Abstract
Sound localization relies on the neural processing of monaural and binaural spatial cues that arise from the way sounds interact with the head and external ears. Neurophysiological studies of animals raised with abnormal sensory inputs show that the map of auditory space in the superior colliculus is shaped during development by both auditory and visual experience. An example of this plasticity is provided by monaural occlusion during infancy, which leads to compensatory changes in auditory spatial tuning that tend to preserve the alignment between the neural representations of visual and auditory space. Adaptive changes also take place in sound localization behavior, as demonstrated by the fact that ferrets raised and tested with one ear plugged learn to localize as accurately as control animals. In both cases, these adjustments may involve greater use of monaural spectral cues provided by the other ear. Although plasticity in the auditory space map seems to be restricted to development, adult ferrets show some recovery of sound localization behavior after long-term monaural occlusion. The capacity for behavioral adaptation is, however, task dependent, because auditory spatial acuity and binaural unmasking (a measure of the spatial contribution to the “cocktail party effect”) are permanently impaired by chronically plugging one ear, both in infancy but especially in adulthood. Experience-induced plasticity allows the neural circuitry underlying sound localization to be customized to individual characteristics, such as the size and shape of the head and ears, and to compensate for natural conductive hearing losses, including those associated with middle ear disease in infancy.
Considerable plasticity exists in the neural circuits that process sensory information. Although plasticity is greatest during development, certain aspects of the mature brain maintain the capacity to reorganize in response to changes in the activity patterns of sensory inputs. Experience-mediated plasticity is most commonly associated with higher-level response properties that are generated within the brain by serial stages of computation. One of the best examples is provided by the neural mechanisms underlying sound localization, which are adapted by experience to the features of the individual.
Identifying the location of sounds produced by potential mates, prey, or predators is one of the most important functions of the auditory system. This ability relies on the extraction of direction-dependent cues generated by the head, torso, and external ears. For localization in the horizontal plane, the separation of the two ears allows mammals to use interaural time differences (ITDs) at low frequencies and interaural level differences (ILDs) at high frequencies (1, 2). These binaural cues also contribute to the ability of listeners to detect and discriminate signals of interest against a background of masking noise (3).
ITDs and ILDs do not, by themselves, provide a sufficient basis for localizing a sound source. For individual frequencies, both cues are spatially ambiguous in that the same binaural disparity value may arise from different directions in space. Moreover, when binaural cues are introduced into sounds presented over headphones, humans report that the sound is lateralized inside the head, rather than localized to a specific direction in the external environment. Additional spatial cues are generated, however, when broadband sounds travel from a source in real space and pass through the external ear. Depending on the direction of the sound source, certain frequency components will be increased or decreased in amplitude, giving rise to specific spectral patterns at each ear (1, 2). Along with the acoustic shadow cast by the head, these spectral cues contribute to the complex pattern of ILDs that are generated in the free field and are responsible for resolving front–back confusions and for localization in the vertical plane, where minimal binaural information is available. Presentation over headphones of virtual acoustic space signals, which incorporate all of the spatial information associated with free-field sound sources, including the filtering characteristics of the external ears, have highlighted the role of spectral cues in producing an externalized perception of auditory space (4). Monaural spectral cues are also presumably responsible for the capacity of unilaterally deaf humans to localize sounds in both azimuth and elevation (5, 6)
Neural Representations of Auditory Space
The initial processing of ITDs and ILDs is carried out predominantly, but not exclusively, in the medial superior olive and the lateral superior olive, respectively (7). These nuclei project to the central nucleus of the inferior colliculus (IC) in the midbrain, which also receives inputs from the contralateral dorsal cochlear nucleus, where monaural spectral cues seem to be processed (8), and from other auditory brainstem nuclei. Although the multiple inputs to the central nucleus of the IC allow further processing to take place (9–11), neurons sensitive to different auditory localization cues remain largely segregated within this nucleus, which reflects the the frequency dependence of the cues and the tonotopic organization of the brainstem auditory nuclei (7, 12). Spatial information is then relayed via the thalamus to the primary auditory cortex. The importance of the primary auditory cortex in sound localization is indicated by the inability of mammals to approach sound sources in the contralateral hemifield after unilateral lesions of this cortical area (13, 14). How sound source location is encoded in the primary auditory cortex remains unclear but is likely to be based on the distribution of activity, in terms of both firing rate and temporal discharge pattern, across assemblies of neurons (15).
In addition to more complex behaviors that involve the cortex, an important function of auditory localization is the guidance of reflexive orienting responses that shift attention toward unexpected sounds in the environment. These seem to be mediated at the level of the superior colliculus (SC), which receives inputs from the external nucleus of the IC, from the nucleus of the brachium of the IC, and from other auditory regions of the brainstem and cortex (16, 17). The SC is of particular interest to studies of sound localization, because, in contrast to the primary auditory cortex, the spatial selectivity of the auditory neurons found there varies systematically to form a map of space (18–20), much like the visual and tactile maps that also exist in the SC and elsewhere in the brain.
