In this study, Andreadi et al. dissect the molecular basis for the L597VBraf mutation's role in RASopathies and cancer in mice. They show that L597VBraf produces a modest activation of the Mek–Erk pathway, sufficient to drive RASopathies but not cancer, and that L597VBraf activates this pathway through its intrinsic Braf kinase activity as well as through transactivation of Craf on both WTKras and G12DKras backgrounds. Thus, L597VBraf contributes to cancer only when coexpressed with another oncogenic mutation.
Keywords: ERK signaling pathway, RASopathies, cancer, oncogene, BRAF
Abstract
L597VBRAF mutations are acquired somatically in human cancer samples and are frequently coincident with RAS mutations. Germline L597VBRAF mutations are also found in several autosomal dominant developmental conditions known as RASopathies, raising the important question of how the same mutation can contribute to both pathologies. Using a conditional knock-in mouse model, we show that endogenous expression of L597VBraf leads to approximately twofold elevated Braf kinase activity and weak activation of the Mek/Erk pathway. This is associated with induction of RASopathy hallmarks including cardiac abnormalities and facial dysmorphia but is not sufficient for tumor formation. We combined L597VBraf with G12DKras and found that L597VBraf modified G12DKras oncogenesis such that fibroblast transformation and lung tumor development were more reminiscent of that driven by the high-activity V600EBraf mutant. Mek/Erk activation levels were comparable with those driven by V600EBraf in the double-mutant cells, and the gene expression signature was more similar to that induced by V600EBraf than G12DKras. However, unlike V600EBraf, Mek/Erk pathway activation was mediated by both Craf and Braf, and ATP-competitive RAF inhibitors induced paradoxical Mek/Erk pathway activation. Our data show that weak activation of the Mek/Erk pathway underpins RASopathies, but in cancer, L597VBraf epistatically modifies the transforming effects of driver oncogenes.
The RAS/RAF/MEK/ERK signaling pathway is a critical mediator of cell growth signals in multiple organisms and cell types. Dysregulation of this pathway is a key characteristic of tumor cells, and components of the pathway are mutational targets in human cancer (Pearson et al. 2001; Davies et al. 2002; Malumbres and Barbacid 2003). In particular, oncogenic BRAF and RAS mutations are detected in ∼7% and ∼30% of samples, respectively, and their common ability to activate the downstream MEK/ERK pathway is thought to account at least in part for the transforming effects of these oncogenes (Davies et al. 2002; Malumbres and Barbacid 2003). Germline mutations in components of the RAS/RAF/MEK/ERK pathway, including BRAF and RAS, are also detected in a group of newly described developmental disorders collectively known as “RASopathies” (Tidyman and Rauen 2009; Rauen et al. 2011). RASopathies include Noonan syndrome (NS), LEOPARD syndrome (LS), Neurofibromatosis type 1 (NF1), Costello syndrome (CS), and cardio–facio–cutaneous syndrome (CFC) and have many overlapping features, including craniofacial abnormalities, cardiac malfunctions, and cutaneous, muscular, and ocular impairments with some increased risk of cancer (Tidyman and Rauen 2009; Rauen et al. 2011).
Of the BRAF mutations detected in human cancer, the high-activity V600EBRAF mutation is by far the most common, being detected in >90% of cases (Davies et al. 2002). However, several other mutations are detected at a lower frequency and have been categorized into high, intermediate, or impaired depending on the level of kinase activity they possess (Wan et al. 2004). Whereas V600EBRAF mutations are mutually exclusive with RAS mutations in human cancer samples, the intermediate- and impaired-activity BRAF mutations are significantly coincident with RAS or other oncogenic driver mutations (http://www.sanger.ac.uk/genetics/CGP/cosmic), suggesting that they may be cooperating rather than driver mutations. In RASopathies, BRAF mutations are detected in ∼75% of mutation-positive CFC patients and at lower frequencies in NS and LS patients (Rodriguez-Viciana et al. 2006; Sarkozy et al. 2009). All of the BRAF mutations are non-V600EBRAF. The majority fall into the high- or intermediate-activity class (Rodriguez-Viciana et al. 2006), with only seven also being found in human cancer samples; namely, G469E, F468S, L485F, F595L, V600G, and K601E in CFC patients (Rodriguez-Viciana et al. 2006; Champion et al. 2011), with the intermediate-activity L597V mutation being detected in both NS and CFC patients (Sarkozy et al. 2009; Pierpont et al. 2010).
How the same mutations can promote developmental abnormalities when constitutively expressed but cancer when acquired somatically is a critical question to address and is likely related to mechanisms of downstream MEK/ERK pathway activation under different contexts. High-activity mutants, such as V600EBRAF, have activity greater than oncogenic RAS-induced WTBRAF and are known to induce tumor development through their intrinsic ability to hyperactivate the MEK/ERK pathway (Davies et al. 2002; Karasarides et al. 2004). The situation is more complex with lower-activity BRAF mutants. Although impaired-activity mutants have lower activity than WTBRAF, they induce ERK activation through the formation of heterodimers with CRAF, leading to its activation (Wan et al. 2004; Kamata et al. 2010). Through analysis of the impaired-activity D594ABraf mutant in mice, we demonstrated that transactivation of Craf in this context was insufficient to drive tumor development per se (Kamata et al. 2010), although, when coexpressed with oncogenic Ras, a cooperating role in tumor development was revealed (Heidorn et al. 2010). Intermediate-activity mutants have activity in between oncogenic RAS-induced WTBRAF and WTBRAF and, following overexpression in COS cells, have been shown to induce MEK/ERK activation but to a lower level than high-activity mutants (Wan et al. 2004). CRAF was also transactivated by these mutants in COS cells, although siRNA depletion studies showed that BRAF but not CRAF was responsible for ERK activation in these situations (Wan et al. 2004). Whether mutants of this class are able to drive tumor development in vivo has not yet been addressed, nor has their role in inducing RASopathy syndromes.
Apart from the MEK/ERK pathway, oncogenic RAS can activate multiple downstream signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)/AKT and RalGDS signaling pathways (Malumbres and Barbacid 2003). Of these various pathways, studies in mice have shown a particular dependency on the MEK/ERK pathway for tumor maintenance driven by oncogenic RAS, despite the fact that this pathway is only weakly activated by the oncogene (Tuveson et al. 2004). In two separate reports, treatment of mice with MEK inhibitors showed significant regression of oncogenic Kras-driven tumors in the lung (Ji et al. 2007; Engelman et al. 2008), although a synergistic effect of combined PI3K inhibition was demonstrated in one of these reports (Engelman et al. 2008). Recent studies using knockout mice have also shown a critical role for the Raf/Mek/Erk pathway in lung tumor initiation downstream from oncogenic Kras (Blasco et al. 2011; Karreth et al. 2011). Elimination of both Erk isoforms or both Mek isoforms completely blocked tumor development. However, while knockout of Craf prevented lung tumor development, Braf knockout had no significant effect, indicating that Braf cannot compensate for the loss of Craf and that oncogenic Kras elicits its oncogenic effects through Craf. Consistent with this, oncogenic RAS has been shown to signal exclusively through CRAF to MEK in melanoma cell lines (Dumaz et al. 2006), and Craf has been shown to be required for tumor initiation and maintenance in the DMBA/TPA skin tumorigenesis model in which tumor development is driven by the acquisition of ras mutations (Ehrenreiter et al. 2009).
