Abstract
Introduction
The aim of this study was to determine whether years of schooling influences regional cortical thicknesses and volumes in Alzheimer’s disease (AD), mild cognitive impairment (MCI), and healthy age-matched controls.
Methods
Using an automated image analysis pipeline, 33 regional cortical thickness and 15 regional volumes measures from MRI images were determined in 121 subjects with MCI, 121 patients with AD, and 113 controls from AddNeuroMed study. Correlations with years of schooling were determined and more highly and less highly educated subjects compared, controlling for intracranial volume, age, gender, country of origin, cognitive status, and multiple testing.
Results
After controlling for confounding factors and multiple testing, in the control group, subjects with more education had larger regional cortical thickness in transverse temporal cortex, insula, and isthmus of cingulate cortex than subjects with less education. However, in the AD group, the subjects with more education had smaller regional cortical thickness in temporal gyrus, inferior and superior parietal gyri, and lateral occipital cortex than the subjects with less education. No significant difference was found in the MCI group.
Conclusion
Education may increase regional cortical thickness in healthy controls, leading to increased brain reserve, as well as helping AD patients to cope better with the effects of brain atrophy by increasing cognitive reserve.
Keywords: Alzheimer’s disease, Cognitive aging, MCI (mild cognitive impairment), Education, MRI
Introduction
Alzheimer’s disease (AD) is the most common type of dementia, but a cure for AD has yet to be discovered. However, it may be possible to prevent or delay the onset of AD by reducing modifiable risks. Education has been proposed as a protective factor that can reduce the risk of developing dementia. The protective effect of education has been supported by numerous epidemiological, imaging, pathological, and neuropsychological studies [1–3]. However, results are not consistent and some conflicting studies have shown no protective effect of education against dementia [4, 5].
Imaging studies have shown that education has a morphological influence on the brain in both non-demented and AD subjects [6–9]. Whole brain and ventricular volumes have mainly been used as measures for evaluating the morphological effects of education on brain [6, 7, 10]. Recently studies have shown that cortical thickness can give complementary information for evaluating brain morphology and is a promising parameter for early diagnosis of AD [11, 12]. With the development of magnetic resonance (MR) post-processing technique, it is now possible to quantitatively measure regional cortical thicknesses and volumes in an automated way which minimizes rater-dependent bias. The relationship between education and regional cortical thicknesses and regional volumes of brain structures has not been studied previously in the same population however. The effect of education on brain structures might help our understanding of the mechanism of how education modulates the risk of AD and is of relevance for policy decisions, especially in low and middle-income countries where education provision is limited by restricted resources.
The purpose of this study was to explore if regional cortical thickness and volume measures can provide evidence to support the protective effect of education against AD in a large sample from the AddNeuroMed study [13, 14].
Methods
Subjects
A total of 121 AD patients (50% female; 74 ± 6 years), 121 mild cognitive impairment (MCI) subjects (65% female; 75 ± 6 years), and 113 age-matched healthy controls (55% female; 73 ± 6 years) were selected from the AddNeuroMed study [13, 14]; a prospective, longitudinal multicenter study to discover biomarkers for AD. Data were collected from six medical centers across Europe: University of Kuopio, Finland; University of Perugia, Italy; Aristotle University of Thessaloniki, Greece; King’s College London, United Kingdom; Medical University of Lodz, Poland; and University of Toulouse, France. The length of formal education was 8 ± 4, 9 ± 4, and 11 ± 5 years for the AD patients, MCI subjects, and controls, respectively. All subjects had successfully undergone MRI and cognitive tests at baseline. Clinical Dementia Rating Scale (CDR) [15] and Mini-Mental State Examination (MMSE) [16] were assessed for each subject. The APOE genotype was determined in 114 AD, 110 MCI, and 108 controls. Informed consent was obtained for all subjects and protocols and procedures were approved by the relevant Institutional Review Board at each data acquisition site and the data coordination site. None of the MCI and AD subjects had other neurological or psychiatric disease, significant unstable systemic illness or organ failure, and alcohol or substance misuse. The diagnosis of dementia was based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) [17] and the diagnosis of AD on the National Institute of Neurologic and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [18]. At baseline all MCI subjects fulfilled the following criteria, in line with consensus criteria for amnestic MCI [19, 20]: i.e., (1) memory complaint by patient, family, or physician; (2) normal activities of daily living; (3) normal global cognitive function as measured by the MMSE (score range between 24–30); (4) Geriatric Depression Scale score less than or equal to 5; (5) subject aged 65 years or above; (6) CDR memory score of 0.5 or 1; and (7) absence of dementia according to the DSM-IV [17] and NINCDS-ADRDA criteria for AD [18]. The controls did not have any neurological or psychiatric disorders and were not taking any psychoactive medication. Classification of the controls and MCI subjects was based on CDR score and clinician’s judgment, rather than on cognitive tests. The CDR total score was 0.5 for MCI subjects and ≥0.5 for AD patients.