Whereas neural maps of visual space and of the body surface arise from topographically ordered projections from the primary afferents innervating the retina and skin, respectively, the map of auditory space has to be computed by tuning neurons to combinations of localization cues that correspond to specific sound directions. In mammals, a systematic variation in sensitivity to ILDs and to monaural spectral cues seems to provide the acoustical basis for the map of auditory space in the SC (1). Given the high-frequency bias of SC neurons, it is less likely that ITDs are involved. Nevertheless, ITD sensitivity has been demonstrated for cat SC neurons over ranges exceeding those produced naturally by the separation of the ears (21). Because the response latencies of auditory neurons tend to be shorter at higher sound levels, the timing of inputs arriving from each ear may differ for high-frequency sounds located to one side of the head. Consequently, apparent sensitivity to ITDs may provide a basis for the encoding of ILDs.
Auditory, visual, and somatosensory inputs converge on the deeper layers of the mammalian SC, whereas the superficial layers are exclusively visual in function. Despite this laminar segregation, the sensory inputs to both regions are topographically aligned, as illustrated in Fig. 1 A and D for the visual representation in the superficial layers and the auditory representation in the deeper layers. Individual neurons in the deeper layers of the SC often receive inputs from more than one sensory modality, and their responses are determined by the spatiotemporal relationship between different sensory stimuli (22). The responses of these neurons tend to be enhanced when different sensory cues are presented in close spatial and temporal proximity, as would be the case for a source that can be both seen and heard, but depressed with stimuli that are widely separated in space or time. These multisensory interactions highlight the importance of aligning the different sensory maps in the SC. Indeed, modulation of auditory responses by eye-position signals may allow intersensory map registration to be largely preserved, even after gaze shifts in awake mammals that would otherwise misalign the visual and auditory maps (23–25). The correspondence between sensory and motor maps in the SC also provides an efficient way of using spatial information from different modalities to guide orienting movements toward novel stimuli.
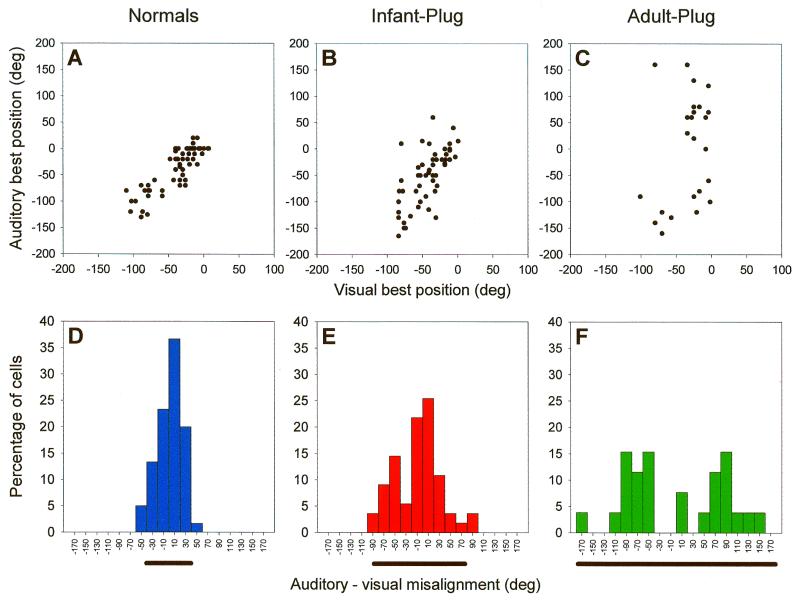
Figure 1.
Effects of chronic monaural occlusion on the registration of the auditory and visual maps in the ferret SC. Each panel shows the relationship between the representations of visual azimuth in the superficial layers and auditory azimuth in the deeper layers of the SC. (A–C) Recordings were made from anesthetized ferrets, and for each vertical electrode penetration, the visual best azimuth is plotted against the auditory best azimuth (measured with 100-ms broadband noise bursts at sound levels of 25–35 dB above unit threshold). deg, degree. (D–F) The frequency histograms plot the angular difference between the visual and auditory best azimuths; the bar below each histogram is centered on the mean misalignment and extends to 2 SDs on either side. (A and D) Data from normal, adult ferrets. (B and E) Data from adult ferrets that had been raised from just before the onset of hearing (which in ferrets occurs ≈4 weeks after birth) with the ear ipsilateral to the recording site occluded. (C and F) Data from adult ferrets that had one ear plugged for a comparable period, this time beginning when they were at least 6 months old. The data shown in B, C, E, and F were obtained with the earplug still in place. F tests revealed that the variance in auditory-visual misalignment is significantly different between each of the three groups. Because the superficial layer visual map showed a high degree of topographic order in each case, the increased scatter in the relationship between the two maps in the plugged animals is indicative of poorer topographic order in the auditory representation. These comparisons indicate some adaptation to the altered cues in the ferrets that were raised with one ear occluded but not in the ferrets that were plugged as adults. Data from juvenile animals are based on those in ref. 36.