All of the above data reinforce the rationale for targeting RAS/RAF/MEK/ERK as an anti-cancer strategy, an initiative that has been under way for several years now. Although targeted therapies against RAS have largely failed (Basso et al. 2006), the availability of selective chemical inhibitors against RAF, MEK, and ERK have provided new therapeutic opportunities (Montagut and Settleman 2009). RAF inhibitors have made the most progress in the clinic, with the ATP-competitive inhibitor vemurafenib (PLX4032) showing remarkable efficacy in the treatment of melanomas with the V600EBRAF mutation (Chapman et al. 2011). The drug increased rates of overall and progression-free survival in patients with previously untreated melanoma, although resistance to the drug eventually emerged (Johannessen et al. 2010; Nazarian et al. 2010; Chapman et al. 2011; Su et al. 2012). Despite this extraordinary success, the ability of a given cancer to respond to vemurafenib and other similar RAF inhibitors is dependent on BRAF mutation status (Hatzivassiliou et al. 2010; Heidorn et al. 2010; Poulikakos et al. 2010). In melanomas with the V600EBRAF mutation, levels of RAS activation are low, and these drugs bind to BRAF monomers, inhibiting their activity (Hatzivassiliou et al. 2010; Heidorn et al. 2010; Poulikakos et al. 2010). However, in WTBRAF cells, activated RAS promotes dimerization of members of the RAF family, and vemurafenib has been shown to activate signaling through the MEK/ERK pathway by transactivating CRAF (Hatzivassiliou et al. 2010; Heidorn et al. 2010; Poulikakos et al. 2010). This has been hypothesized to explain why ∼18% of patients administered with vemurafenib develop squamous cell carcinoma of the skin or keratoacanthoma, with them arising from vemurafenib-induced MEK/ERK pathway activation in cells that have WTBRAF but active RAS (Chapman et al. 2011; Su et al. 2012). RAF/MEK inhibitors also offer huge potential for the treatment of RASopathies, and the suppression of RASopathy symptoms in animal models by MEK inhibitors is highly encouraging in this regard (Schuhmacher et al. 2008; Anastasaki et al. 2009; Sarkozy et al. 2009; Rauen et al. 2011). As with cancers, it will be important to understand the mechanisms underpinning RAS/RAF/MEK/ERK pathway deregulation in individual RASopathy patients as a corollary to the implementation of these novel therapies in the clinic.
We focused our efforts on understanding the contribution of each class of BRAF mutation to cancer and RASopathies by creating conditional knock-in mouse models with the view to informing better treatments for patients suffering from these diseases. Here, we generated a conditional knock-in mouse for the intermediate activity mutant L597VBraf and characterized its physiological effects following endogenous expression. We show that constitutive expression of L597VBraf induces Braf activity intermediate between WTBRaf and V600EBraf and weakly activates the Mek/Erk pathway. However, this is not sufficient to induce mouse embryonic fibroblast (MEF) transformation or tumor development in vivo, although the mice do demonstrate a spectrum of RASopathy hallmarks and have some predisposition to cancer when aged. We addressed cooperation with oncogenic Ras by intercrossing with KrasLSL-G12D mice (Jackson et al. 2001) and found that L597VBraf has a modifying effect on G12DKras-driven MEF transformation and lung tumor formation such that morphological and molecular features are partially transitioned to those driven by V600EBraf. However, both Craf and Braf mediate Mek/Erk pathway activation in the L597VBraf-expressing cells and, as with cells expressing WTBRAF on a mutant RAS background, RAF inhibitors promote Mek/Erk pathway activity rather than inhibiting it. These observations have important implications for the treatment of RASopathy and cancer patients carrying intermediate-activity BRAF mutations.
Results
Generation of L597VBraf-expressing mice
We used a strategy for the generation of Cre-regulated, conditional knock-in LSL-BrafL597V mice (Fig. 1A) similar to that previously reported for LSL-BrafV600E (Mercer et al. 2005) and LSL-BrafD594A (Mercer et al. 2005; Heidorn et al. 2010; Kamata et al. 2010) mice. Constitutive expression of L597VBraf from one allele of Braf was achieved by intercrossing Braf+/LSL-L597V heterozygous mice with mice heterozygous for the CMV-Cre transgene (Schwenk et al. 1995). PCR genotyping was used to confirm inheritance of the LSL-BrafL597V allele as well as Cre-mediated recombination to form the Lox-BrafL597V allele (Supplemental Fig. S1). On a predominantly C57BL6J background (at least five backcross generations), Braf+/Lox-L597V (+/LV) animals were born alive at the expected Mendelian ratio, but some animals were lost after weaning, with ∼70% surviving to adulthood (Fig. 1B).
Figure 1.
L597VBraf-expressing mice show RASopathy phenotypes. (A) Schematic of the gene-targeting event. The Lox–Stop–Lox (LSL) targeting vector was assembled with mouse Braf exon 15 containing the C1789A mutation and minigene Braf cDNA exons 15–18 (gray box). Splice acceptor (SA) and polyadenylation (pA) sequences were cloned on either side of the minigene. Cre recombinase induces deletion of the LSL cassette flanked by LoxP sequences (large black arrows), allowing expression of L597VBraf from the BrafLox-L597V allele.(A–C) PCR primers to detect wild-type, Lox, and LSL alleles are indicated (small black arrows). (B) Survival of littermate Braf+/+ (+/+) and Braf+/Lox-L597V (+/LV) mice containing the CMV-Cre transgene. (C) Weight comparisons of littermate +/+ and +/LV mice with the CMV-Cre transgene. (D) Gross appearance of 3-mo-old +/+ and +/LV female mice. (E) Gross facial appearance of 3-mo-old +/+ and +/LV female mice illustrating the blunt nose of mutant animals (arrowhead). (F) Enlarged heart. H&E-stained cross-sections of hearts of 3-mo-old mice are shown. Bars, 2 mm. The bar chart indicates the heart weight/body weight ratio of 3-mo-old +/+ (n = 6) and +/LV (n = 3) mice. (*) P < 0.01, Student's t-test. (G) Wheat germ agglutinin-stained cross-sections of cardiomyocytes. Bars, 100 μm. Cross-sectional areas were measured (n = 3 samples per genotype, with 100 cells counted per sample), and the average areas are given below for each genotype.
Mice expressing endogenous L597VBraf develop RASopathy hallmarks
All surviving +/LV animals were ∼10%–20% reduced in weight compared with controls (Fig. 1C). In addition, these animals showed multiple NS/CFC phenotypes, including short stature (Fig. 1D), facial dysmorphia (Fig. 1E), and cardiac enlargement with substantial thickening of the ventricular wall and septum (Fig. 1F). Cardiomyocyte cross-sectional area was increased by ∼20% in +/LV mice, indicative of cardiac hypertrophy (Fig. 1G). The surviving +/LV animals developed a spectrum of other pathologies with variable penetrance (Supplemental Table S1). Although they did not develop any signs of advanced cancer, they showed a predisposition to the development of benign tumors, including skin papillomas and intestinal polyps, when aged (Supplemental Fig. S2; Supplemental Table S1).