Data acquisition
Data acquisition took place using six different 1.5-T MR systems (four General Electric, one Siemens, and one Picker). At each site a quadrature birdcage coil was used for RF transmission and reception. A high-resolution sagittal 3D T1-weighted MPRAGE volume was acquired using a custom pulse sequence specifically designed for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study to ensure compatibility across scanners [21]. The parameters were: TR = 9–13 ms, TE = 3.0–4.1 ms, TI = 1,000 ms, flip angle = 8°, voxel size 1.1 × 1.1 × 1.2 mm3. Detailed quality control was carried out on all MR images using previously published criteria [21, 22].
We adopted a three-stage approach to quality control. First, MRI scans were regularly acquired at each of the sites using a urethane test phantom developed by the US-based ADNI consortium. This allowed precise quantification of the geometric accuracy of a scan, together with other quality control measures, including signal-to-noise ratio and image uniformity using the ImageOwl web-based automated quality control system (http://www.imageowl.com) to ensure good performance of each of the scanners during the duration of the study. Second, two volunteers were scanned at each site, and their images were processed using the same analysis tools as the cohort images to ensure compatibility of data [22]. Finally, the MRI sites and data coordination center worked closely together with continuous feedback as data were acquired. All MRI centers were trained to perform quality control at their site at the time the images were acquired with particular emphasis on full brain coverage, image wrap around, subject motion artifacts, contrast between gray and white matter, and image non uniformities [14]. All scanners remained within specification for the ADNI phantom throughout the course of the study. The coefficient of variation for whole brain measures for the two volunteers scanned at each of the sites was 1.7% and the mean (sd) of the coefficients of variation for the smaller anatomical regions reported in this paper was 3.4 (1.9)%. All MR images received a clinical read by an on-site radiologist in order to exclude any subjects with non-AD-related pathologies.
Image analysis
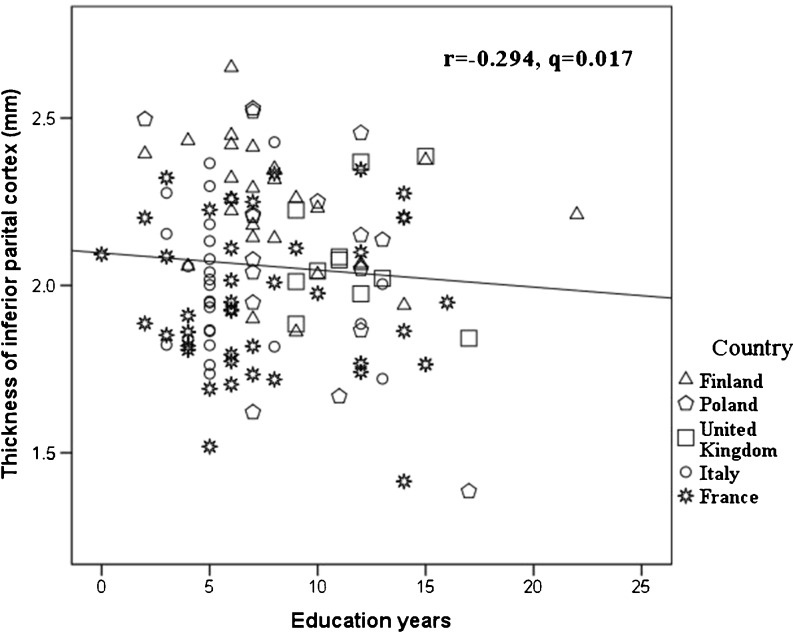
The FreeSurfer structural MRI image processing pipeline (version 4.5.0) which was developed by Fischl and his colleagues was utilized for data analysis [23, 24]. It produced both regional cortical thickness measures and regional volume measures. Cortical reconstruction and volumetric segmentation included removal of non-brain tissue using a hybrid watershed/surface deformation procedure [25], automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including the hippocampus, amygdala, caudate, putamen, and ventricles) [24, 26], intensity normalization [27], tessellation of the gray matter–white matter boundary, automated topology correction [28], and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [23]. Surface inflation [29] was followed by registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across subjects [30] and parcellation of the cerebral cortex into units based on gyral and sulcal structure [31]. This method uses both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface [23]. The maps are created using spatial-intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and are thus capable of detecting submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis [32] and manual measurements [33, 34]. These procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and across field strengths [35]. A sample of scatter plot from our data shows good comparable across the different centers (Fig. 1). The image analysis pipeline copes well with interindividual differences.
Fig. 1.
Years of education was inversely correlated with regional cortical thickness of the inferior parietal gyrus. The data from different countries and scanners were comparable
The cortical thicknesses and regional cortical/subcortical volumes from the automatic processing output were used in the present study, including 33 regional cortical thicknesses and 15 regional volumes. The averaged values of left and right hemispheres were used in the analysis, unless otherwise noted.