Learning to Localize with Your Own Ears
Coordinating the neural maps of space for different sensory modalities involves matching specific values of the auditory localization cues to positions on the retina and the body surface. However, the monaural and binaural cue values corresponding to particular directions in space depend on the size, shape, and separation of the ears and may vary markedly between different individuals (26) and even between the left and right sides of the same subject (27). The cue values also undergo substantial changes during development as the head and external ears grow (1, 28, 29). These changes may contribute to the steady improvement in the ability of human infants to localize sound with increasing age (30). Similarly, the map of auditory space in the SC matures gradually during postnatal life, with normal topographic order emerging at about the same stage that the monaural spectral cues and ILDs attain their adult values (1). It therefore seems unlikely that the neural mechanisms responsible for processing spatial information are adjusted continuously during development to adapt to the changing cues. Nevertheless, adult human listeners localize virtual acoustic space stimuli derived from the head-related transfer functions of their own ears more accurately than those based on the ears of other subjects (31, 32). This finding suggests that neural representations of auditory space are calibrated by experience of the spatial cues provided by the subject's own ears.
Experience Shapes the Development of the Auditory Space Map in the SC
The map of auditory space in the SC has provided a very useful system for investigating experience-dependent plasticity in the neural coding of sound source location. By manipulating the sensory cues available during development, studies in barn owls (33) and mammals (34) have demonstrated the importance of both auditory and visual experience in shaping the spatial tuning of SC neurons.
Experiments in which the visual field was displaced with respect to the head, either optically in owls by prism rearing (35) or surgically in young ferrets by removal of one of the extraocular muscles (34, 36), revealed compensatory shifts in the auditory space map, suggesting that visual cues play a dominant role in establishing intersensory map registration. Much progress has since been made in owls in identifying the basis for the visual calibration of auditory spatial tuning and the time scale over which prism-induced changes can be induced (33). The site and mechanisms of plasticity have received less attention in mammals, although lesion studies suggest that the guiding visual signals may arise during development from the superficial layers of the SC (37). These exclusively visual layers project topographically both to the deeper layers of the SC and to the nucleus of the brachium of the IC, which itself contains a coarse map of sound azimuth (38).
Consistent with the guiding role of vision is the finding that degradation of visual cues during development leads to some abnormalities in the auditory representation. For example, raising ferrets with their eyelids sutured results in a higher incidence of auditory units that are ambiguously tuned to two different sound directions, although otherwise the map seems to be essentially normal (39). The ability of visually deprived ferrets to judge the location of sound sources in the horizontal plane is also no different from that of normally sighted ferrets. In keeping with the SC results, however, these animals do exhibit more front–back confusions than normal controls. On the other hand, we found that visually deprived ferrets actually show an improvement in auditory spatial acuity at lateral sound locations (40), a result that has also been reported in localization studies of lid-sutured cats (41) and blind humans (42). The conclusion from these studies is that, within a multisensory environment, the more reliable and accurate cues available through the visual system are used to coordinate spatial information provided by the auditory system. This ability to combine information across the senses leads to enhanced stimulus detection, localization, and identification as well as faster reactions after stimulus presentation (22). However, in the absence of vision, compensatory changes can take place within the brain in the processing of auditory and other sensory signals.
Auditory map plasticity has also been examined by using experimental procedures that alter the acoustic localization cues available (33, 34). Studies in mammals suggest that if the spatial cues are sufficiently degraded during development, then the map of auditory space in the SC will fail to form properly. For example, removal of the pinna and concha of the external ear disrupts the spectral localization cues such that, compared with those of normal animals, they provide much less information for discriminating between anterior and posterior sound directions (43, 44). Bilateral removal of the pinna and concha in juvenile ferrets impairs both the emergence of topographic order in the auditory space map (43) and the ability of mature animals to localize broadband sounds (44).
An adaptive change in the auditory space map has been observed, however, by raising owls (45) and ferrets (36) with unnatural binaural cues produced by plugging one of the ears. Fig. 1 B and E shows the relationship between the auditory map in the deeper layers and the visual map in the superficial layers of the SC in adult ferrets that had been raised with one ear occluded. These data, which were obtained with the earplug in place, show that the correspondence between the two maps is not as good as in normal ferrets (Fig. 1 A and D) but significantly better than in another group of animals that received a similar period of monaural occlusion as adults (Fig. 1 C and F).
In owls, the basis for this adaptation involves a compensatory shift during development in the tuning of auditory neurons toward the abnormal ITDs and ILDs produced by the earplug (46). Consequently, removal of the plug results in an immediate misalignment of the auditory and visual receptive fields, which, in juvenile owls, gradually decreases over a period of several weeks (45).
Irrespective of whether the ferrets received an earplug in infancy or as adults, we observed closer visual-auditory map alignment after plug removal. This result suggests that monaural occlusion in adult ferrets does not alter the sensitivity of SC neurons to the localization cues, as normal topographic order in the representation is apparent only with a balanced binaural input. The basis for the adaptation observed in the auditory space map in the infant-plugged ferrets is presently unclear, but, as discussed below, the neural plasticity involved is also likely to underlie the effects of early monaural occlusion on sound localization behavior.