L597VBraf is a weak activator of the Mek/Erk pathway but does not transform MEFs
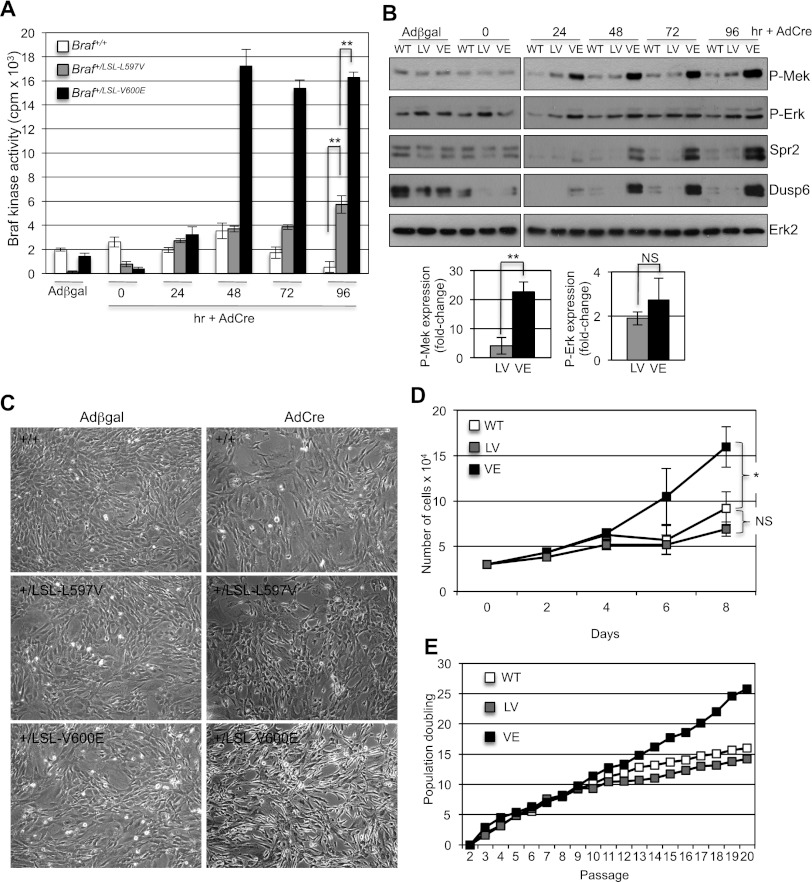
To further investigate the transforming potential of L597VBraf, we induced expression of the mutant protein in MEFs by treating Braf+/LSL-L597V primary MEFs with adenoviral-Cre (AdCre) or adenoviral-βgal (Adβgal). As comparisons, Braf+/+ and Braf+/LSL-V600E MEFs were simultaneously treated. PCR analysis showed that ∼50% recombination of the LSL-L597V and LSL-V600E alleles occurred within 24 h of AdCre treatment, and recombination was virtually complete by 72 h (Supplemental Fig. S3). Previous studies have shown that L597VBRAF is ∼70-fold more active than WTBRAF, while V600EBRAF is ∼500-fold more active when overexpressed (Wan et al. 2004). To assess the activity of L597VBraf when endogenously expressed from one allele of Braf, as occurs in human cancer, we performed Braf kinase assays of MEF protein lysates. V600EBraf expression induced approximately eightfold elevated Braf activity, whereas L597VBraf expression elevated Braf activity by approximately twofold compared with WTBraf (Fig. 2A). While V600EBraf expression gave rise to significant induction of phospho-Mek, phospho-Mek was only slightly elevated in LV cells compared with controls (Fig. 2B). However, phospho-Erk reached levels similar to that in VE cells and was not significantly different (Fig. 2B). To explain this paradox, we analyzed the expression of Sprouty2 and Dusp6, negative regulators of the Mek/Erk pathway (Packer et al. 2009; Pratilas et al. 2009), and found that both were significantly induced by V600EBraf but not by L597VBraf (Fig. 2B). The induction of Dusp6 could explain, in part, the equivalent levels of phospho-Erk in the VE and LV cells, and indeed, we found that phospho-Erk levels are raised in V600EBraf cells when the expression of Dusp6 is down-regulated by siRNA knockdown (Supplemental Fig. S4A). As further confirmation of greater Mek/Erk output by V600EBraf, we also detected higher levels of p90RSK phosphorylation in VE cells compared with LV and wild-type cells (Supplemental Fig. S4B).
Figure 2.
Characterization of MEFs expressing L597VBraf. (A) Braf kinase assays of soluble protein lysates taken from Braf+/+, Braf+/LSL-L597V, and Braf+/LSL-V600E MEFs treated with AdCre for 0–96 h or Adβgal for 96 h. Columns indicate the mean of three samples, and bars indicate the SD. (B) Western blot analysis of protein lysates from Braf+/+, Braf+/LSL-L597V, and Braf+/LSL-V600E MEFs treated with AdCre for 0–96 h or Adβgal for 96 h. Western blots were analyzed with the antibodies indicated. Quantification of Western blot analysis of Mek/Erk phosphorylation for the 96-h time point is shown in the graphs below. P-Mek and P-Erk levels were normalized to Erk2, the fold changes compared with AdCre-infected wild-type (WT) cells are shown, and error bars indicate the SEM. Data were pooled from three MEFs with the same genotype. (C) Representative photographs of Braf+/+, Braf+/LSL-L597V, and Braf+/LSL-V600E MEFs treated with AdCre or Adβgal for 96 h. (D) Growth curves of primary Braf+/+ (wild-type), Braf+/Lox-L597V (LV), and Braf+/Lox-V600E (VE) MEFs over 8 d immediately following 72 h of treatment with AdCre. Mean values of three technical replicates of MEFs of each genotype are shown, and error bars indicate the SEM. These are representative profiles of four different MEFs of each genotype. (E) 3T3 immortalization profiles of primary Braf+/+ (wild-type), Braf+/LSL-L597V (LV), and Braf+/LSL-V600E (VE) MEFs treated with AdCre. Representative profiles of three different MEF lines of each genotypes are shown. For all data, P-values were calculated using the Student's t-test; (*) P < 0.01; (**) P < 0.005; (NS) not significant.
Distinct morphological transformation of primary VE MEFs was observed, as previously reported (Mercer et al. 2005), whereas LV MEFs were not transformed and were similar in morphology to control MEFs (Fig. 2C). In addition, VE primary MEFs had an enhanced growth rate (Fig. 2D) and immortalized at early passage number (Fig. 2E), while LV cells had growth and immortalization profiles similar to control MEFs (Fig. 2D,E). Overall, these data show that L597VBraf is able to induce weakly elevated signaling through the Mek/Erk pathway, and while this is sufficient to induce RASopathy hallmarks, it is not enough to transform primary fibroblasts.
L597VBraf does not induce lung tumor growth in vivo
To examine the cancer phenotype more directly, we focused on the lung, since L597VBRAF mutations are more prevalent in human non-small-cell lung cancer (NSCLC) than any other cancer (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic). The lungs of Braf+/Lox-L597V mice with and without the CMV-Cre transgene were examined by H&E staining. In both cases, no histopathological changes were observed compared with controls (Fig. 3A). We also examined the consequences of AdCre delivery to the lungs of Braf+/LSL-L597V mice by nasal inhalation compared with the lungs of Braf+/+ and Braf+/LSL-V600E mice. While AdCre induced the rapid formation of multiple benign adenomas in the Braf+/LSL-V600E mice (Fig. 3B), as previously reported (Dankort et al. 2007), there were no histopathological changes in the Braf+/LSL-L597V lungs (Fig. 3B). Furthermore, while AdCre delivery gave rise to significant levels of LSL-V600E recombination in the lung, AdCre-mediated LSL-L597V recombination was not detectable (Supplemental Fig. S5A), indicating that L597VBraf expression in the lung does not give a selective growth advantage.
Figure 3.
Analysis of L597VBraf-expressing lung. (A) H&E-stained lung sections taken from Braf+/+;CMV-Cre+/o mice, Braf+/Lox-L597V;CMV-Cre+/o mice, or Braf+/Lox-L597V mice lacking the CMV-Cre transgene. Bars, 100 μm. (B) H&E-stained lung sections taken from Braf+/+, Braf+/LSL-L597V, and Braf+/LSL-V600E mice treated with 1 × 108 plaque-forming units (pfu) by nasal inhalation 8 wk post-AdCre infection. Bars, 100 μm. (C) Braf kinase assays of protein lysates prepared from the lungs of Braf+/+;CMV-Cre+/o (wild-type) and Braf+/Lox-L597V;CMV-Cre+/o (LV) mice as well as protein lysates prepared from Braf+/LSL-V600E mice treated with 1 × 108 pfu of AdCre by nasal inhalation 8 wk post-AdCre infection (VE). Columns indicate the mean of three technical replicates of three different biological samples, and bars indicate the SD. P-values were calculated using the Student's t-test; (**) P < 0.005; (*) P < 0.01. (D) Western blot analysis of protein lysates prepared from the lungs of Braf+/+;CMV-Cre+/o (wild-type), Braf+/Lox-L597V;CMV-Cre+/o (LV), and Braf+/LSL-V600E mice treated with 1 × 108 pfu of AdCre by nasal inhalation 8 wk post-AdCre infection (VE). Western blots were analyzed with the antibodies indicated.