Statistical analysis
The correlation between years of schooling and regional cortical thicknesses/volumes was tested with the partial correlation coefficient for each diagnostic group. To allow for the possibility that education may not correlate linearly with regional thickness/volume, we further dichotomized the subjects into more and less educated groups. Since the years of compulsory education in the countries from which the subjects was recruited varied from 9–11 years, and the median of years of schooling in our study population was 9 years, we chose this as a threshold to separate subjects with more and less education. The difference in presence of ApoE4 between subjects with more and less education was tested with the chi square test. The analysis of covariance (ANCOVA) test was used to compare MRI measures between subjects with more and less education. The intracranial volume (for volume measures, but not for cortical thickness measures), country of origin, age, and gender were used as covariates in the partial correlation analysis and ANCOVA. Previous studies have shown that higher education is related with better cognitive performance [36], and that cognitive performance is sensitive to brain atrophy [37]. To control for the possible influence of cognitive performance on regional cortical thickness/volume, MMSE was also used as a covariate in the analysis. As the cortical thickness and volumes were measured from a large number of locations, it was necessary to correct for multiple testing. False discovery rate (FDR) <0.05 [38, 39] was used to control for type 1 error in the correlation analysis and ANCOVA with the qvalue software package (http://genomics.princeton.edu/storeylab/qvalue). A q value for FDR analysis less than 0.05 was considered as statistically significant.
Results
The demographics and cognitive tests for each group are given in Table 1. The partial correlation coefficients between years of schooling and regional cortical thicknesses and regional volumes are summarized in Table 2, and the regional cortical thicknesses and regional volumes of subjects with more and less education for each group are summarized in Table 3. There were no significant differences in the presence of ApoE4 between subjects with more and less education (p values 0.57, 0.72, and 0.12 in AD, MCI, and control groups, respectively).
Table 1.
Demographics and cognitive test results of the AD, MCI, and control subjects
| Control (n = 113) | MCI (n = 121) | AD (n = 121) | |
|---|---|---|---|
| Age | 73 ± 6 | 74 ± 6 | 75 ± 6 |
| Female/male (% female) | 62/51 (55%) | 60/61 (50%) | 79/42 (65%) |
| Education years; mean ± SD (range) | 11 ± 5 (2–25) | 9 ± 4 (0–20) | 8 ± 4 (0–22) |
| APOE4 carrier/non-carrier | 32/76 | 40/70 | 64/50 |
| CDR | 0 | 0.5 | 1.2 ± 0.5 |
| MMSE | 29 ± 1 | 27 ± 2 | 21 ± 5 |
Table 2.
Correlation between years of schooling and regional cortical thicknesses and volumes after controlling for intracranial volume, age, gender, MMSE, and country of origin
| Control | MCI | AD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | q | r | p | q | r | p | q | |
| Regional cortical thickness | |||||||||
| Entorhinal cortex | 0.108 | 0.266 | 0.488 | 0.085 | 0.367 | 0.732 | −0.179 | 0.054 | 0.089 |
| Parahippocampal gyrus | 0.067 | 0.489 | 0.600 | −0.033 | 0.723 | 0.821 | −0.096 | 0.307 | 0.327 |
| Middle temporal gyrus | 0.165 | 0.088 | 0.348 | 0.161 | 0.084 | 0.682 | −0.283 | 0.002 | 0.022a |
| Superior temporal gyrus | 0.108 | 0.266 | 0.488 | 0.124 | 0.184 | 0.682 | −0.262 | 0.005 | 0.041a |
| Inferior temporal gyrus | 0.067 | 0.489 | 0.600 | 0.078 | 0.402 | 0.732 | −0.237 | 0.010 | 0.046a |
| Temporal pole | 0.165 | 0.088 | 0.348 | −0.046 | 0.625 | 0.793 | −0.190 | 0.041 | 0.087 |
| Transverse temporal cortex | 0.020 | 0.839 | 0.906 | 0.169 | 0.070 | 0.682 | −0.156 | 0.094 | 0.124 |
| Insula | 0.067 | 0.491 | 0.600 | 0.061 | 0.517 | 0.732 | −0.149 | 0.110 | 0.140 |
