Abstract
Aim
To characterize progression of Alzheimer's disease (AD) using proton magnetic resonance spectroscopy (1H MRS).
Methods
Eleven subjects with mild to moderate AD underwent neurocognitive testing and single-voxel 1H MRS from the precuneus and posterior cingulate region at baseline, after 24 weeks of monotherapy with a cholinesterase inhibitor, and after another 24 weeks of combination therapy with open-label memantine and a cholinesterase inhibitor. Baseline metabolites [N-acetylaspartate (NAA), myo-inositol (mI), choline (Cho), and creatine (Cr)] and their ratios in AD subjects were compared with those of an age-matched control group of 28 cognitively normal subjects.
Results
AD subjects had significantly higher mI/Cr and lower NAA, NAA/Cr, NAA/Cho, and NAA/mI. Baseline Alzheimer's Disease Cooperative Study Activities of Daily Living (ADCS-ADL) scores significantly correlated with NAA/Cr, mI/Cr, and NAA/mI. There was an increase in mI and a decrease in NAA/mI, but no significant change in other metabolites or ratios, or neurocognitive measures, when memantine was added to a cholinesterase inhibitor.
Conclusion
Metabolite ratios significantly differed between AD and control subjects. Baseline metabolite ratios correlated with function (ADCS-ADL). There was an increase in mI and a decrease in NAA/mI, but no changes in other metabolites, ratios, or cognitive measures, when memantine was added to a cholinesterase inhibitor.
Key Words: Magnetic resonance spectroscopy, Alzheimer's disease, Activities of daily living, Image biomarker, Memantine, Cholinesterase inhibitors
Introduction
One of the most pressing challenges underlying clinical trials in Alzheimer's disease (AD) is the need to validate reliable surrogate biomarkers of disease progression. Proton magnetic resonance spectroscopy (1H MRS) allows for noninvasive in vivo detection and measurement of brain metabolites, often using the creatine (Cr) peak as a relatively stable reference [1]. N-acetylaspartate (NAA) is a marker of neuronal integrity and/or mitochondrial function. NAA concentrations [2, 3] and NAA/Cr ratios [4, 5, 6, 7] have been reported to be lower in AD patients as compared with normal elderly subjects. Furthermore, decreased NAA/Cr levels may predict future conversion from mild cognitive impairment (MCI) to AD [8, 9, 10, 11]. Myo-inositol (mI) is a glial cell marker, and mI/Cr ratios have been found to be elevated in AD [4] and MCI [12, 13]. Choline (Cho) is a cell membrane marker, and Cho/Cr ratios have also been found to be elevated in AD [7, 12, 14] and MCI [15].
Memantine is a low-to-moderate affinity, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist with strong voltage dependency and rapid blocking/unblocking kinetics. While preclinical evidence suggests that memantine decreases the neuronal toxicity associated with excessive activation of NMDA receptors [16] and protects against beta-amyloid-induced toxicity [17], it is not clear whether this translates into a beneficial effect in patients with AD. Memantine has been shown to be effective for the treatment of moderate to severe AD, both as monotherapy [18] and when added to a cholinesterase inhibitor [19]. However, it has not been proven to be of benefit in mild AD, based on cognitive and functional outcome measures [20, 21].
The purpose of this study was to characterize the progression of AD using 1H MRS and cognitive outcome measures in patients with mild to moderate AD treated with open-label memantine in addition to a stable-dose cholinesterase inhibitor. We compared baseline metabolite levels and ratios in mild to moderate AD subjects to those of age-matched cognitively normal control subjects. We examined correlations between baseline metabolites and ratios, functional status, and cognitive measures within AD subjects. In addition, we explored whether we could detect any differential longitudinal effect on either neurocognitive measures or spectroscopic biomarkers when memantine was added to a cholinesterase inhibitor.