Constitutive expression of V600EBraf in mice gives rise to embryonic lethality (Mercer et al. 2005). Therefore, in order to compare Raf–Mek–Erk signaling between the V600EBraf- and L597VBraf-expressing lungs, protein lysates were analyzed from the lungs of AdCre-infected Braf+/LSL-V600E mice (Fig. 3B, right panel) in comparison with the lungs of Braf+/Lox-L597V;CMV-Cre+/o mice (Fig. 3A, middle panel). The Braf+/Lox-L597V lungs had Braf activity in between that of the Braf+/+ and V600EBraf-expressing samples (Fig. 3C). Phospho-Mek levels were slightly elevated, but to a significantly lower extent than the V600EBraf lung, whereas phospho-Erk levels were significantly higher in the L597VBraf lung than the V600EBraf lung (Fig. 3D), presumably due to the high levels of Dusp6 induced by V600EBraf (Fig. 3D) that can down-regulate phospho-Erk (Supplemental Fig. S4A). This is a slightly different scenario from MEFs where phospho-Erk levels were comparable in the L597VBraf and V600EBraf samples (Fig. 2B), suggesting that there may be tissue-specific differences in regulation of the Mek/Erk pathway. Overall, these data show that, as with MEFs, L597VBraf has weak activity toward the Mek/Erk pathway in the lung, and this is not sufficient to induce tumor development in vivo.
L597VBraf modifies G12DKras-induced MEF transformation
L597BRAF mutations are frequently coincident with other oncogenic driver mutations in human cancer, particularly oncogenic RAS mutations (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic). Given that L597VBraf is not able to transform cells on its own (Fig. 2C), we assessed its role in modifying G12DKras transformation. Braf+/LSL-L597V mice were intercrossed with Kras+/LSL-G12D mice (Jackson et al. 2001), and double heterozygotes were obtained along with single heterozygote controls. Primary MEFs were treated with AdCre along with Braf+/LSL-V600E MEFs, and Cre-mediated recombination of Lox–Stop–Lox (LSL) alleles was confirmed by PCR genotyping (Supplemental Fig. S5B). Consistent with previous observations (Tuveson et al. 2004; Mercer et al. 2005), G12D and VE MEFs showed evidence of transformation, although the V600EBraf-driven morphology was far more distinct than that driven by G12DKras (Fig. 4A). Adding L597VBraf and G12DKras mutations together led to a more striking morphological transformation than either mutation alone, and the double-mutant cells were more similar to VE cells in this regard (Fig. 4A).
Figure 4.
L597VBraf modifies G12DKras MEF transformation. (A) Representative photographs of primary MEFs. (B) Western blot analysis of protein lysates from primary MEFs. Western blots were analyzed with the antibodies indicated. (C) Quantification of Western blot analysis of Mek/Erk phosphorylation and Dusp6/Sprouty2 expression. P-Mek, P-Erk, Dusp6, and Sprouty2 levels were normalized to Erk2, and the fold changes compared with wild-type MEFs are shown, where error bars indicate the SEM. Data were pooled from three MEFs with the same genotype. (D) Growth curves of primary MEFs over 8 d immediately following 72 h of treatment with AdCre. Mean values of three technical replicates of MEFs of each genotype are shown, and error bars indicate the SEM. These are representative profiles of four different MEFs of each genotype. (E) 3T3 immortalization profiles of MEFs treated with AdCre. Representative profiles of three different MEF lines of each genotype are shown. For all data, P-values were calculated using the Student's t-test; (*) P < 0.01; (NS) not significant.
The G12DKras and L597VBraf mutations together induced significantly higher levels of phospho-Mek than either mutation alone (Fig. 4B,C). Phospho-Erk levels were not significantly different between the single- and double-mutant cells, presumably because of alterations in the expression of Dusps (Fig. 4B,C). Indeed, the double-mutant MEFs had significantly higher Dusp6 levels compared with the single-mutant G12D or LV cells (Fig. 4B,C). Sprouty2 levels were higher in the G12D/LV cells compared with the LV cells but were not significantly different between the G12D/LV and G12D cells (Fig. 4C), suggesting that Sprouty2 expression may also be regulated by non-Mek/Erk pathways in MEFs.
Analysis of the growth of primary cells showed that G12D cells had higher growth rates than VE cells, whereas the double-mutant cells grew in between the two (Fig. 4D). The G12D/LV MEFs underwent early immortalization, although the kinetics of immortalization was delayed in comparison with G12D cells, with them immortalizing at a passage number more similar to the VE cells (Fig. 4E). The slower growth and immortalization of VE and G12D/LV MEFs in comparison with G12D cells may be related to the higher Mek/Erk signaling in these cells and the consequent impact on D-type cyclin expression. Indeed, we found that, although the expression of Cyclin d1 and d2 was elevated in VE, G12D, and G12D/LV cells compared with control and LV cells, Cyclin d3 was only elevated in G12D cells (Supplemental Fig. S6). Overall, these data show that L597VBraf enhances G12DKras signaling through the Mek/Erk pathway, and this has the effect of partially converting G12DKras to an oncogene more like V600EBraf.
L597VBraf modifies G12DKras-induced lung tumor development
G12DKras and V600EBraf induce the formation of pulmonary preneoplastic lesions with similar histopathological characteristics (Jackson et al. 2001; Dankort et al. 2007), and both are dependent on the Erk signaling pathway for tumor maintenance (Ji et al. 2007). The major difference between the two is that V600EBraf induces more rapid tumor growth followed by the induction of senescence, whereas G12DKras elicits faster progression to adenocarcinoma. We administered AdCre by nasal inhalation to Kras+/LSL-G12D and Braf+/LSL-V600E mice as well as double-mutant Kras+/LSL-G12D;Braf+/LSL-L597V mice and analyzed lung tumor development at 8 and 12 wk post-AdCre treatment. Cre-mediated recombination was confirmed by PCR (Supplemental Fig. S5A). The VE lung had a higher tumor burden than the G12D lung, and the tumor burden for the G12D/LV lung remained similar to that driven by G12D (Fig. 5A,B). Consistent with previous observations (Jackson et al. 2001; Dankort et al. 2007), G12DKras and V600EBraf induced a spectrum of preneoplastic lesions, including bronchiolar hyperplasia (BH), adenomatous alveolar hyperplasia (AAH), and adenomas, although V600EBraf induced far more adenomas and fewer AAH lesions than G12DKras (Fig. 5A,C). The presence of L597VBraf on top of G12DKras generated significantly more adenomas but fewer AAH lesions (Fig. 5A,C). Mosaic Cre-mediated recombination is a more frequent occurrence with multiple floxed alleles, and indeed, a lower level of recombination of the BrafLSL allele was observed in the G12D/LV lung compared with the VE lung (Supplemental Fig. S5A). This may account in part for the observation that the G12D/LV phenotype is only partially transitioned to the VE lung phenotype (Fig. 5A–C). While occasional adenocarcinoma transitions were observed in the G12D mice (two of 10 mice analyzed), none were observed in the VE mice (zero of 10 mice analyzed) or G12D/LV mice (zero of 11 mice analyzed). Activation of the Mek/Erk pathway and the expression of downstream targets Dusp6 and Sprouty2 were enhanced by combining the L597VBraf mutation with the G12DKras mutation (Fig. 5D). Thus, as in MEFs, L597VBraf modifies G12DKras-driven lung tumor development such that there is a partial transition to tumors with biochemical and histological features more similar to those driven by V600EBraf.
Figure 5.
L597VBraf modifies G12DKras-driven lung tumorigenesis. (A) H&E staining of representative lung sections from Kras+/LSL-G12D, Braf+/LSL-V600E, and Braf+/LSL-L597V;Kras+/LSL-G12D mice treated with 1 × 108 pfu of AdCre 8 wk post-infection. Bars: top panels, 2 mm; bottom panels, 100 μm. (B) Tumor burden in lungs of Kras+/LSL-G12D, Braf+/LSL-V600E, and Braf+/LSL-L597V;Kras+/LSL-G12D mice at 8 and 12 wk post-infection with 1 × 108 pfu of AdCre. Burden was determined as the percent diseased area per total lung area. Data represent the mean of three samples of each genotype at each time point, and error bars indicate the SD. (C) Percent of pulmonary lesions with respect to the total number of lesions in Kras+/LSL-G12D, Braf+/LSL-V600E, and Braf+/LSL-L597V;Kras+/LSL-G12D mice treated with 1 × 108 pfu of AdCre pooled from 8- and 12-wk time points combined. Data represent the mean of six samples of each genotype, and error bars indicate the SD. For B and C, P-values were calculated using the Student's t-test; (**) P < 0.005; (NS) not significant. (D) Western blot analysis of protein lysates from the lungs of Braf+/+, Kras+/LSL-G12D, Braf+/Lox-V600E, and Braf+/Lox-L597V;Kras+/Lox-G12D mice treated with 1 × 108 pfu of AdCre 8 wk post-infection and lysates from lungs of Braf+/LSL-L597V;CMV-Cre+/o mice. Western blots were analyzed with the antibodies indicated.