| Fusiform gyrus | 0.145 | 0.133 | 0.366 | 0.000 | 1.000 | 1.000 | −0.227 | 0.014 | 0.046a |
| Medial orbitofrontal cortex | 0.221 | 0.021 | 0.206 | 0.055 | 0.561 | 0.741 | −0.165 | 0.077 | 0.110 |
| Lateral orbitofrontal cortex | 0.269 | 0.005 | 0.165 | 0.027 | 0.771 | 0.821 | −0.144 | 0.122 | 0.146 |
| Orbital operculum | 0.215 | 0.025 | 0.206 | 0.124 | 0.184 | 0.682 | −0.166 | 0.076 | 0.110 |
| Frontal operculum | 0.133 | 0.170 | 0.383 | 0.115 | 0.220 | 0.726 | −0.183 | 0.050 | 0.087 |
| Superior frontal gyrus | 0.050 | 0.611 | 0.720 | 0.071 | 0.450 | 0.732 | −0.208 | 0.025 | 0.069 |
| Pars triangularis | 0.151 | 0.119 | 0.357 | 0.070 | 0.453 | 0.732 | −0.109 | 0.243 | 0.267 |
| Frontal pole | 0.162 | 0.095 | 0.348 | 0.029 | 0.753 | 0.821 | −0.043 | 0.644 | 0.664 |
| Rostral middle frontal gyrus | −0.007 | 0.939 | 0.952 | 0.146 | 0.117 | 0.682 | −0.197 | 0.034 | 0.080 |
| Caudal middle frontal gyrus | 0.231 | 0.016 | 0.206 | 0.100 | 0.286 | 0.726 | −0.234 | 0.012 | 0.046a |
| Precentral gyrus | 0.075 | 0.443 | 0.600 | 0.124 | 0.186 | 0.682 | −0.182 | 0.050 | 0.087 |
| Superior parietal gyrus | 0.162 | 0.093 | 0.348 | 0.036 | 0.704 | 0.821 | −0.229 | 0.013 | 0.046a |
| Post central gyrus | 0.154 | 0.111 | 0.357 | 0.190 | 0.041 | 0.682 | −0.144 | 0.124 | 0.146 |
| Paracentral gyrus | 0.041 | 0.671 | 0.764 | 0.030 | 0.750 | 0.821 | −0.188 | 0.044 | 0.087 |
| Inferior parietal cortex | −0.074 | 0.444 | 0.600 | 0.075 | 0.423 | 0.732 | −0.294 | 0.001 | 0.017a |
| Supramarginal gyrus | 0.075 | 0.438 | 0.600 | 0.101 | 0.280 | 0.726 | −0.185 | 0.047 | 0.087 |
| Pericalcarine cortex | 0.006 | 0.952 | 0.952 | −0.178 | 0.056 | 0.682 | −0.231 | 0.013 | 0.046a |
| Precuneus cortex | 0.132 | 0.174 | 0.383 | 0.079 | 0.399 | 0.732 | −0.230 | 0.013 | 0.046a |
| Lingual gyrus | 0.104 | 0.286 | 0.4967 | 0.012 | 0.896 | 0.924 | −0.159 | 0.087 | 0.120 |
| Cuneus cortex | 0.018 | 0.851 | 0.906 | 0.081 | 0.386 | 0.732 | −0.214 | 0.021 | 0.063 |
| Lateral occipital cortex | 0.098 | 0.313 | 0.516 | 0.063 | 0.501 | 0.732 | −0.297 | 0.001 | 0.017a |
| Isthmus of cingulate cortex | 0.137 | 0.157 | 0.383 | 0.106 | 0.258 | 0.726 | −0.173 | 0.064 | 0.101 |
| Caudal anterior cingulate cortex | 0.108 | 0.266 | 0.488 | 0.067 | 0.477 | 0.732 | −0.021 | 0.820 | 0.820 |
| Rostral anterior cingulate | 0.067 | 0.489 | 0.600 | 0.059 | 0.532 | 0.732 | −0.112 | 0.230 | 0.262 |
| Posterior cingulate cortex | 0.165 | 0.088 | 0.348 | 0.141 | 0.130 | 0.682 | −0.203 | 0.029 | 0.074 |
| Regional volume | |||||||||
| Hippocampus | 0.082 | 0.400 | 0.750 | 0.081 | 0.387 | 0.893 | −0.095 | 0.312 | 0.736 |
| Amygdala | 0.034 | 0.727 | 0.839 | 0.052 | 0.576 | 0.893 | −0.080 | 0.396 | 0.736 |
| Caudate | −0.115 | 0.237 | 0.750 | 0.063 | 0.500 | 0.893 | −0.038 | 0.686 | 0.736 |
| Accumbens | 0.090 | 0.355 | 0.750 | 0.073 | 0.433 | 0.893 | −0.065 | 0.486 | 0.736 |
| Putamen | −0.039 | 0.685 | 0.839 | −0.013 | 0.893 | 0.893 | −0.032 | 0.733 | 0.736 |
| Pallidum | 0.037 | 0.705 | 0.839 | −0.143 | 0.124 | 0.893 | −0.088 | 0.350 | 0.736 |
| Brain stem | 0.099 | 0.310 | 0.750 | 0.121 | 0.196 | 0.893 | 0.032 | 0.736 | 0.736 |
| Cerebral cortex | 0.051 | 0.601 | 0.839 | 0.024 | 0.797 | 0.893 | −0.081 | 0.387 | 0.736 |
| Cerebellum cortex | 0.056 | 0.568 | 0.839 | 0.085 | 0.362 | 0.893 | 0.147 | 0.116 | 0.736 |
| Thalamus | 0.177 | 0.066 | 0.518 | 0.091 | 0.329 | 0.893 | −0.034 | 0.721 | 0.736 |
| Corpus callosum anterior | −0.115 | 0.235 | 0.750 | 0.029 | 0.760 | 0.893 | −0.043 | 0.644 | 0.736 |
| Corpus callosum central | −0.016 | 0.866 | 0.908 | 0.032 | 0.733 | 0.893 | −0.041 | 0.665 | 0.736 |
| Corpus callosum mid-anterior | 0.176 | 0.069 | 0.518 | 0.138 | 0.140 | 0.893 | −0.047 | 0.618 | 0.736 |
| Corpus callosum mid-posterior | 0.101 | 0.298 | 0.750 | 0.016 | 0.866 | 0.893 | −0.052 | 0.581 | 0.736 |
| Corpus callosum posterior | −0.011 | 0.908 | 0.908 | −0.043 | 0.643 | 0.893 | −0.062 | 0.506 | 0.736 |
aIndicates significant correlation (false discovery rate < 0.05)
Table 3.