Methods
Subjects
We enrolled 11 subjects, aged ≥50 years, with a diagnosis of probable AD according to NINCDS-ADRDA criteria, of mild to moderate severity based on MMSE score ≥15 and ≤26 at screening (mean MMSE score 21.9 ± 3.0), a modified Hachinski score ≤4 at screening, no other clinically significant neurologic, psychiatric, or medical illnesses, and an MRI scan performed as part of the screening procedures consistent with a diagnosis of AD, and without any findings that could confound spectroscopic analysis. AD subjects had been on a stable dose of a cholinesterase inhibitor (9 subjects on donepezil 10 mg daily and 2 subjects on galantamine 16 mg daily) for at least 3 months prior to study entry, and had not been previously treated with memantine. The study included a 24-week observational phase, wherein AD subjects continued stable-dose cholinesterase inhibitor treatment, followed by a 24-week open-label memantine phase, wherein AD subjects received open-label memantine treatment titrated to a dose of 20 mg per day, in addition to their ongoing cholinesterase inhibitor treatment. An age-matched control group of 28 cognitively normal subjects (mean MMSE score 28.4 ± 1.5) was used for comparison to baseline 1H MRS metabolites and ratios in the AD subjects.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol (ClinicalTrials.gov Identifier NCT00551161) was approved by the North Shore-LIJ Health System Institutional Review Board. Written informed consent by a legally authorized representative as well as subject assent was obtained for each patient.
Neurocognitive Testing
AD subjects underwent neurocognitive testing at baseline (t0), after 24 weeks of ongoing monotherapy with a stable-dose cholinesterase inhibitor (t1), and after another 24 weeks of combination therapy with memantine in addition to a stable-dose cholinesterase inhibitor (t2). The testing battery at each visit included the MMSE [22], Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-cog) [23], Alzheimer's Disease Cooperative Study-Activities of Daily Living scale (ADCS-ADL) [24], Trails A and B [25], Animal Naming test and FAS verbal fluency tasks [26], logical memory and visual reproduction subtests from the WMS-R [27], Digit Span subtest from the WAIS-R [28], Rey-Osterrieth Complex Figure copying and recall [29], Judgment of Line Orientation test [30], and Buschke Selective Reminding test [31, 32].
1H MRS
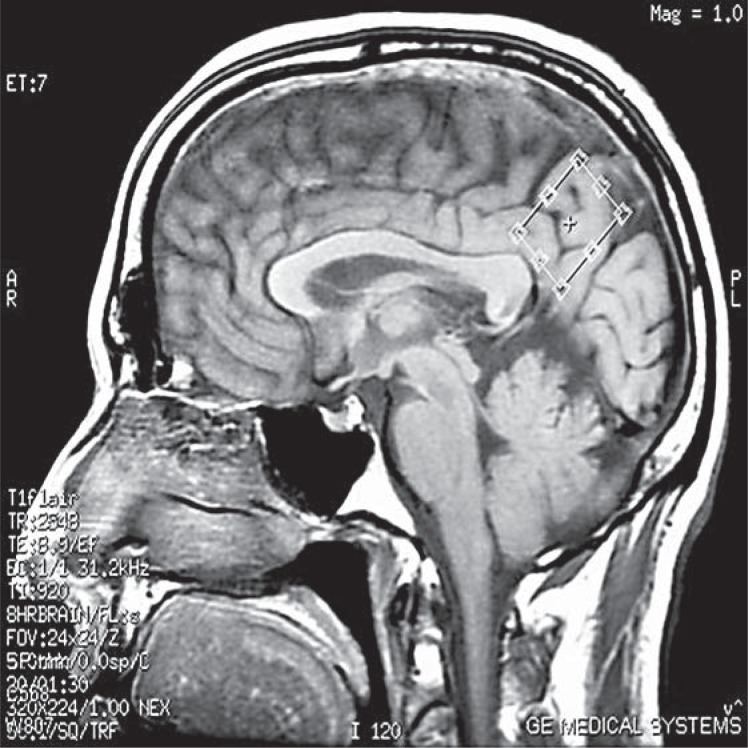
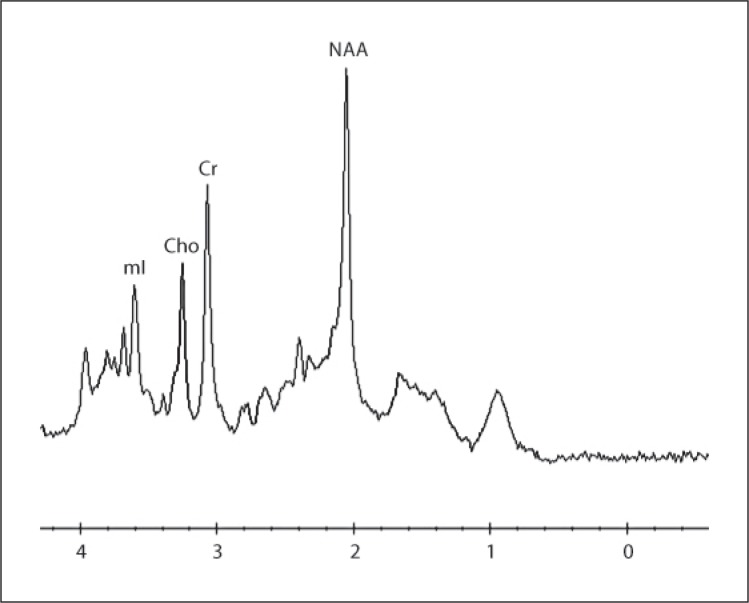
