few longitudinal studies that examine its developmental origins or whether the comorbidity is due to common genetic or environmental risk factors. To fill this gap, we utilized a large sample of female adolescent twins (N = 1280) to examine the developmental course, reciprocal influences, and the genetic and environmental factors underlying the co-occurrence of BPD traits and substance use from age 14 to 18. Rank-order stability was moderate to high for both BPD traits (r = .58) and substance use (r = .51), while mean-levels of substance use increased substantially from age 14 to 18 (d = .77) and BPD traits showed a small decline (d = −.21). BPD traits and substance use exhibited concurrent and prospective associations; however, the longitudinal associations dropped to non-significance after accounting for the temporal stability of each trait. Twin analyses revealed that shared environmental factors accounted for the association between BPD traits and substance use at age 14, but genetic factors account for the association at age 18. These results indicate that, at least in adolescence, the comorbidity between BPD traits and substance use is a consequence of common risk factors rather than due to one being a casual antecedent of the other.
Keywords: Borderline Personality Disorder, Substance Use, Longitudinal Change, Development, Behavioral Genetics
There is a strong association between borderline personality disorder (BPD) and substance use and substance use disorders (SUDs) (Trull, Sher, Minks-Brown, Durbin, & Burr, 2000). Compared to people without a BPD diagnosis, individuals that meet criteria for BPD 5 to 10 times more likely to meet criteria for a lifetime drug or alcohol dependence diagnosis (Grant et al., 2008; Trull, Jahng, Tomko, Wood, & Sher, 2010). Longitudinal studies have also shown that psychiatric patients with BPD are 2 to 3 times more likely to develop a new SUD diagnosis, compared to patients with other personality disorders (Links, Heslegrave, Mitton, Vanreekum, & Patrick, 1995; Stepp, Trull, & Sher, 2005; Tragesser, Sher, Trull, & Park, 2007; Tragesser, Trull, Sher, & Park, 2008; Trull, Waudby, & Sher, 2004). These results extend to subclinical BPD as well. Studies with samples of community young adults have found that BPD traits predict alcohol use-related problems two years later (Stepp, et al., 2005; Tragesser, et al., 2007). Especially important is that relative to individuals with only a BPD or SUD diagnosis in isolation, individuals with comorbid BPD and SUDs exhibit a more severe and persistent course; more criminal, health risk (e.g., intravenous needle use), and suicidal behaviors; worse treatment compliance and higher rates of premature treatment termination and relapse (Bornovalova & Daughters, 2007; Bosch van den, Verheul, Schippers, & Brink van den, 2002; Darke et al., 2007; Linehan et al., 1999; Links, et al., 1995; Martinez-Raga, Marshall, Keaney, Ball, & Strang, 2002; Stone, 1990; Yen et al., 2003). The public health cost and dysfunction associated with the co-occurrence of these disorders highlight the need for a better understanding of the factors underlying their comorbidity.
While the cross-sectional association is well established, few studies have traced the developmental origins of the association between BPD and SUDs, specifically, in adolescence when these problem behaviors first emerge. First, adolescence is marked by rapid physiological and environmental changes. For instance, this is a critical window for neural development that affects emotion regulation and decision-making (Steinberg, 2004, 2007). Additionally, there are increased social and cognitive demands on adolescents (e.g., social relationships become more complex, academic tasks more demanding) – whereas the psychological processes needed to complete these tasks might lag well behind. As such, it is not surprising that adolescence is characterized by steep increases in various forms of psychopathology and problem behavior (Bongers, Koot, van der Ende, & Verhulst, 2003; Cicchetti & Rogosch, 2002; Hicks et al., 2007; Kelley, Schochet, & Landry, 2004). However, there are also individual differences during this period such that many young people navigate adolescence with relatively little difficulty (Arnett, 1999). Given this variability in adjustment, examining the reciprocal influence between BPD traits and substance use among adolescents has the potential to be especially useful in understanding the common etiology of both phenotypes.
Next, prior to understanding the comorbidity between BPD traits and SUDs, each phenotype should be examined within the context of normative developmental change. For example, BPD traits tend to remain relatively stable through adolescence and decline during the transition from adolescence to young adulthood (Bornovalova, Hicks, Iacono, & McGue, 2009; Winograd, Cohen, & Chen, 2008). In contrast, substance use increases dramatically throughout adolescence, with the prevalence of SUDs peaking in young adulthood (SAMHSA, 2010). Especially necessary—but currently lacking—are longitudinal studies that measure both BPD traits and substance use at two (or more) time points, as such a design would provide the ability to test whether BPD traits predict increases in substance use (or vice versa) after accounting for the normative developmental increases in substance use during adolescence. Such a cross-lagged longitudinal design then would provide the initial step in determining whether BPD traits and substance use act as causal antecedents of each other, or alternatively are merely correlates of each other due to another common risk factor such as impulsivity or poor emotion regulation (Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009).
The etiological basis of the BPD-SUD comorbidity is also poorly understood, specifically, the extent to which their association is due to common genetic or environmental risk factors. Whereas among adults, twin and family studies suggest a common genetic (and to a lesser degree, environmental) basis for BPD and substance use (Kendler, Myers, & Reichborn-Kjennerud, 2011; White, Gunderson, Zanarini, & Hudson, 2003), longitudinal work with younger samples is lacking. Here again, developmental context must be considered as the heritability of BPD traits and substance use-related phenotypes changes over the course of adolescence and young adulthood. For example, initiation of substance use exhibits large shared environmental effects (i.e., environmental effects that contribute to similarities among relatives) and modest heritability (McGue, Elkins, & Iacono, 2000; Rhee et al., 2003), while quantity and frequency measures of substance use in adolescence and young adulthood exhibit moderate heritability and modest shared environmental effects (Kendler, Schmitt, Aggen, & Prescott, 2008; Rhee, et al., 2003), and SUDs in adulthood exhibit moderate heritability and no shared environmental effects (Goldman, Oroszi, & Ducci, 2005). Though the effects are weaker for BPD traits, there is also some evidence of modest to moderate shared environmental effects in adolescence that decline through young adulthood as genetic effects increase over the same period (Bornovalova, et al., 2009). The finding that the heritability of BPD traits and substance use-related phenotypes changes over the course of adolescence and young adulthood suggests that the genetic and environmental contributions to their co-occurrence might also exhibit a similar pattern, that is, shared environmental effects early in adolescence followed by increasing genetic effects in later adolescence and young adulthood.