Regional cortical thicknesses and absolute regional volumes of more and less educated subjects in AD, MCI, and control groups
| Control | MCI | AD | ||||
|---|---|---|---|---|---|---|
| Less educated (n = 41) | More educated (n = 72) | Less educated (n = 69) | More educated (n = 52) | Less educated (n = 77) | More educated (n = 44) | |
| Regional cortical thickness (mm) | ||||||
| Entorhinal cortex | 3.3 ± 0.5 | 3.4 ± 0.3 | 3.0 ± 0.5 | 3.1 ± 0.5 | 2.7 ± 0.5 | 2.7 ± 0.5 |
| Parahippocampal gyrus | 2.5 ± 0.3 | 2.6 ± 0.3 | 2.4 ± 0.3 | 2.4 ± 0.3 | 2.2 ± 0.3 | 2.2 ± 0.3 |
| Middle temporal gyrus | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.3 | 2.4 ± 0.3a |
| Superior temporal gyrus | 2.5 ± 0.2 | 2.5 ± 0.1 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2a |
| Inferior temporal gyrus | 2.7 ± 0.2 | 2.7 ± 0.1 | 2.6 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.3 | 2.4 ± 0.3a |
| Temporal pole | 3.5 ± 0.3 | 3.6 ± 0.2 | 3.3 ± 0.4 | 3.4 ± 0.4 | 3.1 ± 0.5 | 3.1 ± 0.5 |
| Transverse temporal cortex | 2.0 ± 0.2 | 2.1 ± 0.2a | 1.9 ± 0.2 | 2.0 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.3 |
| Insula | 2.8 ± 0.2 | 2.9 ± 0.2a | 2.7 ± 0.2 | 2.8 ± 0.2 | 2.6 ± 0.3 | 2.6 ± 0.2 |
| Fusiform gyrus | 2.5 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.3 ± 0.3 | 2.3 ± 0.2 |
| Medial orbitofrontal cortex | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.3 | 2.1 ± 0.2 |
| Lateral orbitofrontal cortex | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.2 |
| Orbital operculum | 2.5 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 |
| Frontal operculum | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.3 | 2.2 ± 0.2 |
| Superior frontal gyrus | 2.5 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.2 |
| Pars triangularis | 2.2 ± 0.1 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.2 |
| Frontal pole | 2.6 ± 0.2 | 2.7 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.3 |
| Rostral middle frontal gyrus | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.3 | 2.0 ± 0.2 |
| Caudal middle frontal gyrus | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.3 | 2.1 ± 0.2 |
| Precentral gyrus | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.4 | 2.0 ± 0.2 |
| Superior parietal gyrus | 2.0 ± 0.1 | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.4 | 1.8 ± 0.2 a |
| Post central gyrus | 1.8 ± 0.2 | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.3 | 1.7 ± 0.2 |
| Paracentral gyrus | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.3 | 1.9 ± 0.2 |
| Inferior parietal cortex | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2a |
| Supramarginal gyrus | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.3 | 2.1 ± 0.2 |
| Pericalcarine cortex | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Precuneus cortex | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.2 | 1.9 ± 0.3 | 1.9 ± 0.2 |
| Lingual gyrus | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.1 |
| Cuneus cortex | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.1 |
| Lateral occipital cortex | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.2 | 1.9 ± 0.2a |
| Isthmus of cingulate cortex | 2.3 ± 0.2 | 2.4 ± 0.2a | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.3 | 2.1 ± 0.3 |
| Caudal anterior cingulate cortex | 2.6 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.3 | 2.6 ± 0.2 | 2.5 ± 0.3 | 2.5 ± 0.3 |
| Rostral anterior cingulate | 2.8 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.3 | 2.8 ± 0.2 | 2.7 ± 0.3 | 2.7 ± 0.3 |
| Posterior cingulate cortex | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 |