AD subjects underwent MRI with single-voxel 1H MRS at baseline (t0), after 24 weeks of ongoing monotherapy with a stable-dose cholinesterase inhibitor (t1), and after another 24 weeks of combination therapy with memantine in addition to a stable-dose cholinesterase inhibitor (t2). A 10.8 cm3 (2 × 2 × 2.7 cm) mid-sagittal oblique precuneus and posterior cingulate region 1H MRS voxel was prescribed, with the long axis parallel to the parieto-occipital sulcus (fig. 1). Double-spin-echo (PRESS) spectra were acquired on a GE Twinspeed 3T MRI scanner with HDx technology, using an 8-channel-phased array head coil (GE Healthcare), with 128 excitations acquired at TR = 1,600 ms and TE = 30 ms (fig. 2). Metabolite ratios were quantified automatically with vendor-supplied software. Another 128 excitations were acquired at TR = 1,600 ms and TE = 144 ms. This allowed use of the water signal intensity at TE = 144 ms (W144) to correct the water signal intensity at TE = 30 ms (W30) for T2 losses as follows: (1) W30 = W0*e(–30/T2); (2) W144 = W0*e(–144/T2); (3) T2 = 114/ln(W30/W144), and (4) W0 = W30*e(30/T2), where W0 is the water signal intensity extrapolated to TE = 0 ms. Individual metabolite levels were calculated as the ratio of the metabolite signal intensity to the water signal intensity corrected for T2 losses (W0). These metabolite/ T2-corrected water ratios were multiplied by a scaling factor of 106 to derive the individual metabolite levels reported herein.
Fig. 1.
Mid-sagittal precuneus and posterior cingulate region 1H MRS voxel positioned with the long axis parallel to the parieto-occipital sulcus.
Fig. 2.
1H MRS spectrum acquired at TR = 1,600 ms and TE = 30 ms.
Statistical Analysis
Comparisons between AD patients and normal control subjects were made using the Mann-Whitney test for continuous factors and Fisher's exact test for gender. For each measurement, the Wilcoxon signed-rank test was used to examine whether there was a change in that measurement between visits, and between the first and last visit (t1 vs. t0, t2 vs. t1, and t2 vs. t0). The Wilcoxon signed-rank test was also used to examine whether the change between t0 and t1 differed from the change between t1 and t2 [(t2 – t1) – (t1 – t0)]. This analysis was carried out in order to examine whether the rate of change differed while on monotherapy as compared with combination therapy. Spearman correlation coefficients were computed in order to determine the degree of correlation between each neurocognitive measurement and each 1H MRS metabolite level and metabolite ratio at baseline. For the changes in measurements between visits, as described above, Spearman correlation coefficients were computed in order to determine the degree of correlation between the changes in each neurocognitive measurement and the changes in each metabolite level and ratio. Due to the exploratory nature of these analyses, no adjustment for multiple testing was made; hence, p < 0.05 was considered significant. Although it would have been preferable to carry out an omnibus analysis, due to the small sample size, the descriptive approach described above was used.