Current Study
We sought to begin to answer these questions by using a large sample of female twins assessed during middle (age 14) and late adolescence (age 18) for both BPD traits and substance use. The strength of the current design is that we were able to measure both BPD traits and substance use at two time points during a key developmental period when these problem behaviors begin to emerge and increase in prevalence. Indeed, as we allude above, we focused on these two specific timepoints because they bracket the beginning and end of middle adolescence – one of the highest risk periods for the initiation of general psychopathology and substance use disorders. We had three specific goals:
Examine normative change in BPD traits and substance use from age 14 to 18, that is, estimate the amount of mean-level change and rank-order stability.
Test whether BPD traits at age 14 had a causal effect on substance use at age 18 (and vice versa). This was done by fitting a cross-lagged model that estimates the effect of BPD traits at age 14 on substance use at age 18, after accounting for the stability of substance use from age 14 to 18 (and vice versa). A significant “cross-lagged” effect is consistent with one phenotype having a causal effect on the other. If the phenotypes are correlated but the cross-lagged effects are not significant, this suggests the two phenotypes share common risk factors, but are not causal antecedents of each other.
Use the genetically informative twin sample to estimate the genetic and environmental influences on BPD traits and substance use and their stability, as well as the genetic and environmental influences underlying their comorbidity at age 14 and 18. Given greater shared environmental influences on each phenotype in mid-adolescence and greater genetic influences in late adolescence, we predicted shared environmental influences would account for their comorbidity at age 14 and genetic influences would account for their comorbidity at age 18.
Method
Sample
Participants were 1280 female twins from the ongoing Minnesota Twin Family Study (MTFS). The MTFS is a population-based longitudinal study of male and female twins and their families. Families with a twin birth were identified using Minnesota public birth records for two time periods— January 1, 1977 to December 31, 1984 and January 1, 1988 to December 31, 1994— and recruited into the study the year the twins turned 11-years old. Twins are then invited to return for follow-up assessments every 3-4 years. A more comprehensive description of the MTFS sample and methods are available elsewhere (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Keyes et al., 2009).
Self-report personality data—used to assess BPD traits—was first collected at the first follow-up assessment. Therefore, we utilized data from the follow-up 1 (target age 14) and follow-up 2 (target age 18) assessments. We were only able to include female twins, because personality data was not available for male twins at age 14. Among the 640 twin pairs studied, 390 were monozygotic (MZ) and 250 dizygotic (DZ). Zygosity was determined by agreement among three estimates: MTFS staff evaluations of the twins’ physical similarity; parents’ completion of a standard zygosity questionnaire; and degree of sibling similarity as measured by an algorithm based on ponderal and cephalic indices and fingerprint ridge count. If the three estimates did not agree, a serological analysis was conducted (see (Iacono, et al., 1999) for a full description of the methods). Additionally, zygosity for all DZ twins has been confirmed using a genome-wide association study. Consistent with the Minnesota demographics during the study periods, over 96% of the twins were of European American ancestry. The mean age was 14.90 (SD = 0.59) years for the follow-up 1 assessment, and 18.21 (SD = 0.67) years for the follow-up 2 assessment.To control for the slight variability in age, all measures were centered on the target age of the assessment.
Assessment
Minnesota Borderline Personality Disorder scale
(MBPD)1 (Bornovalova, Hicks, Patrick, Iacono, & McGue, 2011). The MBPD underwent a thorough validation procedure that included five separate samples. Candidate items were identified in two samples—inner-city drug users and undergraduates—by examining correlations between all MPQ items and diagnostic and self-report measures of BPD. Candidate items that were significantly correlated with BPD measures in both samples were retained for further analyses in a third sample of community young adults. Various validation analyses were conducted with a special emphasis on potential BPD items providing incremental prediction over general negative affect as measured by MPQ Stress Reaction scale. The final 19 items were drawn from the MPQ Stress Reaction, Alienation, Control, Aggression, Well-Being, and Absorption scales. In support of its convergent validity, MBPD scores were correlated strongly with scores on the Personality Assessment Inventory-Borderline scale (PAI-BOR; (Morey, 1991) in the undergraduate sample in both males (r = .74) and females (r = .82). Among substance users, the MBPD was correlated with the self-report Inventory for Interpersonal Problems-BPD scale (Lejuez, Aklin, Zvolensky, & Pedulla, 2003; Pilkonis, Yookung, & JM, 1996) (males, r = .59; females, r = .62), and with a DSM-IV interview-based diagnosis of BPD in the drug user sample (males, r = .69; females, r = .60). The degree to which MBPD was associated with the self-report and diagnostic BPD scales did not differ by gender indicating that the scale is tapping the same construct across males and females.
Bornovalova et al. (2011) also examined the association between MBPD scores and external criterion variables in the community and drug user samples, as well as a sample of male and female prisoners. In terms of convergent validity, MBPD scores correlated with several known correlates of BPD including trauma history (r = .27), symptoms of post-traumatic stress disorder (r = .56), and multiple measures of antisocial behavior (rs = .19-.42), internalizing distress (rs = .31-.48), and drug/alcohol use severity (rs = .25-.42). MBPD scores also exhibited incremental validity over MPQ Negative Emotionality scores in predicting these external criterion variables. MBPD scores also exhibited theoretically coherent associations with multiple measures of normal-range personality constructs including negative affect (rs = .47-.64), positive affect (r = −.39), and disinhibition (rs = .26-.32). Internal consistency was high across the five study samples (α =.81 to.83). For the current sample, the internal consistency was .86 at age 14 and .88 at age 18. Finally, the MBPD showed adequate discriminant validity. Specifically, MBPD scores exhibited stronger correlations with interview-based BPD symptoms (r = .69 and 60 for males and females, respectively) than symptoms of adult antisocial (the adult criteria for antisocial personality disorder; males, r = .33; females, r = .42), conduct disorder (males, r = .32; females, r = .49), and self-reported depressive symptoms (males, r = .44; females, r = .37). Similarly, in a sample of male and female prisoners, the MBPD showed higher correlations with the regression-estimated PAI-BOR scale (males, r = .90; females, r = .92) than with interview-based symptoms of conduct disorder (males, r = .18; females, r = .42) and adult antisocial behavior (males, r = .23; females, r = .37).