| Regional volume (ml) | ||||||
| Hippocampus | 3.5 ± 0.5 | 3.6 ± 0.4 | 3.2 ± 0.6 | 3.3 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.4 |
| Amygdala | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Caudate | 3.3 ± 0.6 | 3.3 ± 0.5 | 3.3 ± 0.5 | 3.3 ± 0.6 | 3.3 ± 0.7 | 3.2 ± 0.7 |
| Accumbens | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Putamen | 4.6 ± 0.5 | 4.7 ± 0.5 | 4.7 ± 0.6 | 4.5 ± 1.1 | 4.5 ± 0.6 | 4.5 ± 0.9 |
| Pallidum | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.4 | 1.4 ± 0.2 |
| Brain stem | 19.4 ± 2.3 | 19.8 ± 1.9 | 19.4 ± 2.2 | 19.6 ± 2.6 | 18.9 ± 3.1 | 18.8 ± 2.1 |
| Cerebral cortex | 198.1 ± 22.4 | 198.9 ± 16.1 | 192.5 ± 18.7 | 196.2 ± 18.3 | 179.5 ± 21.7 | 180.8 ± 22.7 |
| Cerebellum cortex | 45.6 ± 5.4 | 46.2 ± 4.5 | 45.6 ± 5.3 | 46.1 ± 5.3 | 44.2 ± 6.4 | 45.6 ± 5.8 |
| Thalamus | 5.9 ± 0.7 | 5.9 ± 0.5 | 5.8 ± 0.6 | 5.9 ± 0.7 | 6.0 ± 2.7. | 5.7 ± 0.7 |
| Corpus callosum anterior | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.4 | 0.7 ± 0.1 |
| Corpus callosum central | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.7 | 0.3 ± 0.1 |
| Corpus callosum mid-anterior | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.9 | 0.3 ± 0.1 |
| Corpus callosum mid-posterior | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.3 | 0.3 ± 0.1 |
| Corpus callosum posterior | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
aIndicates significant difference (FDR < 0.05) between more and less educated subjects after adjusting for age, gender, country of origin, and MMSE. The intracranial volume was also adjusted for volumes
Control group
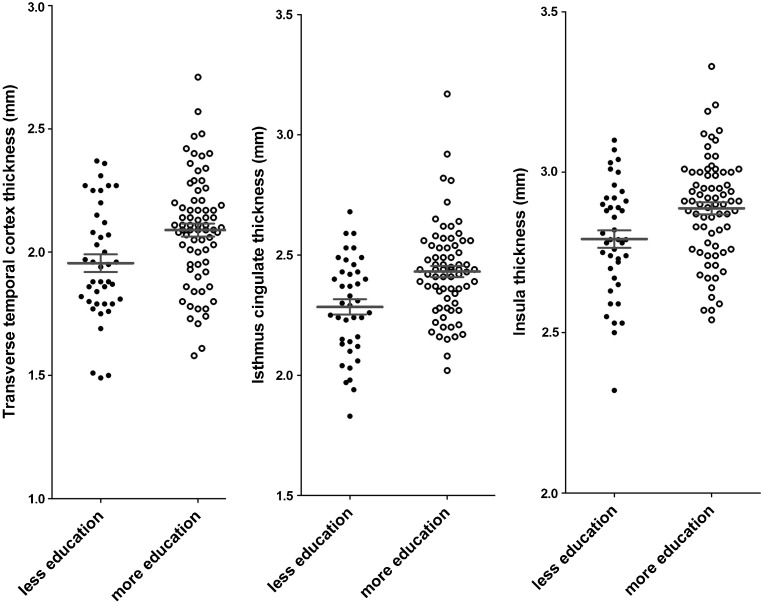
After adjusting for confounders and multiple testing, no significant correlation between years of schooling and regional cortical thicknesses/volumes was found. However, when the controls were dichotomized into more and less educated groups (years of schooling 14 ± 3 vs. 6 ± 2 years), the thicknesses of the temporal pole, transverse temporal gyrus, and isthmus of cingulate cortex were significantly larger in the subjects with more education than in those with less education (Fig. 2).
Fig. 2.
The healthy controls with more education had greater cortical thickness than those with less education at the transverse temporal cortex, isthmus cingulate, and insula
MCI group
After adjusting for confounders and multiple testing, no significant correlation between years of schooling and regional cortical thicknesses/volumes was found. The regional cortical thicknesses/volumes did not differ significantly between the subjects with more education than those with less education (years of schooling 13 ± 3 vs. 6 ± 2 years).
AD group
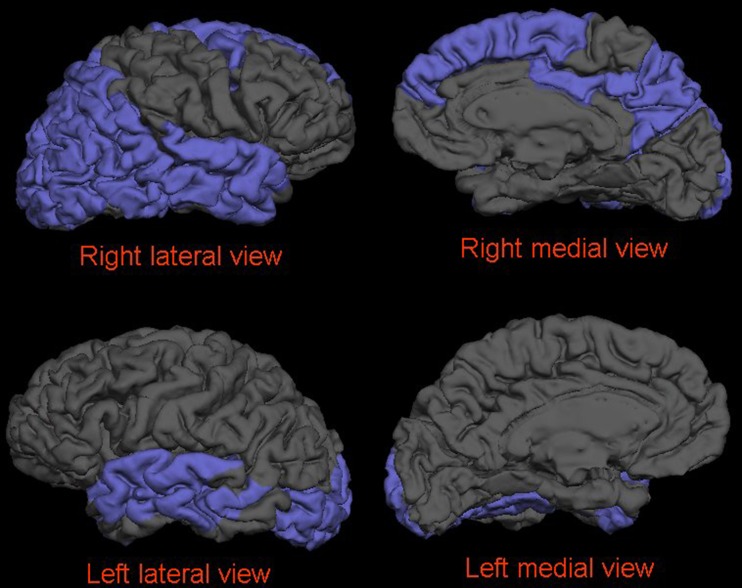
After controlling for confounders and multiple testing, in the AD group, the years of schooling were inversely correlated with regional cortical thicknesses of the inferior, middle and superior temporal gyri, fusiform gyrus, caudal middle frontal gyrus, inferior and superior parietal gyri, pericalcarine cortex, precuneus cortex, and lateral occipital cortex. We further separately analyzed the correlation between years of schooling and regional cortical thickness for the left and right hemispheres. The results are presented in Fig. 3. The regional cortex was significantly thinner in the inferior, superior, and middle temporal gyri, superior and inferior parietal gyri, and lateral occipital cortex in the patients with more education than those with less education (years of schooling 12 ± 3 vs. 5 ± 2 years).