Results
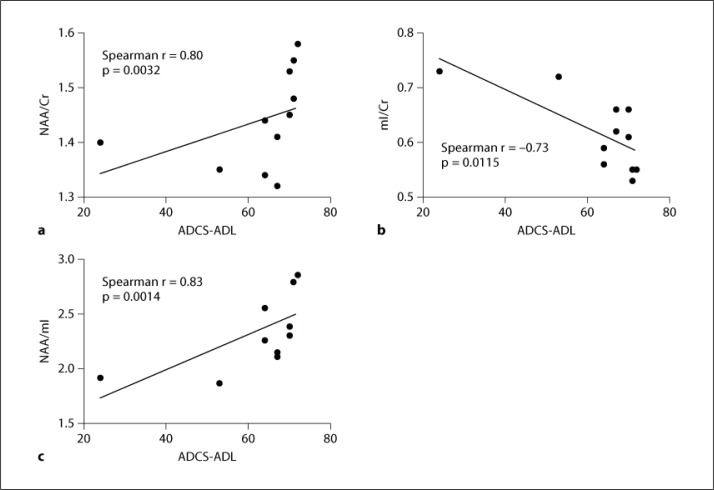
Baseline demographics and 1H MRS metabolites and metabolite ratios for AD subjects as compared with control subjects are summarized in table 1. As compared with control subjects, AD subjects had significantly higher baseline mI/Cr (p = 0.0115) and significantly lower baseline NAA (p = 0.0351), NAA/Cr (p = 0.0287), NAA/Cho (p = 0.0169), and NAA/mI (p = 0.0063). There was a trend towards increased Cho/Cr in AD subjects as compared with normal controls (p = 0.0602). In the AD subjects, baseline ADCS-ADL scores were significantly correlated with baseline NAA/Cr (Spearman r = 0.80, p = 0.0032; fig. 3a), mI/Cr (Spearman r = −0.73, p = 0.0115; fig. 3b), and NAA/mI (Spearman r = 0.83, p = 0.0014; fig. 3c), but not with individual metabolite levels. Baseline cognitive measures were not significantly correlated with baseline metabolite levels or ratios.
Table 1.
Baseline demographics and 1H MRS metabolites (normalized to T2-corrected water signal intensity) and metabolite ratios for AD subjects as compared with cognitively normal control subjects
| Controls (n = 28) | AD (n = 11) | p value | |
|---|---|---|---|
| Gender (M/F) | 10/18 | 7/4 | 0.1582 (n.s.)a |
| Age, years | 75.2±2.8 | 76.1±7.5 | 0.4613 (n.s.)b |
| MMSE | 28.4±1.5 | 21.9±3.0 | <0.0001a |
| NAA | 697±50 | 651±68 | 0.0351a |
| Cr | 462±32 | 452±45 | 0.674 (n.s.)a |
| Cho | 275±26 | 285±31 | 0.629 (n.s.)a |
| mI | 258±34 | 280±48 | 0.230 (n.s.)a |
| NAA/Cr | 1.51±0.09 | 1.44±0.09 | 0.0287a |
| Cho/Cr | 0.60±0.06 | 0.63±0.06 | 0.0602 (n.s.)a |
| mI/Cr | 0.56±0.07 | 0.62±0.07 | 0.0115a |
| NAA/Cho | 2.55±0.23 | 2.30±0.29 | 0.0169a |
| NAA/mI | 2.74±0.33 | 2.37±0.35 | 0.0063a |
Data represent mean ± SD.
Mann-Whitney test;
Fisher's exact test. n.s. = Nonsignificant.