Transcriptome profiling
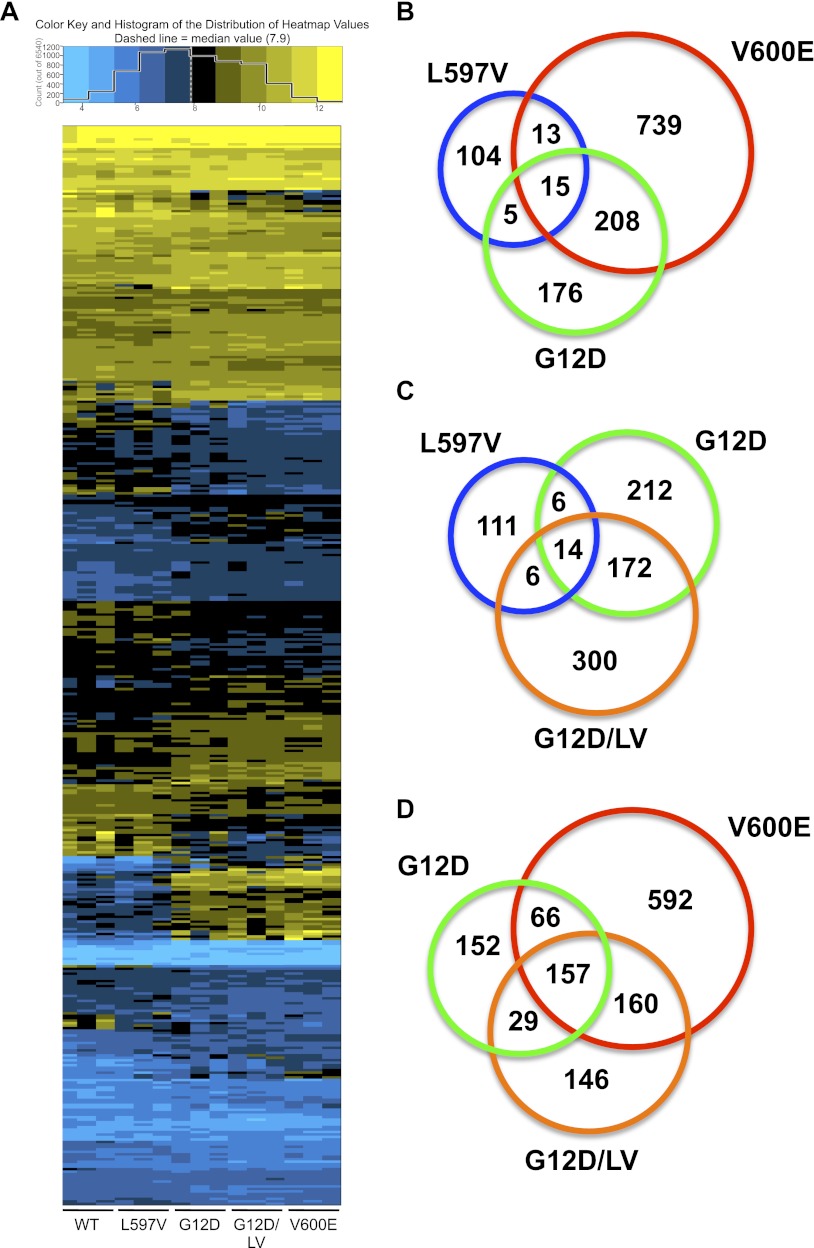
In order to perform a more direct assessment of the impact of L597VBraf on G12DKras, we undertook microarray comparison of genes expressed in immortalized wild-type, LV, VE, G12D, and G12D/LV MEFs. Following microarray normalization and summarization, analysis of variance (ANOVA) with a 0.01 false discovery rate (FDR) threshold was used to identify genes significantly altered compared with wild-type controls (Supplemental Table S2). For all genotypes, more genes were up-regulated than were down-regulated (Fig. 6A). There were 137 gene changes in the LV samples, 404 in the G12D samples, 492 in the G12D/LV samples, and 975 in the VE samples (Fig. 6B,C). Thus, consistent with the morphology data (Fig. 4A), L597VBraf had a weaker molecular effect than either of the other mutations, whereas V600EBraf had the strongest molecular effect. Less than 20% of the gene expression changes observed in the LV MEFs were shared with G12D or VE MEFs, whereas ∼50% of the gene changes induced by G12D were shared by VE (223 genes) (Fig. 6A,B). Given that previous studies in melanoma cells have shown that the gene expression signature induced by V600EBRAF is attributable to signaling through the MEK/ERK pathway (Packer et al. 2009), these data suggest that approximately half of the gene changes induced by G12DKras arise through signaling through this pathway, and the weak effect of L597VBraf on the Mek/Erk pathway is insufficient to induce cognate transcriptional changes.
Figure 6.
Microarray analysis. (A) Heat map of 436 genes significantly differentially expressed in G12D/LV MEFs compared with wild-type MEFs at a cutoff raw P-value of <0.01 in each of the biological samples. Values were generated through Affymetrix RMA normalization of all of the arrays, and the expression represents the absolute level of expression. The scale is log2, and the median expression level for the whole genome is ∼7.9. Genes are ordered by magnitude of differential expression. (B–D) Venn diagrams indicating numbers of shared genes differentially expressed in each of the samples indicated compared with wild-type samples at a cutoff raw P-value of <0.01.
Of the gene changes observed in the G12D/LV samples, ∼40% were also found in single-mutant samples alone, predominantly within the G12D cohort (Fig. 6C,D), indicating that the combination of the two mutations is able to mirror the molecular effects of either mutation alone to some extent, but additional molecular changes are induced on top of this. Indeed, the G12D/LV samples had more gene expression changes shared in common with VE than G12D (∼65% compared with 40%) (Fig. 6C,D), suggesting that L597VBraf may subvert some of the signaling induced by G12DKras away from other Ras–effector pathways toward the Mek/Erk pathway. In addition, ∼30% of the gene changes in the G12D/LV samples were not shared with either VE or G12D (146 of 492 genes) (Fig. 6D), suggesting that L597VBraf may act to regulate Mek/Erk-independent and Ras-independent signaling pathways.
Gene ontology analysis performed using GenMAPP (http://www.genmapp.org/go_elite) showed that the gene changes shared by VE, G12D, and G12D/LV (Supplemental Table S3) were enriched for those involved in inactivation of the MAPK pathway, although this was not quite statistically significant (P = 0.0574, adjusted for multiple hypothesis testing); presumably, this occurs as a response to hyperactivation of the Mek/Erk pathway in these cells (Fig. 4B). No other enrichments were observed in other data sets except for gene changes unique to G12D/LV that showed a preponderance for genes involved in RNA binding and translation (Supplemental Table S4).
L597VBraf signals through Braf and Craf
Impaired-activity BRAF mutants are known to transactivate CRAF (Wan et al. 2004; Kamata et al. 2010). To assess whether this is also the case for intermediate-activity mutants, Craf activity was assessed in LV MEFs and lungs and compared with wild-type as well as VE, G12D, and G12D/LV samples. Craf activity was significantly elevated by ∼5.4-fold and ∼1.7-fold in the LV lungs and MEFs, respectively, compared with wild-type samples, while Craf activity was not significantly elevated in the VE samples (Fig. 7A,B). The kinase activities of both Braf and Craf were slightly elevated by G12D, but there was striking induction of Craf activity in the G12D/LV lungs and MEFs of ∼20-fold and ∼8.7-fold, respectively (Fig. 7A,B). Craf and Braf siRNA was performed on immortalized MEFs to identify the contribution of each isoform to downstream Mek/Erk activation. As previously determined for cells expressing V600EBraf, siRNA knockdown of Braf but not Craf significantly suppressed Mek phosphorylation (Fig. 7C). In G12D cells, Craf or Braf siRNA alone significantly suppressed Mek phosphorylation, although there was a greater suppression following combined Braf/Craf siRNA knockdown (Fig. 7C). The LV and G12D/LV cells responded in a way similar to the G12D cells. In several cases, alterations in phospho-Erk levels did not always correlate with phospho-Mek levels; this is likely attributable to changes in expression of Dusps arising as a result of Braf/Craf down-regulation.