Fig. 3.
After controlling for confounders and multiple testing, in the AD group, the years of schooling were inversely correlated with regional cortical thicknesses of the bilateral lateral occipital cortex, middle and superior temporal gyri, left fusiform gyrus, and right caudal middle and superior frontal gyri, inferior and superior parietal gyri, inferior temporal gyrus, posterior cingulate cortex, and precuneus cortex
Discussion
Two kinds of reserve against brain damage have been advocated: brain reserve [40] and cognitive reserve [41]. The concept of brain reserve was first introduced by Roth, Tomlinson, and Blessed [42] and was elaborated upon by Satz [43]. It refers to individuals with larger brain volumes who can sustain brain damage before reaching a threshold for clinical symptoms. Cognitive reserve refers to the ability of the brain to compensate for brain damage by using preexisting cognitive processing approaches or recruiting compensatory approaches [41]. To date the concept of brain reserve has received very limited support (for review see [44]). It is likely that both brain and cognitive reserves are involved in providing reserve against brain damage and cognitive decline [44].
One of the most interesting finding in the present study is that after adjusting for cognitive performance (MMSE), i.e., at similar cognitive performance, some areas of regional cortex were significantly thinner in AD patients with high education than those with less education (regional cortical thicknesses of middle and superior temporal gyri, inferior parietal cortex, and lateral occipital cortex). This implies that the AD patients with more education have better ability than the patients with less education to compensate for the effects of atrophy. This finding strongly supports the concept of an increased cognitive reserve in the subjects with more education. The thinner regional cortex in AD patients with more education might be one reason to explain why deterioration progresses more rapidly in AD patients with more education [45].
More educated AD patients had thinner regional cortices in the present study. This finding is supported by a diffusion–tensor imaging study which showed that years of schooling are associated with reduced white matter integrity of media temporal lobe [9] and is in line with the study of Querbes et al. [12] who found that people with more education have a thinner cortex than people with less education [12]. Our study further narrows this finding to AD patients. The finding that AD patients had smaller regional cortical thicknesses is further supported by a recent study which showed that education is associated with cortical thinning in the frontal, temporal, and parietal association cortexes; precuneus; medial, frontal, and basal temporal regions; orbitofrontal; and cuneus [46]. However, morphological influence of education on the brain in non-demented people is controversial in the literature. In previous volumetric studies, non-demented people with more education or better socioeconomic status have been shown to have more atrophy [6, 7]. Piras et al. reported that years of schooling are significantly positively correlated with regional brain volumes and inversely correlated with mean diffusivity of several deep gray matter structures, indicating larger regional volumes and higher level of neural reserve in non-demented people [8]. A recent study showed that people with high early life IQ had larger brains than people with low IQ in the aged healthy group [10]. In our study, after controlling for confounders and multiple testing, there was no significant correlation between years of schooling and regional cortical thicknesses/volumes in the healthy control group, which is supported by a recent study which showed no effect of education on regional cortical thickness in a linear regression analysis in healthy people [46]. However, when dichotomizing the controls into more and less educated groups with a cutoff of 9 years of schooling, the thicknesses of the temporal pole, transverse temporal gyrus, and isthmus of cingulate cortex were significantly larger in the subjects with more education than those with less education.
It is interesting that the healthy controls with more education had larger regional cortical thicknesses than those with less education after controlling for confounding factors, but that they did not show any significant correlation between regional cortical thicknesses and years of schooling. This finding may be explained as education increases brain reserve in a nonlinear way. However, this potential nonlinear effect should be replicated in a population with the same education system to be truly robust.
In the present study, the controls with more education had thicker regional cortices, but there was no significant difference in the MCI subjects. In AD patients, the subjects with more education had thinner regional cortices. A recent study showed that in non-demented people, higher socioeconomic status is associated with more rapid brain volume loss in those who developed dementia later [6]. This might account for the different finding in our diagnostic groups.
It is notable that there were positive findings for regional cortical thickness measures, but a lack of positive findings for regional volume measures. In previous studies, a significant correlation was found between education and whole brain volume or volume of peripheral cerebrospinal fluid [6, 7]. However, in the study of Coffey et al., there was no significant correlation between regional volumes and education [7]. In a recent study, Seo et al. had similar findings to ours using regional cortical thicknesses as a morphological proxy [46]. It thus seems that regional cortical thickness is a better parameter for studying brain and cognitive reserve than brain volume, especially when results are divided into subregions of the brain.