Behavioral Evidence for Plasticity of Sound Localization
In addition to allowing for growth-related changes in localization cues, plasticity of the auditory system allows for some degree of adaptation to the altered auditory input produced by a conductive hearing loss. A common form of conductive loss, particularly prevalent in early childhood (ref. 47; Fig. 2), is middle ear disease (MED; also called otitis media with effusion). In MED, pressure changes associated with Eustachian tube malfunction result in the secretion of fluid into the middle ear cavity. This fluid impairs the impedance-matching function of the middle ear and both attenuates and delays sound passing to the cochlea (D. E. H. Hartley and D.R.M., unpublished observation). Because MED is usually either unilateral or bilaterally asymmetric, it changes the balance of input between the two ears, thereby generating abnormal binaural cues during infancy. There is therefore considerable interest in determining whether conductive hearing losses lead to changes in central auditory function, as suggested by the neurophysiological studies of the developing space map in the SC.
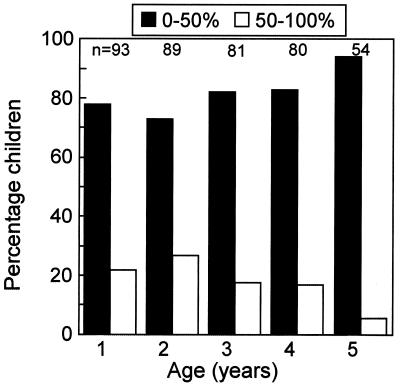
Figure 2.
Incidence of MED in the first 5 years of life (from a study performed in Oxford, 1993–1998). A group of children with normal birth histories were examined every month from birth with bilateral otoscopy and tympanometry to detect the occurrence of fluid in the middle ear (see ref. 47 for further details). The histogram bars show, for each year, the percentage of children who had fluid, in one or both ears, on less than (black bars) or more than (white bars) 50% of the examinations in that year. Most of the children who had high rates of MED in 1 year also had high rates in the other years. Overall, about 15% of the children in our sample had MED for more than half of the first 5 years of their lives (S. C. Hogan and D.R.M., unpublished data).
Research into the effects of MED on human hearing has been hampered by the usually poor data available on the precise history of the disease and, of course, by the lack of control over either the severity or the time course. To address these problems, we have studied the behavioral effects of monaural occlusion on various measures of localization in humans and ferrets. Earplugs produce sound attenuation and delays that are comparable to those produced by severe MED, but they do so in a far more controllable way.
Studies in adult humans have shown that, as expected, earplugs or other devices fitted to the external ear disrupt auditory localization, with differing degrees of adaptation reported during the variable and often relatively short periods used for manipulating the localization cues (6, 48–51). Studies in animals permit longer-term ear plugging, which can also be carried out during infancy, and have demonstrated considerable adaptation in certain circumstances. By measuring the accuracy of sound-evoked head-orienting responses, Knudsen and colleagues (52) showed that juvenile owls gradually learn to reinterpret the abnormal spatial cues produced by plugging one of their ears. We examined the effects of monaural occlusion on the ability of ferrets to perform a spatial identification task, in which they were trained to approach the location of a sound source in the azimuthal plane (Fig. 3A). Ferrets that were raised with one ear occluded could perform this task just as accurately as normal controls, indicating that they had compensated for the presence of the earplug (Fig. 3 B and C).
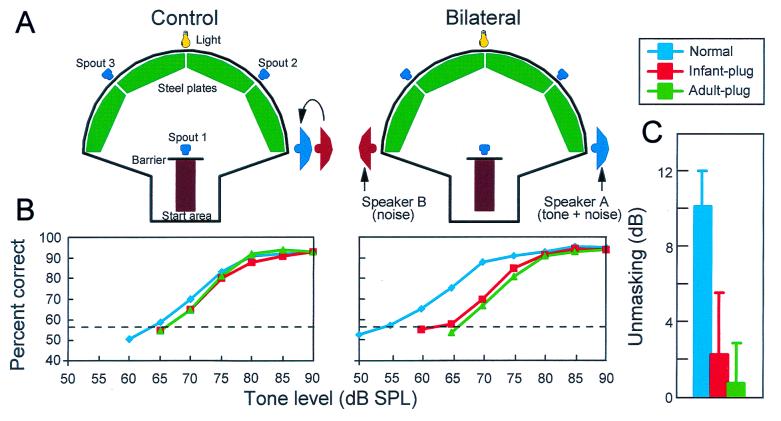
Figure 3.
Effects of chronic monaural occlusion in infancy on auditory localization. (A) Schematic view of chamber used to measure the spatial identification ability of ferrets. The animals were trained to stand on the start platform and initiate a trial by licking the center spout. Each trial consisted of a Gaussian noise burst (0–30 kHz, 100-ms duration) presented quasirandomly from 1 of 12 speakers placed at 30° intervals in the azimuthal plane. Within each testing session, five sound levels ranging randomly from 56 to 84 dB sound pressure level were used to minimize loudness cues. Ferrets were rewarded for approaching and licking the spout associated with the speaker that had been triggered. (B) Stimulus-response plots showing the combined data of three normal adult ferrets (Normals) and three ferrets that had been raised and tested with the left ear occluded with a plug that produced 30–50 dB attenuation (Infant plug). These plots illustrate the distribution of responses (ordinate) as a function of stimulus location (abscissa). The size of the dots indicates, for a given speaker angle, the proportion of responses made to different spout locations. deg, degree. (C) Polar plots showing the percentage of correct responses made to each of the 12 speaker locations. Comparison of the percentage scores and error magnitudes showed that ferrets reared with a plug in one ear perform as well as normally reared animals.