Figure 7.
L597VBraf signals through Craf and Braf. (A) Raf kinase assays of protein lysates isolated from the lungs of Braf+/+ (wild-type), Braf+/LSL-V600E (VE), Kras+/Lox-G12D (G12D), and Braf+/LSL-L597V;Kras+/LSL-G12D (G12D/LV) mice treated with 1 × 108 pfu of AdCre 8 wk post-infection as well as protein lysates from lungs of Braf+/LSL-L597V;CMV-Cre+/o (LV) mice. The mean of three samples is shown, and error bars represent the SD. (B) Raf kinase assays of protein lysates isolated from primary MEFs following 72 h post-AdCre treatment. The mean of three samples is shown, and error bars represent the SEM. (A,B) Student's t-test in comparison with respective Braf/Craf kinase activities for wild-type samples; (*) P < 0.01; (**) P < 0.005; (NS) not significant. (C) Immortalized MEFs of each genotype were transfected with scrambled (Scr) siRNA or Craf, Braf, or both siRNAs, and Western blots were analyzed with the antibodies indicated. Quantification of Mek/Erk phosphorylation following siRNA treatment is shown in the graphs on the right. P-Mek and P-Erk levels were normalized to Erk2, and the fold changes compared with Scr-treated samples are shown, where error bars indicate the SEM. Data were pooled from three experiments. (D) Immortalized MEFs of each genotype were treated with carrier (C), U0126 (U0), PD184352 (PD), PLX4720 (PLX), or SB590885 (885) for 4 h, and Western blots were analyzed with the antibodies indicated. Quantification of Mek/Erk phosphorylation following RAF inhibitor treatment is shown in the graphs below. P-Mek and P-Erk levels were normalized to Erk2, and the fold changes compared with carrier-treated samples are shown, where error bars indicate the SEM. Data were pooled from six samples, representing three different cell lines of each genotype treated with PLX4720 or SB590885. For Western blot quantitations in C and D, P-values were calculated using the Student's t-test; (*) P < 0.01; (**) P < 0.005; (***) P < 0.001. (E) Craf:Braf heterodimer formation. LV and G12D/LV MEFs were treated with either PD184352/PLX4720 (P/X) or carrier control (C), protein lysates were harvested and immunoprecipitated for Braf, and immunoprecipitates were analyzed for Braf and Craf. As a control, the G12D/LV samples were also immunoprecipitated without (−) primary antibody and analyzed with the same antibodies.
RAF inhibitors induce paradoxical Mek/Erk activation
Small molecule inhibitors targeting BRAF are now in clinical use as anti-cancer therapies (Chapman et al. 2011), and MEK inhibitors have proven effective at ameliorating disease phenotypes in RASopathy models (Schuhmacher et al. 2008; Anastasaki et al. 2009). Human cancer and RASopathy cell lines with the L597VBRAF mutation are not currently available, and so we analyzed BRAF/MEK inhibitor responses using mouse cells. Each of the immortalized LV, VE, G12D, and G12D/LV MEFs was treated with either MEK inhibitors (U0126 and PD184352) or two ATP competitive RAF inhibitors (PLX4720 and SB590885). Mek/Erk activity was blocked in all cell lines in response to the MEK inhibitors (Fig. 7D). RAF inhibitors significantly suppressed Mek/Erk phosphorylations in the VE cells, as expected, but Mek/Erk phosphorylations were significantly induced in the LV, G12D, and G12D/LV cells (Fig. 7D). Like WTBRAF, L597VBRAF formed a heterodimer with CRAF in HEK293T cells following transient transfection (Supplemental Fig. S7), and furthermore, heterodimer formation between endogenous Braf and Craf was strongly induced in LV and G12D/LV MEFs following dual treatment of these cells with PLX4720 and PD184352 (Fig. 7E).
Discussion
The L597VBRAF mutation is a relatively unique mutation because it is acquired somatically in cancer samples yet is also mutated in RASopathy conditions. Here we identified the molecular basis for the involvement of the mutation in these two pathologies. Using a knock-in mouse model, we show that L597VBraf can induce weak activation of the Mek/Erk pathway and that this is sufficient to drive RASopathy hallmarks but not cancer. L597VBraf only contributes to cancer when it is coexpressed with another oncogenic mutation, and in this study we demonstrate a modifying effect on G12DKras-driven oncogenesis. We also found that RAF inhibitors induce paradoxical activation of the Mek/Erk pathway in L597VBraf mutant cells, cautioning against the use of vemurafenib/PLX4032 or other similar RAF inhibitors in the treatment of RASopathies or cancers carrying the mutation.
L597VBRAF is the best-characterized mutation affecting residue L597. Previous studies have shown that it has intermediate kinase activity when overexpressed in COS cells (Wan et al. 2004), and, using endogenous expression from one allele of Braf, we confirmed the intermediate nature of L597VBraf and its weak impact on the Mek/Erk pathway (Fig. 2). The fact that RASopathy hallmarks can be induced by L597VBraf but not cancer suggests that activation of downstream signaling pathways, particularly the Mek/Erk pathway, needs to pass a key threshold for transformation to occur. L597VBRAF—and presumably other BRAF mutations present in RASopathies—clearly cannot activate downstream pathways past this point. For cancer, acquisition of a second mutation is a requirement for tipping the balance, and this may explain why L597BRAF mutations are coincident with other low MEK/ERK-activating mutants such as S259ACRAF in occasional human cancers in addition to driver oncogenes with higher activity toward the MEK/ERK pathway (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic). BRAF mutant human RASopathy patients (Sarkozy et al. 2009; Tidyman and Rauen 2009) and L597VBraf-expressing mice show some predisposition to cancer when aged (Supplemental Table S1); such lesions may arise as a result of “second hits” being acquired in genes that allow the transforming threshold to be surpassed.
It has been estimated that only seven to 15 somatic mutations in key “driver” genes are absolutely required for tumor development (Beerenwinkel et al. 2007), with the remainder being “passenger” mutations or bystanders that do not contribute to the carcinogenesis process. However, this is likely to be a gross oversimplification, since it does not account for the existence of genetic interactions that can modify drivers through epistatic mechanisms (Ashworth et al. 2011). While it is difficult to functionally prove the existence of such modifiers in human cancers, recent data from a transposon screen for genes involved in promoting Apc-driven intestinal tumorigenesis identified modifiers of the canonical Wnt pathway (March et al. 2011). We also previously described a functional interaction between the impaired-activity D594ABraf mutation and oncogenic Kras in the induction of rapid onset melanoma in mice (Heidorn et al. 2010; Kamata et al. 2010). In this study, we characterized the intermediate-activity Braf mutant L597VBraf and found that it falls into the category of “epistatic modifier,” as it does not act as an oncogenic driver by itself but is able to interact with G12DKras to induce high levels of signaling through the Mapk pathway as well as through Mapk-independent pathways.
L597VBraf induces a shift from AAH to adenoma lesions in the G12DKras mutant lung (Fig. 5). Since adenomas are thought to arise through increased proliferation of AAH followed by the induction of senescence (Kerr 2001; Dankort et al. 2007), these data suggest that L597VBraf enhances the proliferation/senescence of G12DKras mutant alveolar type II pneumocytes in vivo. Similarly, morphological transformation and growth of G12DKras MEFs are more similar to that driven by V600EBraf when coexpressed with L597VBraf (Fig. 4). All in all, L597VBraf induces a partial transition from a G12DKras mutant phenotype to a more V600EBraf-like phenotype, as confirmed at the molecular level by microarray analysis (Fig. 6). This is thought to be partly attributable to increased signaling through the Mek/Erk pathway, as together, L597VBraf and G12DKras raise Mek/Erk activity levels to those similar to V600EBraf. In spite of this, the consequences for tumor development in the lung are somewhat paradoxical, as although enhanced adenoma formation is observed in the L597VBraf;G12DKras mutant lung compared with the G12DKras mutant lung (Fig. 5A), as with the V600EBraf mutant lung, there is reduced adenocarcinoma progression. Thus, the selective drive for the evolution of human cancers with both the L597VBRAF and G12DKRAS mutations must occur in the initiation stage, regardless of the consequences for subsequent cancer progression.