Substance Use
In the current study, we utilized a composite measure of substance use quantity/frequency was used rather than symptoms of abuse/dependence as SUDs are uncommon in community-based samples in middle adolescence, especially for females (SAMHSA, 2010). Thus, participants reported on quantity and frequency of tobacco, alcohol, and marijuana use over the previous 12 months using an 11-item computerized substance use assessment at ages 14 and 18. All questions used a Likert-type scale ranging from 0 (no use of substances) to 6 (everyday or nearly every day). Means and frequency of use of each individual drug type and any substance use are reported in Table 1.
Table 1. Means and Longitudinal Change of BPD and Substance Use from Age 14 to 18.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Age 14 | % past-year use |
Age 18 | % past-year use |
Mean-level Change (Cohen’s d) |
|||
| Variable | Mean | SD | Mean | SD | |||
| BPD Traits | 41.00 | 9.58 | --- | 39.11 | 8.66 | --- | −.21 |
| Substance Use Composite | .43 | .94 | 21.6 | 1.33 | 1.44 | 55.7 | .81 |
| Tobacco Use | .49 | 1.24 | 17.5 | 1.22 | 1.88 | 36.1 | .59 |
| Alcohol Use | .47 | 1.04 | 19.9 | 1.60 | 1.68 | 53.5 | .83 |
| Marijuana Use | .19 | .83 | 7.4 | .49 | 1.27 | 19.5 | .32 |
Note: BPD = Borderline Personality Disorder. Raw means of each substance are provided on a 6-point likert scale (0 = never to 6 = daily). Frequencies of past year use are dichotomized as “used in past year” and “didn’t use in past year”. Mean-level change analyses conducted on log-transformed tobacco, alcohol, marijuana, and substance use composite variables.
The measure of overall substance use was calculated by taking the mean of all alcohol, nicotine, and marijuana items. We collapsed across substances for several reasons. First, substance use in adolescence is non-specific (i.e., high rate of using multiple substances) such that a composite measure of use across multiple substances provides the best index of overall risk (Hicks, et al., 2007). Additionally, multiple substances of abuse share a general (rather than specific) vulnerability (Rhee, et al., 2003). In the current sample, indices of alcohol, tobacco, and marijuana use were highly correlated; the mean inter-item correlation was .60 and .52 at age 14 and 18, respectively (range r = .48 to .65). Additionally, each item loaded > .8 on the first principal component at both age 14 and 18; thus, the substance use composite was equally influenced by each substance. Finally, we replicated the analyses in the current study with each individual substance rather than the substance use composite. We found that the significance or lack thereof for each analysis doesn’t change in any case. Thus, the findings aren’t an artifact of creating a composite or the method by which the composite was created. However, the composite was preferred because it required only one set of analyses and largely produced more robust findings than any individual substance. The composite substance use measure had a range of 0 to 6 with α = .79 and .76 at age 14 and 18, respectively. The natural log transformation was used to reduce the skewness and kurtosis of the variable at the two ages. At age 14, the skew decreased from 2.47 to 1.80, and the kurtosis decreased from 5.75 to 1.97. At age 18, the skew decreased from 0.71 to 0.21, and the kurtosis decreased from −0.72 to −1.53.
To further demonstrate its validity, we also correlated the log-transformed substance use composites at both ages with mean DSM-IV drug and alcohol abuse symptoms and mean DSM-IV drug and alcohol dependence symptoms. At age 14, the correlations between the substance use composite and symptoms of abuse and dependence were .37 and .68 (both p’s < .001), respectively. At age 18, the correlations between the substance use composite and symptoms of abuse and dependence were .43 and .56 (both p’s < .001), respectively. This evidence indicates that our current substance use composite is a good indicator of substance use problems.
Statistical Analysis
Longitudinal Stability, Change, and Phenotypic Associations
Two sets of analyses were conducted to examine patterns of change and stability in BPD traits and substance use from middle to late adolescence. First, mean-level change from age 14 to 18 was evaluated using Cohen’s d (M1-M2/SD). Rank-order stability was assessed via the test-retest Pearson correlation coefficients for the BPD and substance use scores from the age 14 and 18. Significance levels were adjusted with linear mixed models in SPSS to account for the non-independence of the twin observations.
Next, we examined the cross-sectional and prospective associations between BPD traits and substance use by computing zero-order correlations between BPD traits and substance use at each age and across time. We then fit a cross-lagged panel model using Mplus (Muthen & Muthen, 2007). This model allowed us to examine the prospective association between BPD traits and substance use from age 14 to 18 (cross-lagged effects) after adjusting for the association between BPD traits and substance use at age 14, and the stability of BPD traits and substance use from age 14 to 18. The Mplus COMPLEX analysis was used to account for non-independence of observations (i.e., correlations among members of a single family).
Biometric Analyses
First, we estimated within-trait, cross-twin correlations used to estimate heritability (Table 2). These correlations are used to estimate genetic and environmental influences on a phenotype. Genetic influences are inferred if the MZ correlation is greater than the DZ correlation for a given trait. Shared environmental influences are inferred if the DZ correlation is greater than ½ the MZ correlation. Non-shared environmental influences are inferred when the MZ correlation is less than 1.0. Next, we used standard biometric models to estimate the additive genetic, shared environmental, and non-shared environmental influences on MBPD and substance use scores at ages 14 and 18. The additive genetic component (a2) is the effect of individual genes summed over loci on trait variance. Shared environmental effects (c2) are non-genetic factors that increase similarity between members of a twin pair. Non-shared environmental effects (e2) are factors that contribute to differences between members of a twin pair. Measurement error is also included in the estimate of e2. Preliminary analyses indicated that, in some cases, the shared environmental parameter approached zero. In these cases, we fit nested models that dropped this parameter and compared the resulting model fit on the −2 log likelihood value (Δ-2LL, which follows a chi-square distribution) and the Bayesian Information Criterion (BIC). The BIC is a function of a model’s Χ2 value and df, and penalizes the model fit for the retention of unnecessary parameters. This fit index is not interpreted in isolation; rather it is used to compare alternative models such that lower BIC scores are indicative of better fit. When comparing models, a difference in BIC of 0-2 is considered weak evidence in support of the model with the lower BIC value, a difference of 2-6 is considered positive evidence, a difference of 6-10 is considered strong evidence, and a difference over 10 is considered very strong evidence (Raftery, 1995).