Little after effect was apparent after plug removal, and the ferrets continued to localize almost just as accurately. Subsequently, the performance of these animals showed some improvement, and reinsertion of the earplug resulted in a small shift in their responses toward the side of the unplugged ear. Although consistent with an effect on binaural processing, these changes are much less marked than those produced by acute monaural occlusion in adult ferrets (Fig. 4). As with the effects of plugging one ear during infancy on the responses of ferret SC neurons, the adaptation to the earplug does not seem to be based simply on a systematic shift in the association between binaural cue values and directions in space. Instead, it may be that greater emphasis is placed on other cues that are not disrupted by ear plugging, a possibility that may also explain the adaptation reported in adult humans after monaural occlusion (48, 49).
Figure 4.
Auditory localization data from three normally reared ferrets that had the left ear plugged in adulthood. These ferrets were tested extensively in the spatial identification task before ear plugging and therefore achieved higher scores than the normal control group illustrated in Fig. 3. Before plug panel (Left) shows performance of the animals with 100-ms noise bursts in the last test before insertion of the earplug. Data obtained when the earplug was in place (With Plug) are also shown (Center and Right). These tests were carried out during the day after insertion of the plug (Immediate) and again 6 months later (6 months). In the intervening period, the plug remained in place, but the ferrets were not tested. (A) Stimulus-response plots showing the distribution of errors at each speaker location. deg, degree. (B) Polar plots showing the percentage of correct responses at each speaker location. The ferrets initially performed poorly after occlusion of one ear in adulthood, even at much longer stimulus durations. Despite the improvement in performance observed over time, these animals achieved significantly lower percentage scores and made larger localization errors with one ear plugged, compared with their performance before the ear was occluded.
Sound localization behavior in adult barn owls is not adjusted by long-term plugging of one ear (52). This result is in accordance with the earplug-induced plasticity of auditory spatial tuning in the midbrain, which, as shown in Fig. 1, seems to be regulated developmentally. However, our behavioral studies in ferrets have shown that auditory localization by adult ferrets does improve after several months of monaural occlusion, although not to the level exhibited by the same animals before plugging (Fig. 4). We have also observed a more rapid improvement in performance by training adult-plugged ferrets more extensively, but without feedback about the accuracy of performance, over the first few weeks after insertion of the earplug. These observations raise intriguing questions about the factors that contribute to plasticity of sound localization and the neural mechanisms by which they are brought about.
Effects of Conductive Hearing Loss on Other Measures of Spatial Hearing
Studies of natural conductive impairments in humans suggest that their effects on binaural hearing may depend on the nature of the task that is being measured (53). To determine what aspects of sound localization are altered by plugging one ear, we have examined the effects of monaural occlusion on two other measures of binaural hearing. We found that long-term monaural occlusion, either in infancy or in adulthood, impairs the ability of ferrets to discriminate between two broadband sounds in the azimuthal plane (Fig. 5). However, ferrets reared and tested with one ear occluded achieved significantly better scores than those plugged for a similar period as adults. This result suggests a degree of adaptation, at least during infancy, that may also explain the normal MAAs reported in humans with mild long-term conductive losses (5). The compensatory changes involved seem, however, to be much less complete than those observed in the spatial identification task.
Figure 5.
Effects of chronic monaural occlusion on auditory spatial acuity. (A) Schematic view of chamber used to measure minimum audible angles (MAA) in ferrets. The animals were trained to initiate a trial by standing on the start platform and licking the center spout. This action triggered a noise burst (800 Hz to 24 kHz, 100-ms duration) from one of the two speakers placed symmetrically about the anterior midline. Ferrets were rewarded for licking the water spout in the same hemifield as the active speaker. The separation of the speakers was reduced between testing sessions according to the method detailed in ref. 44. Psychometric functions were measured by reducing the angular separation of the speakers from 90° to 6° or until performance did not differ from chance (56.5%, binomial distribution). (B) Mean (± SD) psychometric functions for normal ferrets (Normals, n = 6), ferrets plugged in infancy (Infant-plug, n = 4), and ferrets plugged as adults (Adult-plug, n = 3). These data were obtained with the earplug in place. deg, degree. (C) MAAs (defined as the speaker separation corresponding to 75% correct performance) were calculated from the psychometric function data. Note that adult-plugged ferrets failed to reach 75% correct, even at the maximum speaker separation of 90°; their MAAs are therefore indicated by >90°.
We have also examined the effects of unilateral ear plugging in ferrets on free-field binaural unmasking (54). This measure of binaural hearing depends on interaural phase detection and underpins the ability of listeners to distinguish separate spatial streams of acoustic information. Binaural unmasking is probably the most common test used to assess binaural hearing in humans. Indeed, studies in children have shown that, after a history of recurrent MED, binaural unmasking can remain below normal levels beyond the recovery of peripheral hearing (refs. 55 and 56; Fig. 6). In contrast to the effects of visual deprivation on the development of central visual function, however, the binaural consequences of long-term MED are reversible. Thus, children gradually reacquire normal hearing in the months to years after the cessation of MED (refs. 57 and 58; Fig. 6).