In addition to a transition to a more V600EBraf-like molecular profile, L597VBraf together with G12DKras induce the expression of several genes that are not shared by V600EBraf or G12DKras alone (Fig. 6D), suggesting the activation of Mek/Erk-independent and/or Ras-independent signaling pathways. This observation may be related to the fact that Craf is strongly activated in the double-mutant cells but is weakly activated in the G12DKras cells and is low in the V600EBraf cells (Fig. 7A,B). Craf is known to operate through a number of Mek/Erk-independent signaling pathways (Niault et al. 2009), and conceivably, activation of these pathways may account for the unique sets of genes induced by L597VBraf combined with G12DKras, although Craf has not previously been connected with genes involved in translation or RNA processing, which seem to be particularly enriched in this data set (Supplemental Table S4). Craf activity is also weakly elevated in the L597VBraf-expressing single-mutant MEFs, but these cells show a phenotype different from our previous analysis of MEFs expressing the impaired activity mutant D594ABraf (Kamata et al. 2010). Craf transactivation in this situation was shown to lead to immortalization of MEFs associated with induction of aneuploidy, and this was reversed by Craf inhibition. The reason for the difference between the two may be related to the fact that Craf is more strongly activated by the D594ABraf mutation (approximately fivefold greater than wild-type MEFs) than the L597VBraf mutation (∼1.7-fold greater than wild-type MEFs). Alternatively, we have not yet ruled out a role of suppressed Braf activity in contributing to the evolution of aneuploidy in D594ABraf-expressing cells.
Throughout this study, we found that there was a good correlation between Raf activity and levels of Mek phosphorylation, but Erk phosphorylation was more variable. As demonstrated in other studies (Pratilas et al. 2009), this is related to the expression of Dusp6 and Sprouty2, negative regulators of the Mek/Erk pathway. Both are transcriptional targets of the pathway (Packer et al. 2009; Pratilas et al. 2009), and Sprouty2 has been shown to act as a tumor suppressor at least in the context of G12DKras-mediated lung tumorigenesis (Shaw et al. 2007). Dusp6 is a dual-specificity phosphatase that acts downstream from Mek to inactivate Erk (Keyse 2008), whereas Sprouty2 acts at multiple levels of the Erk pathway, one way being through direct interaction with Raf (Kim and Bar-Sagi 2004). In MEFs and the lung, V600EBraf expression was found to induce very high levels of expression of these proteins (Figs. 2, 3), whereas L597VBraf did not at all, indicating higher Erk pathway output by V600EBraf and no feedback inhibition in the L597VBraf cells. Although levels of phospho-Mek were significantly higher in the V600EBraf mutant cells, phospho-Erk levels were similar in the two. This suggests that the pathway is sensitive to feedback inhibition below Mek at the level of Erk in the V600EBraf mutant cells, presumably through the action of Dusps, but insensitive to feedback inhibition upstream of Mek. The mechanism of insensitivity upstream of Mek may be related to the fact that the active Braf kinase conformation of V600EBraf cannot bind to Sprouty2 (Brady et al. 2009). Regardless of the mechanism, feedback regulation of the Erk pathway offers exquisite control of the pathway and is important in regulation of the ultimate biological outputs of the pathway.
Using siRNA, we show that L597VBraf activates the Mek/Erk pathway through its intrinsic Braf kinase activity as well as through transactivation of Craf on both the WTKras and G12DKras backgrounds (Fig. 7C). This is a scenario similar to G12DKras cells expressing WTBraf and WTCraf (Blasco et al. 2011; Karreth et al. 2011) but different from cells expressing V600EBraf that signal entirely through its intrinsic activity. As with WTBraf, the likely mechanism for Craf transactivation by L597VBraf is through dimerization, membrane localization, and interaction with Ras.GTP. Given this observation, it is not surprising that ATP-competitive RAF inhibitors (PLX4720 and SB590885) activate the Mek/Erk pathway in L597VBraf mutant cells (Fig. 7D). This finding has important clinical implications, since it suggests that response to vemurafenib (PLX4032) is dependent on not just whether a tumor has a WTBRAF or V600EBRAF allele, but also the type of BRAF mutation and the level of mutant BRAF kinase activity acquired. Mutants such as V600EBRAF with activity approximately eightfold greater than WTBRAF clearly allow response to vemurafenib, but mutants with approximately twofold greater activity, such as L597VBRAF, do not. It will be interesting to assess what threshold of BRAF activity is required to allow response to vemurafenib, and related to this is the question of whether mutant BRAF isoforms undergo dimerization and the levels of RAS activation achieved in cells with different levels of BRAF activity.
Materials and methods
Mouse strains and genotyping
All animal experiments were carried out under U.K. Home Office License authority. Braf+/LSL-L597V mice were generated in the same way as Braf+/LSL-V600E (Mercer et al. 2005) and Braf+/LSL-D594A (Heidorn et al. 2010; Kamata et al. 2010) mice, except Braf exon 15 contained the C1789A mutation. The Kras+/LSL-G12D mice were as previously reported (Jackson et al. 2001) and were obtained from the Mouse Models of Human Cancers Consortium (MMHCC) Mouse Repository (http://www.nih.gov/science/models/mouse/resources/mmhcc.html). All strains were maintained by backcrossing onto the C57BL6J background, and phenotype analysis was performed for mice that had been maintained for more than five generations on this background strain. Genotyping of Braf+/LSL-L597V, Braf+/LSL-V600E, Braf+/Lox-L597V, Braf+/Lox-V600E, and Cre alleles was performed using the primer systems previously reported (Mercer et al. 2005). The KrasLSL-G12D allele was genotyped using primers 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′ and 5′-CCTTTACAAGCGCACGCAGATGTAGA-3′. To monitor Cre recombination, the KrasLox-G12D allele was genotyped with primers 5′-TGACACCAGCTTCGGCTTCCT-3′ and 5′-TCCGAATTCAGTGACTACAGATGTACAGA-3′. Infection of lungs with AdCre (University of Iowa) was performed as described (Jackson et al. 2001; Dankort et al. 2007).
Histology and tissue staining
Tissues were processed for histology and stained as described (Mercer et al. 2005). For cardiomyocyte analysis, cell membranes were stained with FITC-conjugated wheat germ agglutinin (WGA) (Sigma). For quantification, H&E- and WGA-stained sections were assessed using Image J software (http://rsbweb.nih.gov/ij).
Cells and treatments
MEFs were isolated as reported (Mercer et al. 2005) and maintained in DMEM with 10% FCS and penicillin/streptomycin. MEFs were infected with AdCre or Adβgal by addition of ∼1 × 108 plaque-forming units (pfu) directly to the culture medium. For growth assays, cells were plated at a density of 3 × 104 in triplicate and recounted every 2 d for 8 d. For immortalization assays, MEFs were plated at 3 × 105 cells per 6-cm plate in triplicate, counted, and replated every 3 d. For Braf and Craf siRNA, ON-TARGETplus SMARTpool siRNAs (Dharmacon) were used and transfected using Lipofectamine 2000 as previously described (Noble et al. 2008). For inhibitor treatments, MEFs at ∼80% confluency were treated with 10 μM U0126, 1 μM PD184352, 0.3 μM PLX4720, or 1 μM SB590885 for 4 h or a volume of DMSO equivalent as the carrier control.