Table 2. Cross-Twin, Cross-Trait Correlations (rTwinA-TwinB) for BPD traits and Substance Use at Age 14 and 18.
|
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monozygotic Pairs | Dizygotic Pairs | ||||||||||||||||
| Twin A | Twin B | Twin A | Twin B | ||||||||||||||
|
|
|||||||||||||||||
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | ||
| Twin A | 1. BPD traits age 14 | --- | .58 | .35 | .18 | .46 | .37 | .20 | .24 | --- | .53 | .41 | .21 | .39 | .32 | .37 | .31 |
| 2. BPD traits age 18 | --- | .16 | .18 | .42 | .48 | .10 | .22 | --- | .16 | .23 | .20 | .28 | .20 | .13 | |||
| 3. Substance Use age 14 | --- | .54 | .26 | .17 | .71 | .52 | --- | .44 | .34 | .19 | .62 | .0 | |||||
| 4. Substance Use age 18 | --- | .13 | .12 | .50 | .75 | --- | .20 | .14 | .30 | .50 | |||||||
|
|
|||||||||||||||||
| Twin B | 5. BPD traits age 14 | --- | .60 | .23 | .20 | --- | .59 | .45 | .28 | ||||||||
| 6. BPD traits age 18 | --- | .17 | .16 | --- | .26 | .20 | |||||||||||
| 7. Substance Use age 14 | --- | .54 | --- | .55 | |||||||||||||
| 8. Substance Use age 18 | --- | --- | |||||||||||||||
Note: In the triangular elements (e.g., Twin A columns with Twin A rows), the bold and italicized correlations are the within-trait, cross-time or stability correlations (e.g., the correlation between BPD at age 14 with BPD at age 18); bold correlations are the cross-trait, within-time or comorbidity correlations (e.g., the correlation between BPD at age 14 with Substance Use at age 14); the italicized correlations are the cross-trait, cross-time or prospective correlations (e.g., the correlation between BPD at age 14 with Substance Use at age 18). In the rectangular elements (e.g., Twin A rows and Twin B columns), the bold and italicized correlations are the within-trait, cross-twin correlations that are used to estimate heritability (e.g., MZ > DZ correlation indicates a genetic effect). BPD = Borderline Personality Disorder. All ps < .01
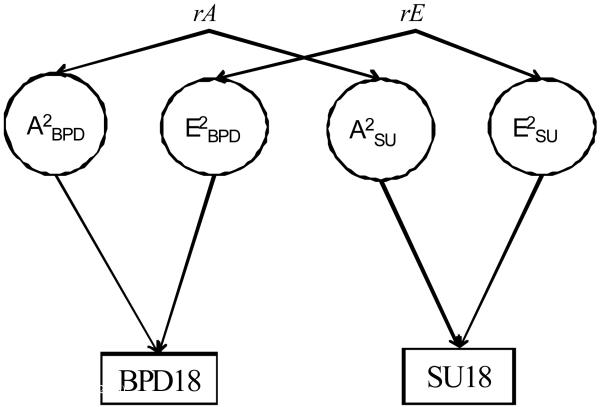
To test the magnitude of genetic and environmental influences on the stability of BPD traits and SU, we fit a series of bivariate Cholesky models. These models allow the genetic and environmental influences on, for example, BPD traits at 14 to correlate with the same influences on BPD traits at 18. The magnitude of the genetic, shared environmental and nonshared environmental correlations between BPD traits at 14 and BPD traits at 18 identifies the extent to which such influences are common to both phenotypes. These models also calculate the percentage of covariance between BPD traits and substance traits attributable to genetic and environmental influences (Neale & Cardon, 1992). Finally, to evaluate the influence of common genetic and environmental factors on the BPD-substance use comorbidity at ages 14 and 18—and from age 14 to age 18—we again fit a series of bivariate Cholesky models. Figure 1 provides a visual representation of the Cholesky models. All biometric analyses were conducted using the Mx computer program (Neale, Boker, Xie, & Maes, 2004) and were fit to the raw data using full information maximum likelihood that adjusts parameters for missing data.
Figure 1.
General bivariate model showing genetic (rA) and environmental (rE) correlations between BPD traits and substance use at age 18. BPD, Borderline Personality Disorder traits; SU, Substance Use; A, additive genetic effects; C, shared environmental effects; E; nonshared environmental effects.
Results
Longitudinal Change in BPD Traits and Substance Use
Table 1 provides the means, standard deviations, and effect sizes for changes in BPD traits and substance use (across different substances as well as for the total substance use composite) from age 14 to 18. Paired t-tests indicated a small but significant decline in BPD traits from age 14 to 18 (t = −5.67; p < .001). In contrast, there were moderate to large and significant increases in all types of substance use (tobacco: t = 12.98; p < .001; alcohol: t = 18.99; p < .001; substance use composite: t = 20.48; p < .001), with the exception of marijuana, which evidenced a significant but small mean-level increase (t = 7.80; p < .001). We also examined the test-retest correlations of the phenotype scores across the two time points. Both BPD traits and substance use exhibited moderate to high rank-order stability from age 14 to 18 (r = .58 and .51, p < .001, respectively).
Cross-Sectional and Longitudinal Associations between BPD Traits and Substance Use
Table 2 provides the phenotypic correlations among BPD traits and substance use at age 14 and 18, separated by twin A and B and zygosity. For the full sample, there were small to moderate associations between BPD traits and substance use at age 14 and 18 (r = .36 and .20, p’s < .001, respectively). The correlation between BPD traits and substance use was significantly greater at age 14 than at age 18 (contrast z = 3.27; p < .01). In terms of prospective associations, BPD traits at age 14 had a modest association with substance use at age 18 (r = .23, p < .001), and substance use at age 14 had a modest association with BPD traits at 18 (r = .20, p < .001). As displayed in Figure 2, after adjusting for the temporal stability for each trait from age 14 and to 18—and their overlap at age 14—the cross-lagged effects were nearly zero. That is, controlling for developmental stability, BPD traits at age 14 did not predict changes in substance use from age 14 to 18, and vice versa.
Figure 2.