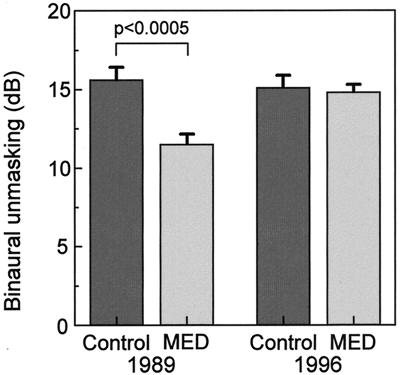
Figure 6.
Binaural hearing in children with a history of MED. Children aged 6–12 years, presenting at the Otolaryngology Department, Radcliffe Infirmary, Oxford, for tympanostomy surgery to relieve MED were paired, in 1989, with control children who had no known history of MED (from ref. 55). These two groups were examined for binaural unmasking—the difference in the detection threshold of a 500-Hz tone presented against a binaural noise stimulus when the tone is either in or out of phase between the two ears. This test is thought to provide a measure of the binaural contribution to the “cocktail party effect.” Children with a history of MED had impaired binaural hearing, even though their absolute thresholds were normal at the time of testing. These findings together suggest that a history of unilateral or asymmetric hearing loss can lead to impaired central auditory function. In 1996, 7 years later (from ref. 58), about two-thirds of the same children were re-examined. Both groups now had normal levels of binaural unmasking, suggesting that prolonged normal binaural experience can lead to the restoration of central auditory function.
Compared with normal adults, monaurally occluded ferrets achieved low levels of unmasking with the earplug in place, even after many months of plugging (Fig. 7). There was some indication that ferrets reared with one ear occluded showed less impairment than animals that had been plugged as adults (Fig. 7 B and C). However, in contrast to the localization experiments, these animals showed little sign of adaptation to the unilateral conductive hearing loss produced by the earplug. Like the children recovering from MED, they also had poor binaural unmasking for some months after removal of the earplugs.
Figure 7.
Effect of chronic monaural occlusion on binaural unmasking in ferrets. (A) Adult ferrets were trained to detect a 500-Hz tone (delivered from speaker A) in the presence of a noise (delivered from speakers A and B) when a light flashed. The noise stimuli were presented continuously. On 50% of trials, a tone was presented when the ferret contacted spout 1. Success in the task was measured by the ferret correctly identifying the presence or absence of the tone (by going to spouts 2 or 3, respectively). In the control condition (tones and noise interaurally in phase), both speakers were positioned on the ferret's right side. In the bilateral condition, the noise was made out of phase with the tone by moving speaker B (noise alone) to the ferret's left side. The difference in tone threshold between the two conditions measured binaural unmasking. (B) Ferrets learned to perform this task at a high level; the dashed line shows performance that was statistically above chance (binomial distribution). However, normal ferrets, like normal humans (Fig. 6), consistently produced thresholds that were about 10 dB better in the bilateral condition, showing binaural unmasking. (C) After 3–12 months of unilateral (left) ear plugging, there was little or no difference in performance between the control and the bilateral conditions, while the plug was in place. This lack of difference was true both for ferrets plugged before the normal onset of hearing (Infant-plug) and for ferrets receiving equivalent experience in adulthood (Adult-plug). Note, however, that one of the four infant-plugged ferrets had 8 dB of residual unmasking. When the plug was finally removed, both groups had impaired unmasking, despite normal pure tone sensitivity in the previously plugged ear. Like humans who have had surgery for a conductive hearing loss (Fig. 6), the unplugged ferrets gradually recovered normal binaural unmasking over a period of several months (from ref. 54).
These studies show that different aspects of spatial hearing are affected in different ways by a unilateral conductive hearing loss. Their findings are consistent with the notion that adaptation of sound localization by ferrets (and humans) may be caused less by compensatory changes in binaural hearing and more by a learned improvement in the use of other cues, such as monaural spectral cues, that are unaffected by the conductive hearing loss.
Summary
Sound localization is calibrated by experience, particularly during development when head growth changes the relationship between auditory cues and directions in space. Studies of the maturation of intersensory map registration in the SC have shown that the auditory representation can accommodate abnormal auditory or visual cues induced by experimental manipulations of the sensory inputs. Behavioral correlates of these experience-induced changes in neuronal response properties are seen in the capacity of juvenile ferrets to adjust sound localization to the altered acoustic cues produced by chronic occlusion of one ear. The effects of plug removal suggest that, in both cases, the adaptation observed may be at least partly based on a greater dependence on monaural spectral cues. Other measures of binaural hearing show much less adaptation after long-term monaural occlusion. Indeed, given the similarity between the effects of plugging one ear in ferrets and MED in human infants on binaural unmasking, it would be of interest to examine whether children with a history of recurrent MED exhibit adaptive changes in their ability to localize sound.