Our study has certain limitations. The frequency of presence of ApoE4 was different among the three groups and it is important to bear in mind that APOE4 genotype is a significant contribution to cortical thinning in AD and MCI. The difference in the frequency of the presence of ApoE4 might confound the results of the different groups. We do not have regional cortical thicknesses/volumes at the subject’s schooling ages, so it is not possible to exclude the presence of selection bias due to individual difference in innate brain volume. Therefore, the finding that healthy aged people with more education have larger regional cortices needs to be confirmed. Our data were collected from six European countries with different education systems. Using 9 years as a cutoff value to dichotomize less or more educated subjects might be one reason for the discrepancy between the results of the correlation analysis and group comparison. We did not control for cardiovascular disease and cardiovascular risk factors (hypertension and diabetes), occupational attainment, and unhealthy life-styles. These factors may be associated with both risk of dementia and cortical volumes. Studies have demonstrated that each of these confounding factors independently contributes to reserve [3, 45, 47, 48]. Finally we used MMSE as a global cognitive functions measure. The MMSE has very low variance and ceiling effects in control subjects might affect the correlation analysis in the control group.
Conclusion
Education appears to have a differential impact on cortical thickness in healthy controls and AD patients. It may increase regional cortical thickness in healthy controls, leading to increased brain reserve, as well as helping AD patients to cope better with the effects of brain atrophy by increasing cognitive reserve.
Acknowledgments
This work was funded by EU, AddNeuroMed/Innovative Medicines LSHB-CT-2005-518170. YL was funded by Health Research Council of The Academy of Finland, grant 121038. SL and AS were supported by funding from the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Kings College London. HS has received funding from Health Research Council of The Academy of Finland, grant 121038, EVO grant 5772720 from Kuopio University Hospital, and Strategic funding from University of Eastern Finland.
Conflict of interest
We declare that we have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Yawu Liu and Valtteri Julkunen contributed equally to this work.
References
- 1.Brayne C, Ince PG, Keage HA, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 2.Perneczky R, Wagenpfeil S, Lunetta KL, et al. Education attenuates the effect of medial temporal lobe atrophy on cognitive function in Alzheimer’s disease: the MIRAGE study. J Alzheimers Dis. 2009;17:855–862. doi: 10.3233/JAD-2009-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngandu T, von Strauss E, Helkala EL, et al. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 4.Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 2007;53:82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- 5.Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology. 2008;70:1732–1739. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- 6.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- 7.Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/WNL.53.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp. 2010;32:282–289. doi: 10.1002/hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teipel SJ, Meindl T, Wagner M, et al. White matter microstructure in relation to education in aging and Alzheimer’s disease. J Alzheimers Dis. 2009;17:571–583. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- 10.Sole-Padulles C, Bartres-Faz D, Junque C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30:1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Paajanen T, Zhang Y, et al. Analysis of regional MRI volumes and thicknesses as predictors of conversion from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2010;31:1375–1385. doi: 10.1016/j.neurobiolaging.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Querbes O, Aubry F, Pariente J, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovestone S, Francis P, Strandgaard K. Biomarkers for disease modification trials—the innovative medicines initiative and AddNeuroMed. J Nutr Health Aging. 2007;11:359–361. [PubMed] [Google Scholar]
- 14.Simmons A, Westman E, Muehlboeck S, et al. MRI measures of Alzheimer’s disease and the AddNeuroMed study. Ann N Y Acad Sci. 2009;1180:47–55. doi: 10.1111/j.1749-6632.2009.05063.x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Association AP. Diagnostic and statistical manual of mental disorders. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons A, Westman E, Muehlboeck S, et al. The AddNeuroMed framework for multi-centre MRI assessment of longitudinal changes in Alzheimer’s disease: experience from the first 24 months. Int J Ger Psych. 2011;26:75–82. doi: 10.1002/gps.2491. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 27.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- 33.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 34.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 36.Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, Chang CC. Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health. 2010;14:100–107. doi: 10.1080/13607860903071014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fjell AM, Amlien IK, Westlye LT, Walhovd KB. Mini-mental state examination is sensitive to brain atrophy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:252–258. doi: 10.1159/000241878. [DOI] [PubMed] [Google Scholar]
- 38.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J Roy Stat Soc B. 2004;66:187–205. doi: 10.1111/j.1467-9868.2004.00439.x. [DOI] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 40.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/WNL.43.1_Part_1.13. [DOI] [PubMed] [Google Scholar]
- 41.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 42.Roth M, Tomlinson BE, Blessed G. The relationship between quantitative measures of dementia and of degenerative changes in the cerebral grey matter of elderly subjects. Proc R Soc Med. 1967;60:254–260. doi: 10.1177/003591576706000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. doi: 10.1037/0894-4105.7.3.273. [DOI] [Google Scholar]
- 44.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 45.Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer’s disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- 46.Seo SW, Im K, Lee JM, et al. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol Aging. 2011;32:200–209. doi: 10.1016/j.neurobiolaging.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Mortel KF, Meyer JS, Herod B, Thornby J. Education and occupation as risk factors for dementias of the Alzheimer and ischemic vascular types. Dementia. 1995;6:55–62. doi: 10.1159/000106922. [DOI] [PubMed] [Google Scholar]
- 48.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. doi: 10.1001/jama.1994.03510370056032. [DOI] [PubMed] [Google Scholar]