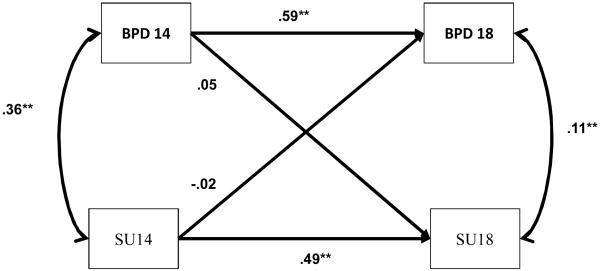
Cross-Lagged Model Testing Pathways between BPD and substance use at ages 14 and 18. BPD, Borderline Personality Disorder traits; SU, Substance Use. The double-headed arrows (far right and far left) are residual correlations between BPD and substance use at each age; the single-headed arrows represent standardized path estimates of cross-time effects between BPD traits and substance use at age 14 and age 18 (e.g., substance use at age 14 on BPD traits at age 18). * p < .05; ** p < .01.
Genetic and Environmental Effects on BPD Traits and Substance Use at Ages 14 and 18
The various within- and cross-twin and trait correlations are presented in Table 2. Table 3 presents the estimates of genetic and environmental contributions to BPD traits and substance use at age 14 and 18. At age 14, BPD traits and substance use had moderate additive genetic and shared environmental influences and large non-shared environmental influences. At age 18, substance use was moderately influenced by genetic, shared and non-shared environmental factors, while BPD traits were strongly influenced by genetic factors and moderately by non-shared environmental factors with almost no shared environment effects. Notably, dropping the shared environmental influence component on BPD traits at age 18 did not result in differences in the -2LL (Δ-2LL = 0.01, p = .94), but a significant improvement in model fit as indexed by changes in the BIC (ΔBIC = −3.09) (Raftery, 1995).
Table 3. Variance Component Estimates for BPD and Substance Use at Age 14 and 18.
| Age 14 | Age 18 | |||||
|---|---|---|---|---|---|---|
| Phenotype | A | C | E | A | C | E |
| BPD traits | .25 (.001,.51) |
.23 (.01,.44) |
.52 (.44,.60) |
.48 (.19, .57) |
.01 (.00,.26) |
.51 (.43,.60) |
| Substance Use | .44 (.28,.61) |
.33 (.16,.48) |
.23 (.19,.28) |
.49 (.26,.76) |
.25 (.00,.47) |
.26 (.21,.32) |
Note: BPD = Borderline Personality; A = additive genetic effects with 95% confidence intervals [CIs]; C = shared environmental effects; E = nonshared environmental effects.
Next, we examined the genetic and environmental influences on the stability of BPD traits and substance use. As reported in Table 4, the bivariate choleksy model showed that there was a large genetic correlation between BPD traits at age 14 and BPD traits at age 18, as well as a moderate nonshared environmental correlation (the shared environmental effect was fixed to zero because it accounted for almost no variance in BPD traits at age 18).2 Genetic influences accounted for about 2/3rd of the stability of BPD traits from age 14 to 18. In contrast, both genetic and shared environmental influences contributed to the stability of substance use from age 14 to 18, with a moderate genetic correlation and large shared environmental correlation.
Table 4. Genetic and Environmental Influences on the BPD-Substance Use Covairance at Ages 14 and 18.
| Genetic and Environmental Correlations | % covariance due to A |
% covariance due to C |
% covariance due to E |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | rA | rC | rE | |||
| BPD14-BPD18 | 1.00 (.80, 1.00) |
-- | .34 (.24, .43) |
71 | -- | 29 |
| SU14-SU18 | .46 (.19,.76) |
.94 (.57, 1.00) |
.10 (−0.03,.22) |
38 | 57 | 4 |
| BPD14-SU14 | .15 (−.36, .55) |
1.00 (.53, 1.00) |
.14 (.03, .25) |
15 | 72 | 13 |
| BPD18-SU18 | .38 (.20, .67) |
-- | .00 (−.12, .12) |
100 | -- | 0 |
Note: BPD = Borderline Personality Disorder traits; SU = Substance Use; A = additive genetic effects with 95% confidence intervals [CIs]; C = shared environmental effects; E = nonshared environmental effects. Because shared environmental effects accounted for almost no variance in BPD traits at age 18, this parameter was fixed to zero in the bivariate models of BPD18-SU18.
Finally, we examined the genetic and environmental influences contributing to both BPD traits and substance use. Bivariate Cholesky models (Table 4) indicated that there was a large shared environmental correlation between BPD traits and substance use at age 14, as well as a small (but significant) nonshared environmental correlation, and a non-significant genetic correlation. Shared environmental effects accounted for most the association between BPD traits and substance use at age 14. At age 18, genetic effects accounted for most of the association between BPD traits and substance use with a moderate genetic correlation and a non-significant nonshared environmental correlation.3
Discussion
We utilized a large sample of female twins to examine the developmental course, reciprocal influences, and genetic and environmental effects on the cross-sectional and prospective associations between BPD traits and substance use from age 14 to 18. This is among the first studies to examine the longitudinal relationship between the two phenotypes during adolescence and the first to explore genetic and environmental influences on their co-occurrence in adolescence.
Analysis of the developmental trends indicated that mean-levels of BPD traits remained relatively stable from middle to late adolescence, and individuals who reported the most BPD traits at age 14 tended to continue to do so at age 18. In contrast, substance use increased substantially from age 14 to 18. Moreover, girls who drank, smoke, and used marijuana the most in middle adolescence also tended to report the highest levels of substance use in late adolescence. As to heritability, genetic factors exerted increasing influence on BPD traits from middle to late adolescence, whereas the impact of shared environmental factors fell to almost zero. Substance use also exhibited a pattern of increasing genetic and decreasing shared environmental effects, though the shift was more subtle as there continued to be moderate shared environmental effects at age 18. Moreover, a combination of genetic and nonshared environmental factors influenced the stability of BPD traits, whereas shared environmental and genetic factors contributed to the stability of SU.
Cross-sectional and cross-lagged analyses indicated that BPD traits and substance use were modestly to moderately correlated at each age, and the presence of one phenotype at age 14 predicted the presence of the other at age 18. However, the longitudinal associations were nearly zero after accounting for their earlier cross-sectional association and temporal stability of each phenotype. This suggests that the association between BPD traits and substance use are correlates rather causal antecedents of each other, and that their association is due to broader risk factors for psychopathology such as behavioral disinhibition (Beauchaine, et al., 2009). These findings are also consistent with the few longitudinal studies that have examined the association between BPD and substance-related phenotypes that is, an association with initial status, but not with change over time (Cohen, Henian, Crawford, Brook, & Gordon, 2007; Rohde, Lewinsohn, Kahler, Seeley, & Brown, 2001).