Compensatory adjustments occurring at the level of the auditory space map in the SC in response to long-term monaural occlusion have thus far been observed only during early postnatal life, suggesting that the need for plasticity may decline once the head and ears have attained their adult size. Although behavioral adaptation to ear plugging is also most pronounced during infancy, studies of sound localization in ferrets and humans indicate that the capacity of the brain to reinterpret auditory localization cues extends into adult life. Moreover, as with other recent examples of adult plasticity, training seems to facilitate this process.
The auditory space map in the SC provides a very useful system for examining the neural basis for the plasticity of sound localization, at least in relation to orienting behavior. However, it is likely that attention will have to switch to the cortex if we are to understand fully the principles underlying adaptation of the more complex localization behaviors used in this study.
Acknowledgments
We are grateful to J. Schnupp for comments on this paper and to Jemma Hine, Sarah Hogan, Leslie Hulvershorn, Ze Dong Jiang, Oliver Kacelnik, Richard Lanyon, Hiroaki Matsuda, and Susan Spires for their contributions to the psychophysical studies. Our research is supported by the Wellcome Trust (through a Senior Research Fellowship to A.J.K.), by the Medical Research Council (D.R.M.), by Defeating Deafness, and by the Oxford McDonnell–Pew Centre for Cognitive Neuroscience.
Abbreviations
- IC
inferior colliculus
- ILD
interaural level difference
- ITD
interaural time difference
- MAA
minimum audible angle
- MED
middle ear disease
- SC
superior colliculus
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.King A J, Carlile S. In: The Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1995. pp. 279–293. [Google Scholar]
- 2.Wightman F L, Kistler D J. In: Human Psychophysics. Yost W A, Popper A N, Fay R R, editors. New York: Springer; 1993. pp. 155–192. [Google Scholar]
- 3.Moore B C J. An Introduction to the Psychology of Hearing. San Diego: Academic; 1997. [Google Scholar]
- 4.Wightman F L, Kistler D J. J Acoust Soc Am. 1989;85:868–878. doi: 10.1121/1.397558. [DOI] [PubMed] [Google Scholar]
- 5.Häusler R, Colburn S, Marr E. Acta Otolaryngol Suppl. 1983;400:1–62. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- 6.Slattery W H, III, Middlebrooks J C. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 7.Irvine D R F. In: The Mammalian Auditory Pathway: Neurophysiology. Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 153–231. [Google Scholar]
- 8.Yu J J, Young E D. Proc Natl Acad Sci USA. 2000;97:11780–11786. doi: 10.1073/pnas.97.22.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwada S, Batra R, Yin T C T, Oliver D L, Haberly L B, Stanford T R. J Neurosci. 1997;17:7565–7581. doi: 10.1523/JNEUROSCI.17-19-07565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Kelly J B. J Neurosci. 1992;12:4530–4539. doi: 10.1523/JNEUROSCI.12-11-04530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlpine D, Jiang D, Shackleton T M, Palmer A R. J Neurosci. 1998;18:6026–6039. doi: 10.1523/JNEUROSCI.18-15-06026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran R, Davis K A, May B J. J Neurophysiol. 1999;82:152–163. doi: 10.1152/jn.1999.82.1.152. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins W M, Masterton R B. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh G L, Kelly J B. J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- 15.Middlebrooks J C. In: The New Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 2000. pp. 425–436. [Google Scholar]
- 16.King A J, Jiang Z D, Moore D R. J Comp Neurol. 1998;390:342–365. [PubMed] [Google Scholar]
- 17.Oliver D L, Huerta M F. In: The Mammalian Auditory Pathway: Neuroanatomy. Webster D B, Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 168–221. [Google Scholar]
- 18.King A J, Hutchings M E. J Neurophysiol. 1987;57:596–624. doi: 10.1152/jn.1987.57.2.596. [DOI] [PubMed] [Google Scholar]
- 19.King A J, Palmer A R. J Physiol. 1983;342:361–381. doi: 10.1113/jphysiol.1983.sp014856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middlebrooks J C, Knudsen E I. J Neurosci. 1984;4:2621–2634. doi: 10.1523/JNEUROSCI.04-10-02621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin T C T, Hirsch J A, Chan J C K. J Neurophysiol. 1985;53:746–758. doi: 10.1152/jn.1985.53.3.746. [DOI] [PubMed] [Google Scholar]
- 22.Stein B E, Meredith M A. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 23.Hartline P H, Pandey Vimal R L, King A J, Kurylo D D, Northmore D P M. Exp Brain Res. 1995;104:402–408. doi: 10.1007/BF00231975. [DOI] [PubMed] [Google Scholar]