Immunoblotting and kinase assays
Protein lysates were prepared by previously published methods (Huser et al. 2001; Mercer et al. 2005). The antibodies used were as follows: phospho-Erk1/2 (Cell Signaling Technologies, #9101S), Erk2 (Santa Cruz Biotechnology, #SC-1647), Craf (BD Biosciences, #610153), phospho-Mek1/2 (Cell Signaling Technologies, #9154S), Gapdh (Millipore, #MAB374), Sprouty2 (Abcam, #AB50317), Dusp6 (Abcam, #AB76310), and Braf (Santa Cruz Biotechnology, #SC-5284). Raf kinase activity was measured as described (Huser et al. 2001; Wan et al. 2004), and the primary antibodies used for immunoprecipitation were Braf (as above) and Craf (Santa Cruz Biotechnology, SC-133). Western blots were quantitated using ImageJ software.
RNA extraction, labeling, and microarray processing
RNA from three biological replicates of immortalized MEFs of each genotype was prepared using a Qiagen RNeasy kit according to the manufacturer's recommendations and quality was assessed using a Bioanalyser 2100 (Agilent). RNA labeling and hybridization to Affymetrix GeneChip Mouse Gene 1.0ST arrays were performed by standard protocols (http://www.gladstone.ucsf.edu/gladstone/site/genomicscore/section/380).
Bioinformatic analysis
Microarrays were normalized for array-specific effects using Affymetrix's Robust Multi-Array (RMA) normalization. Normalized array values were reported on a log2 scale. For statistical analyses, all array probe sets where no experimental groups had an average log2 intensity of >3.0 were removed. Linear models were fitted for each gene using the Bioconductor limma package in R (Gentleman et al. 2004; Smyth 2004). Moderated t-statistics, fold change, and the associated P-values were calculated for each gene. To account for the fact that thousands of genes were tested, FDR-adjusted values were calculated using the Benjamini-Hochberg method (Benjamini and Hochberg 1995).
Acknowledgments
We thank Linda Yee for providing microarray support, Kees Straatman for help with image analysis, and Biomedical Services at Leicester for their invaluable contribution. This work was funded by a Cancer Research UK program grant (C1362/A6969) and Royal Society Wolfson Merit Award to C.A.P.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.193458.112.
References
- Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE 2009. Kinase-activating and kinase-impaired cardio–facio–cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum Mol Genet 18: 2543–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A, Lord CJ, Reis-Filho JS 2011. Genetic interactions in cancer progression and treatment. Cell 145: 30–38 [DOI] [PubMed] [Google Scholar]
- Basso AD, Kirschmeier P, Bishop WR 2006. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res 47: 15–31 [DOI] [PubMed] [Google Scholar]
- Beerenwinkel N, Antal T, Dingli D, Traulsen A, Kinzler KW, Velculescu VE, Vogelstein B, Nowak MA 2007. Genetic progression and the waiting time to cancer. PLoS Comput Biol 3: e225 doi: 10.1371/journal.pcbi.0030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57: 289–300 [Google Scholar]
- Blasco RB, Francoz S, Santamaria D, Canamero M, Dubus P, Charron J, Baccarini M, Barbacid M 2011. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell 19: 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SC, Coleman ML, Munro J, Feller SM, Morrice NA, Olson MF 2009. Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Res 69: 6773–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion KJ, Bunag C, Estep AL, Jones JR, Bolt CH, Rogers RC, Rauen KA, Everman DB 2011. Germline mutation in BRAF codon 600 is compatible with human development: De novo p.V600G mutation identified in a patient with CFC syndrome. Clin Genet 79: 468–474 [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. 2011. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M 2007. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev 21: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. 2002. Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, Bastian BC, Springer C, Marais R 2006. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66: 9483–9491 [DOI] [PubMed] [Google Scholar]
- Ehrenreiter K, Kern F, Velamoor V, Meissl K, Galabova-Kovacs G, Sibilia M, Baccarini M 2009. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell 16: 149–160 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, et al. 2008. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14: 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. 2004. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 5: R80 doi: 10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, et al. 2010. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464: 431–435 [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, et al. 2010. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R, Pritchard C 2001. MEK kinase activity is not necessary for Raf-1 function. EMBO J 20: 1940–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA 2001. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15: 3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Wang Z, Perera SA, Li D, Liang MC, Zaghlul S, McNamara K, Chen L, Albert M, Sun Y, et al. 2007. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res 67: 4933–4939 [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, et al. 2010. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468: 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T, Hussain J, Giblett S, Hayward R, Marais R, Pritchard C 2010. BRAF inactivation drives aneuploidy by deregulating CRAF. Cancer Res 70: 8475–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, et al. 2004. B-RAF is a therapeutic target in melanoma. Oncogene 23: 6292–6298 [DOI] [PubMed] [Google Scholar]
- Karreth FA, Frese KK, Denicola GM, Baccarini M, Tuveson DA 2011. C-Raf is required for the initiation of lung cancer by K-Ras. Cancer Discov 1: 128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM 2001. Pulmonary preinvasive neoplasia. J Clin Pathol 54: 257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM 2008. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27: 253–261 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D 2004. Modulation of signalling by Sprouty: A developing story. Nat Rev Mol Cell Biol 5: 441–450 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M 2003. RAS oncogenes: The first 30 years. Nat Rev Cancer 3: 459–465 [DOI] [PubMed] [Google Scholar]
- March HN, Rust AG, Wright NA, Ten Hoeve J, de Ridder J, Eldridge M, van der Weyden L, Berns A, Gadiot J, Uren A, et al. 2011. Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis. Nat Genet 43: 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer K, Giblett S, Green S, Lloyd D, DaRocha Dias S, Plumb M, Marais R, Pritchard C 2005. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res 65: 11493–11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut C, Settleman J 2009. Targeting the RAF–MEK–ERK pathway in cancer therapy. Cancer Lett 283: 125–134 [DOI] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al. 2010. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468: 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niault T, Sobczak I, Meissl K, Weitsman G, Piazzolla D, Maurer G, Kern F, Ehrenreiter K, Hamerl M, Moarefi I, et al. 2009. From autoinhibition to inhibition in trans: The Raf-1 regulatory domain inhibits Rok-α kinase activity. J Cell Biol 187: 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble C, Mercer K, Hussain J, Carragher L, Giblett S, Hayward R, Patterson C, Marais R, Pritchard CA 2008. CRAF autophosphorylation of serine 621 is required to prevent its proteasome-mediated degradation. Mol Cell 31: 862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer LM, East P, Reis-Filho JS, Marais R 2009. Identification of direct transcriptional targets of (V600E)BRAF/MEK signalling in melanoma. Pigment Cell Melanoma Res 22: 785–798 [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH 2001. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Rauen KA, Seidenberg MS 2010. Effects of germline mutations in the Ras/MAPK signaling pathway on adaptive behavior: Cardiofaciocutaneous syndrome and Noonan syndrome. Am J Med Genet A 152A: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N 2010. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464: 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N 2009. (V600E)BRAF is associated with disabled feedback inhibition of RAF–MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci 106: 4519–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen KA, Banerjee A, Bishop WR, Lauchle JO, McCormick F, McMahon M, Melese T, Munster PN, Nadaf S, Packer RJ, et al. 2011. Costello and cardio–facio–cutaneous syndromes: Moving toward clinical trials in RASopathies. Am J Med Genet C Semin Med Genet 157: 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, McCormick F, Rauen KA 2006. Germline mutations in genes within the MAPK pathway cause cardio–facio–cutaneous syndrome. Science 311: 1287–1290 [DOI] [PubMed] [Google Scholar]
- Sarkozy A, Carta C, Moretti S, Zampino G, Digilio MC, Pantaleoni F, Scioletti AP, Esposito G, Cordeddu V, Lepri F, et al. 2009. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: Molecular diversity and associated phenotypic spectrum. Hum Mutat 30: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher AJ, Guerra C, Sauzeau V, Canamero M, Bustelo XR, Barbacid M 2008. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest 118: 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AT, Meissner A, Dowdle JA, Crowley D, Magendantz M, Ouyang C, Parisi T, Rajagopal J, Blank LJ, Bronson RT, et al. 2007. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev 21: 694–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3 doi: 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT, et al. 2012. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 366: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA 2009. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev 19: 230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. 2004. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5: 375–387 [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, et al. 2004. Mechanism of activation of the RAF–ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116: 855–867 [DOI] [PubMed] [Google Scholar]