Biometric analyses showed that shared environmental effects primarily accounted for the comorbidity between BPD traits and substance use at age 14, while genetic effects primarily accounted for their co-occurrence at age 18. The pattern of increasing genetic and decreasing shared environmental effects is often seen for problem behavior phenotypes (Bergen, Gardner, & Kendler, 2007). Indeed, a large body of literature documents the influence of shared environmental factors such as parent-child conflict (Burt, Krueger, McGue, & Iacono, 2003; Burt, McGue, Krueger, & Iacono, 2005), socioeconomic status (Miech, Caspi, Moffitt, Bradley, & Silva, 1999), and peer group influences (Dishion & Owen, 2002) on problem behavior in early adolescence, but these factors have considerably less influence in late adolescence and early adulthood. Moreover, there is some evidence that this pattern of increasing genetic and decreasing shared environmental effects extends to the comorbidity (defined as common factor variance) among problem behaviors. For example, McGue et al. (2006) reported that the heritability of early adolescent problem behavior (substance use, sexual intercourse, and police contact before age 15) was only modestly heritable (a2 = .20) with moderate shared environmental effects (c2 = .40) while the comorbidity among externalizing disorders at age 20 (antisocial behavior and alcohol, nicotine, illicit drug dependence) was highly heritable (a2 = .75) with no significant shared environmental effects.
These developmental trends may be due to a shift from passive gene-environment correlation effects in childhood and early adolescence to active gene-environment correlation processes in late adolescence and adulthood (Scarr & McCartney, 1983). Passive gene-environment correlation effects refer to the non-independence between aspects of a child’s rearing environment shaped by their parents and the genetic risk inherited from their parents such as parental discipline and monitoring. Active gene-environment correlation effects refer to heritable characteristics (e.g., temperament) being associated with the selection of certain environments. Gene-environment correlations also affect heritability estimates, specifically, passive gene-environment correlations result in higher estimates of shared environmental variance, while active gene-environment correlations result in higher estimates of additive genetic variance (Purcell, 2002). The finding of large, non-specific shared environmental risk present (McGue, et al., 2006) could be due to correlated risk between inherited liability for behavioral disinhibition and shared environmental risk factors (e.g., common parenting practices, peers, schools, neighborhoods) in middle adolescence. In turn, the shift to a predominately common genetic risk underlying various problem behaviors in late adolescence and adulthood may be due to a greater role of selection processes in exposure to environmental risk.
More broadly, the findings that BPD traits share a common vulnerability with substance use disorders (rather than one serving as a causal factor for the other) links the current study with the larger literature on the structure and common vulnerabilities to mental disorders. In particular, the hierarchical framework of broad and heritable Internalizing and Externalizing liabilities that underlie the comorbidity among syndromes that share phenomenological similarities. Externalizing is characterized by high novelty seeking, impulsivity, and lack of constraint (Iacono, Malone, & McGue, 2008; Sher & Trull, 1994), while internalizing indexes a propensity to experience negative emotions and can be further broken down into the factors of fear and distress (Krueger & Markon, 2006; Watson, 2005). This model has been replicated in multiple studies (e.g., Kessler et al, 2011; Krueger & Markon, 2006; Kendler et al, 2011), and several studies also report that both factors show high heritability (Bornovalova, Hicks, Iacono, & McGue, 2010; Hicks, Krueger, Iacono, McGue, & Patrick, 2004; Kendler, Prescott, Myers, & Neale, 2003; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011). Phenotypic models indicate that whereas BPD cross-loads on both Internalizing and Externalizing (Eaton et al., 2011; James & Taylor, 2008), substance use disorders consistently load on Externalizing only. Similar findings are reported by twin studies studies. For instance, Kendler and colleagues (2011) reported that both BPD and drug and alcohol abuse showed genetic overlap with Axis I externalizing disorders. As such, it is likely that the comorbidity between BPD and substance use stems mainly from a common underlying liability to externalizing psychopathology.
Our results have both theoretical and clinical implications. First, understanding of the relative effect of genetic and environmental factors on BPD traits and substance use at different ages can help guide the selection of mediators that suggest explanatory mechanisms. For example, to account for the association between BPD traits and substance use at age 14, one would focus on putative shared environmental effects such as peers, parenting variables, and neighborhoods factors. Further, the importance of shared environmental effects on BPD traits and substance use in middle adolescence suggests the need to consider shared environmental risk factors in treatment and prevention.
Additionally, the failure to find cross-lagged associations between BPD traits and substance misuse suggests that prevention and intervention specifically targeting BPD traits or substance misuse may not prevent the other. Rather, our results suggest the need to identify the underlying vulnerabilities common to both disorders (e.g., behavioral disinhibition) and then provide comprehensive, individualized interventions that target this liability. For example, multisystemic therapy—a broad-based ecological intervention for behavioral problems that targets multiple levels of a high-risk child’s environment (behavior, health, family, school, peers, neighborhood)—might be useful in targeting the general propensity toward disinhibited problem behavior in late adolescence and adulthood (Curtis, Ronan, & Borduin, 2004; Henggeler & Schaeffer, 2010; Schoenwald, Ward, Henggeler, Pickrel, & Patel, 1996).
The study also has two important limitations. First, this research focused solely on female twins. It is uncertain if the pattern and underlying genetic and environmental effects on the association between BPD traits and substance use co-occurrence is the same for males. Second, although our self-report measure of BPD traits was developed and validated across several diverse samples (Bornovalova, et al., 2011), the findings should be replicated using a multi-assessment method, multi-informmant design (including interview-based methods), as previous work suggests that each different assessment method and informant provides unique information about the individuals (Hopwood et al., 2008; Oltmanns & Turkheimer, 2009).
Despite limitations, the current work provides interesting initial results that suggest a number of follow-up studies. For instance, future work might aim to examine the hypotheses presented above regarding the changing nature of gene-environment correlations, specifically, if there is transition from passive to active gene-environment correlation processes from middle to late adolescence. Work of this kind is likely to contribute substantially to knowledge of the shared etiology between BPD traits and substance use, and in turn to methods for preventing and treating this difficult and persistent comorbidity.