- 24.Jay M F, Sparks D L. Nature (London) 1984;309:345–347. doi: 10.1038/309345a0. [DOI] [PubMed] [Google Scholar]
- 25.Populin L C, Yin T C T. In: Psychophysical and Physiological Advances in Hearing. Palmer A R, Rees A, Summerfield A Q, Meddis R, editors. London: Whurr; 1998. pp. 441–448. [Google Scholar]
- 26.Middlebrooks J C. J Acoust Soc Am. 1999;106:1480–1492. doi: 10.1121/1.427176. [DOI] [PubMed] [Google Scholar]
- 27.Carlile S, Pralong D. J Acoust Soc Am. 1994;95:3445–3459. doi: 10.1121/1.409965. [DOI] [PubMed] [Google Scholar]
- 28.Clifton R K, Gwiazda J, Bauer J A, Clarkson M G, Held R M. Dev Psychol. 1988;24:477–483. [Google Scholar]
- 29.Moore D R, Irvine D R F. Acta Otolaryngol. 1979;87:434–440. doi: 10.3109/00016487909126447. [DOI] [PubMed] [Google Scholar]
- 30.Werner L A, Gray L. In: Development of the Auditory System. Rubel E W, Popper A N, Fay R R, editors. New York: Springer; 1998. pp. 12–79. [Google Scholar]
- 31.Middlebrooks J C. J Acoust Soc Am. 1999;106:1493–1510. doi: 10.1121/1.427147. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel E M, Arruda M, Kistler D J, Wightman F L. J Acoust Soc Am. 1993;94:111–123. doi: 10.1121/1.407089. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen E I. J Comp Physiol A. 1999;185:305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- 34.King A J. BioEssays. 1999;21:900–911. doi: 10.1002/(SICI)1521-1878(199911)21:11<900::AID-BIES2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Knudsen E I, Brainard M S. Science. 1991;253:85–87. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- 36.King A J, Hutchings M E, Moore D R, Blakemore C. Nature (London) 1988;332:73–76. doi: 10.1038/332073a0. [DOI] [PubMed] [Google Scholar]
- 37.King A J, Schnupp J W H, Thompson I D. J Neurosci. 1998;18:9394–9408. doi: 10.1523/JNEUROSCI.18-22-09394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnupp J W H, King A J. J Neurophysiol. 1997;78:2717–2731. doi: 10.1152/jn.1997.78.5.2717. [DOI] [PubMed] [Google Scholar]
- 39.King A J, Carlile S. Exp Brain Res. 1993;94:444–455. doi: 10.1007/BF00230202. [DOI] [PubMed] [Google Scholar]
- 40.King A J, Parsons C H. Eur J Neurosci. 1999;11:3945–3956. doi: 10.1046/j.1460-9568.1999.00821.x. [DOI] [PubMed] [Google Scholar]
- 41.Rauschecker J P, Kniepert U. Eur J Neurosci. 1994;6:149–160. doi: 10.1111/j.1460-9568.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 42.Röder B, Teder-Sälejärvi W, Sterr A, Rösler F, Hillyard S A, Neville H J. Nature (London) 1999;400:162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- 43.Schnupp J W H, King A J, Carlile S. J Neurophysiol. 1998;79:1053–1069. doi: 10.1152/jn.1998.79.2.1053. [DOI] [PubMed] [Google Scholar]
- 44.Parsons C H, Lanyon R G, Schnupp J W H, King A J. J Neurophysiol. 1999;82:2294–2309. doi: 10.1152/jn.1999.82.5.2294. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen E I. J Neurosci. 1985;5:3094–3109. doi: 10.1523/JNEUROSCI.05-11-03094.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mogdans J, Knudsen E I. J Neurosci. 1992;12:3473–3484. doi: 10.1523/JNEUROSCI.12-09-03473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogan S C, Stratford K J, Moore D R. Br Med J. 1997;314:350–353. doi: 10.1136/bmj.314.7077.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer R W, Matuzsa J L, Blackmer R F, Glucksberg S. J Acoust Soc Am. 1966;40:441–444. [Google Scholar]
- 49.Florentine M. J Am Audiol Soc. 1976;1:243–251. [PubMed] [Google Scholar]
- 50.Hofman P M, Van Riswick J G A, Van Opstal A J. Nat Neurosci. 1998;1:417–421. doi: 10.1038/1633. [DOI] [PubMed] [Google Scholar]
- 51.McPartland J L, Culling J F, Moore D R. Hear Res. 1997;113:165–172. doi: 10.1016/s0378-5955(97)00142-1. [DOI] [PubMed] [Google Scholar]
- 52.Knudsen E I, Esterly S D, Knudsen P F. J Neurosci. 1984;4:1001–1011. doi: 10.1523/JNEUROSCI.04-04-01001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilmington D, Gray L, Jahrsdoerfer R. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 54.Moore D R, Hine J E, Jiang Z D, Matsuda H, Parsons C H, King A J. J Neurosci. 1999;19:8704–8711. doi: 10.1523/JNEUROSCI.19-19-08704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore D R, Hutchings M E, Meyer S E. Audiology. 1991;30:91–101. doi: 10.3109/00206099109072874. [DOI] [PubMed] [Google Scholar]
- 56.Pillsbury H C, Grose J H, Hall J W., III Arch Otolaryngol Head Neck Surg. 1991;117:718–723. doi: 10.1001/archotol.1991.01870190030008. [DOI] [PubMed] [Google Scholar]
- 57.Hall J W, III, Grose J H, Pillsbury H C. Arch Otolaryngol Head Neck Surg. 1995;121:847–852. doi: 10.1001/archotol.1995.01890080017003. [DOI] [PubMed] [Google Scholar]
- 58.Hogan S C, Meyer S E, Moore D R. Audiol Neurootol. 1996;1:104–111. doi: 10.1159/000259189. [DOI] [PubMed] [Google Scholar]