Acknowledgments
Data for this project were collected at the University of Minnesota. This work was supported by National Institute of Drug Abuse Grants DA05147, DA005147, and DA013240, and National Institute on Alcohol Abuse and Alcoholism grant AA09367. Brian M. Hicks was supported by National Institute of Drug Abuse grant K01 DA025868. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Notably, as is the case with many large scale studies, there may be concern about data and reporting overlap across manuscripts. We have conducted two other studies using this measure and the current sample. First, we examined the normal developmental trajectory of BPD traits and the genetic/environmental influences on this trajectory across four time points: age 14, 17, 20, and 24 (Bornovalova et al, 2009). Second, we utilized a genetically-informed design to examine whether childhood trauma serves as a “causal” influence on BPD traits at age 24, or is the link between childhood trauma and BPD better explained by third variables (liability to internalizing or externalizing problems) (Bornovalova, et al, under review). As such, while there is some overlap in the measure and sample (although this overlap is minimal), each paper is quite separate in its question and scope.
Fixing the shared environmental parameter to zero did not result in significant changes in the −2LL value (Δ-2LL = 0.59; p = .44). It resulted in a significant improvement in model fit (ΔBIC = −2.82).
We also conducted the biometric analyses with the substance use composite split up into three specific substance indices (marijuana, alcohol, nicotine). Similar to the substance use composite, shared environmental effects accounted for most the association between BPD traits each drug at age 14, whereas genetic effects accounted for most of the association between BPD traits and each drug at age 18.
No conflict of interest exists for any of the authors.
References
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Beauchaine T, Klein D, Crowell S, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21(Special Issue 03):735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen S, Gardner C, Kendler K. A meta-analysis of age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Bongers I, Koot H, van der Ende J, Verhulst F. The normative development of child and adolescent problem behavior. Journal of Abnormal Psychology. 2003;112(2):179–192. doi: 10.1037/0021-843x.112.2.179. [DOI] [PubMed] [Google Scholar]
- Bornovalova M, Daughters S. How does Dialectical Behavior Therapy facilitate treatment retention among individuals with comorbid borderline personality disorder and substance use disorders? Clinical Psychology Review. 2007;27:923–943. doi: 10.1016/j.cpr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Bornovalova M, Hicks B, Iacono W, McGue M. Stability, change, and heritability of borderline personality disorder traits from adolescence to adulthood: A longitudinal twin study. Development and Psychopathology. 2009;21:1335–1353. doi: 10.1017/S0954579409990186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova M, Hicks B, Iacono W, McGue M. Familial transmission and heritability of childhood disruptive disorders. Am J Psychiatry. 2010;167(9):1066–1074. doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova M, Hicks B, Patrick C, Iacono W, McGue M. Development and validation of the Minnesota Borderline Personality Disorder scale. Assessment. 2011;18(2):234–252. doi: 10.1177/1073191111398320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch van den L, Verheul R, Schippers G, Brink van den W. Dialetical Behavior Therapy of borderline patients with and without substance use problems: Implementation and long term effects. Addictive Behaviors. 2002;27:911–923. doi: 10.1016/s0306-4603(02)00293-9. [DOI] [PubMed] [Google Scholar]
- Burt S, Krueger R, McGue M, Iacono W. Parent-child conflict and the co-morbidity among childhood externalizing disorders. Archives of General Psychiatry. 2003;60:505–513. doi: 10.1001/archpsyc.60.5.505. [DOI] [PubMed] [Google Scholar]
- Burt S, McGue M, Krueger R, Iacono W. Sources of covariation among the child-externalizing disorders: informant effects and the shared environment. Psychological Medicine. 2005;35(08):1133–1144. doi: 10.1017/S0033291705004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70(1):6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cohen P, Henian C, Crawford T, Brook J, Gordon K. Personality disorders in early adolescence and the development of later substance use disorders in the general population. Drug and Alcohol Dependence. 2007;88S:S71–S84. doi: 10.1016/j.drugalcdep.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N, Ronan K, Borduin C. Multisystemic Treatment: A Meta-Analysis of Outcome Studies. Journal of Family Psychology. 2004;18(3):411–419. doi: 10.1037/0893-3200.18.3.411. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Williamson A, Mills K, Havard A, Teesson M. Patterns and correlates of attempted suicide by heroin users over a 3-year period: Findings from the Australian treatment outcome study. Drug & Alcohol Dependence. 2007;87:146–152. doi: 10.1016/j.drugalcdep.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Owen LD. A longitudinal analysis of friendships and substance use: Bidirectional influence from adolescence to adulthood. Developmental Psychology. 2002;38(4):480–491. doi: 10.1037//0012-1649.38.4.480. [DOI] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Keyes KM, Skodol AE, Markon KE, Grant BF, Hasin DS. Borderline personality disorder co-morbidity: relationship to the internalizing-externalizing structure of common mental disorders. Psychological Medicine. 2011;41(05):1041–1050. doi: 10.1017/S0033291710001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nature Review Genetics. 2005;6(521-532):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grant B, Chou S, Goldstein R, Huang B, Stinson F, Saha T, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinincal Psychology. 2008;69:533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler S, Schaeffer C. Treating serious emotional and behavioural problems using multisystemic therapy. Australian & New Zealand Journal of Family Therapy. 2010;31(2):149–164. [Google Scholar]
- Hicks B, Blonigen D, Kramer M, Krueger R, Patrick C, Iacono W, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116(3):433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks B, Krueger R, Iacono W, McGue M, Patrick C. Family Transmission and Heritability of Externalizing Disorders: A Twin-Family Study. Arch Gen Psychiatry. 2004;61(9):922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hopwood C, Morey L, Edelen M, Shea M, Grilo C, Sanislow C, et al. A comparison of interview and self-report methods for the assessment of borderline personality disorder criteria. Psychological Assessment. 2008;20:81–85. doi: 10.1037/1040-3590.20.1.81. [DOI] [PubMed] [Google Scholar]
- Iacono W, Carlson S, Taylor J, Elkins I, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono W, Malone S, McGue M. Behavioral dishinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- James LM, Taylor J. Revisiting the structure of mental disorders: Borderline personality disorder and the internalizing/externalizing spectra. British Journal of Clinical Psychology. 2008;47(4):361–380. doi: 10.1348/014466508X299691. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk Taking and Novelty Seeking in Adolescence: Introduction to Part I. Annals of the New York Academy of Sciences. 2004;1021(1):27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, Reichborn-Kjennerud T. The Structure of Genetic and Environmental Risk Factors for Syndromal and Subsyndromal Common DSM-IV Axis I and All Axis II Disorders. Am J Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Myers J, Reichborn-Kjennerud T. Borderline personality disorder traits and their relationship with dimensions of normative personality: a web-based cohort and twin study. Acta Psychiatrica Scandinavica. 2011;123(5):349–359. doi: 10.1111/j.1600-0447.2010.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Prescott C, Myers J, Neale M. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler K, Schmitt B, Aggen S, Prescott C. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch Gen Psychiatry. 2011;68:90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes M, Malone S, Elkins I, Legrand L, McGue M, Iacono W. The enrichment study of the Minnesota twin family study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Research and Human Genetics. 2009;12:489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R, Markon K. Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B, Van Hulle C, Singh A, Waldman I, Rathouz P. Higher order genetic and environmental structure of prevalent forms of child and adolescent psychology. Archives of General Psychiatry. 2011;68:181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Zvolensky M, Pedulla C. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Linehan M, Schimdt H, III, LA D, Craft J, Kanter J, Comtois K. Dialetical behavior therapy for patients with borderline personality disorder and drug-dependence. The American Journal of Addiction. 1999;8:279–292. doi: 10.1080/105504999305686. [DOI] [PubMed] [Google Scholar]
- Links P, Heslegrave R, Mitton J, Vanreekum R, Patrick J. Borderline psychopathology and recurrences of clinical disorders. Journal of Nervous and Mental Disease. 1995;183:582–586. doi: 10.1097/00005053-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Marshall E, Keaney F, Ball D, Strang J. Unplanned versus planned discharges from in-patient alcohol detoxification: retrospective analysis of 470 first-episode admissions. Alcohol and Alcoholism. 2002;37:277–281. doi: 10.1093/alcalc/37.3.277. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono W. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono W, Krueger R. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Caspi A, Moffitt T, Bradley W, Silva P. Low socioeconomic status and mental disorders: A longitudinal study of selection and causation during young adulthood. American Journal of Sociology. 1999;104:112–147. [Google Scholar]
- Morey L. Personality Assessment Inventory: Professional manual. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Muthen L, Muthen B. Mplus User’s Guide. 5th ed. Muthen & Muthen; Los Angeles: 2007. [Google Scholar]
- Neale M, Cardon L. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- Neale M, Boker S, Xie G, Maes H. Mx: Statistical Modeling 6th ed rev. Department of Psychiatry: Virginia Commonwealth University; Richmond: 2004. [Google Scholar]
- Oltmanns T, Turkheimer E. Person Perception and Personality Pathology. Current Directions in Psychological Science. 2009;18:32–36. doi: 10.1111/j.1467-8721.2009.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkonis P, Yookung K, JM P. Scales for personality disorders developed from the Inventory of Interpersonal Problems. Journal of Personality Disorders. 1996;10:355–369. [Google Scholar]
- Purcell S. Variance components models for gene-environment Interaction in twin analysis. Twin Research and Human Genetics. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Raftery A. Bayesian Model Selection in Social Research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Rhee S, Hewitt J, Young S, Corley R, Crowley T, Stallings M. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn P, Kahler C, Seeley J, Brown R. Natural course of alcohol use disorders from adolescence to young adulthood. American Academy of Child and Adolescent Psychiatry. 2001;40:83–90. doi: 10.1097/00004583-200101000-00020. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD: 2010. Office of Applied Studies, NSDUH Series H-38A, HSS Publications No. SMA 10-4586 Findings. [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype greater then environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schoenwald S, Ward D, Henggeler SW, Pickrel S, Patel H. Multisystemic therapy treatment of substance abusing or dependent adolescent offenders: Costs of reducing incarceration, inpatient, and residential placement. Journal of Child and Family Studies. 1996;5:431–444. [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103(1):92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk Taking in Adolescence: What Changes, and Why? Annals of the New York Academy of Sciences. 2004;1021(1):51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk Taking in Adolescence. Current Directions in Psychological Science. 2007;16(2):55–59. [Google Scholar]
- Stepp S, Trull T, Sher K. Borderline personality features predict alcohol use problems. Journal of Personality Disorders. 2005;19:711–722. doi: 10.1521/pedi.2005.19.6.711. [DOI] [PubMed] [Google Scholar]
- Stone M. Successful outcome and psychiatric pratice. Guilford; New York: 1990. [Google Scholar]
- Tragesser S, Sher K, Trull T, Park A. Personality disorder symptoms, drinking motives, and alcohol use and consequences: Cross-sectional and prospective mediation. Experimental and Clinical Psychopharmacology. 2007;15:282–292. doi: 10.1037/1064-1297.15.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tragesser S, Trull T, Sher K, Park A. Drinking motives as mediators in the relation between personality disorder symptoms and alcohol use disorder. Journal of Personality Disorders. 2008;22:525–537. doi: 10.1521/pedi.2008.22.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull T, Jahng S, Tomko R, Wood P, Sher K. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders. 2010;24:412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull T, Sher K, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull T, Waudby C, Sher K. Alcohol and substance use disorders and personality disorder symptoms. Experimental and Clinical Psychopharmacology. 2004;12:65–75. doi: 10.1037/1064-1297.12.1.65. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- White C, Gunderson J, Zanarini M, Hudson J. Family studies of borderline personality disorder:a review. Harvard Review of Psychiatry. 2003;11:8–19. doi: 10.1080/10673220303937. [DOI] [PubMed] [Google Scholar]
- Winograd G, Cohen P, Chen H. Adolescent borderline symptoms in the community: Prognosis for functioning over 20 years. Journal of Child Psychology and Psychiatry. 2008;49:933–941. doi: 10.1111/j.1469-7610.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- Yen S, Shea T, Pagano M, Sanislow CA, Grilo CM, McGlashan TH, Skodol AE, et al. Axis I and Axis II disorders as predictors of prospective suicide attempts: Findings from the Collaborative Longitudinal Personality Disorders Study. Journal of Abnormal Psychology. 2003;112(3):375–381. doi: 10.1037/0021-843x.112.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]