Fig. 3.
Baseline ADCS-ADL scores correlated with baseline NAA/Cr (a), mI/Cr (b), and NAA/mI (c) ratios.
Metabolites and metabolite ratios for AD subjects at each time point as well as changes in metabolites and metabolite ratios are summarized in table 2. There was an increase in mI (p = 0.032) and a decrease in NAA/mI (p = 0.042) over the 24-week phase of combination therapy as compared with the 24-week phase of cholinesterase inhibitor monotherapy. There were no significantly different changes in the other metabolites or metabolite ratios. Also, there was no significantly different change in neurocognitive test performance over the 24-week phase of combination therapy as compared with the 24-week phase of cholinesterase inhibitor monotherapy.
Table 2.
Mean (± SD) metabolite levels (normalized to T2-corrected water signal intensity) and metabolite ratios for AD subjects at t0, t1, and t2
| t0 | t1 | t2 | t1 − t0 | t2 − t1 | [(t2 − t1) − (t1 − t0)] | p value1 | |
|---|---|---|---|---|---|---|---|
| NAA | 651±68 | 678±94 | 651±81 | 27±41 | −26±76 | −54±102 | 0.240(n.s.) |
| Cr | 452±45 | 452±48 | 450±63 | 0±13 | −2±33 | −2±36 | 0.831(n.s.) |
| Cho | 285±31 | 282±32 | 289±39 | −2±14 | 6±17 | 9±12 | 0.067(n.s.) |
| mI | 280±48 | 277±49 | 289±57 | −3±23 | 13±21 | 16±23 | 0.032 |
| NAA/Cr | 1.44±0.09 | 1.50±0.10 | 1.46±0.17 | 0.06±0.06 | −0.04±0.14 | −0.09±0.15 | 0.106(n.s.) |
| Cho/Cr | 0.63±0.06 | 0.63±0.06 | 0.64±0.05 | −0.01±0.03 | 0.02±0.04 | 0.02±0.06 | 0.246(n.s.) |
| mI/Cr | 0.62±0.07 | 0.61±0.09 | 0.64±0.08 | −0.01±0.05 | 0.03±0.04 | 0.04±0.08 | 0.142(n.s.) |
| NAA/Cho | 2.30±0.29 | 2.42±0.37 | 2.28±0.26 | 0.11±0.17 | −0.14±0.30 | −0.26±0.39 | 0.083(n.s.) |
| NAA/mI | 2.37±0.35 | 2.53±0.59 | 2.32±0.51 | 0.15±0.34 | −0.20±0.31 | −0.35±0.50 | 0.042 |
The Wilcoxon signed-rank test was used to examine whether the change between t0 and t1 differed from the change between t1 and t2 [(t2 − t1) − (t1 − t0)]. n.s. = Nonsignificant.
Discussion
This is a small, exploratory study, and the findings may be limited by the sample size of AD subjects. Despite the relatively small number of subjects and the relatively high mean baseline MMSE score (21.9 ± 3.0) in our mild to moderate AD subjects, we were able to demonstrate significant differences in 1H MRS metabolite ratios as compared with cognitively normal age-matched control subjects. All of the differences in metabolite ratios were in the expected directions based on prior cross-sectional studies (higher baseline mI/Cr and Cho/Cr, and lower baseline NAA/Cr, NAA/Cho, and NAA/mI) [4, 5, 6, 7, 12, 13, 14, 15].
We found that baseline 1H MRS metabolite ratios in the precuneus and posterior cingulate region correlated with function as measured by the ADCS-ADL. Functional imaging studies have suggested that the precuneus/posterior cingulate cortex is a pivotal node in the default network [33] and plays a central role in a wide spectrum of highly integrated tasks [34]. This region is affected relatively early in AD [35]. Since the operative definition of AD dementia, in contradistinction to MCI, hinges upon the presence of functional impairment, it is plausible that biochemical changes in this region, as reflected by altered 1H MRS metabolite ratios, would correlate with reduced functional performance.
We did not observe any overall significant change in neurocognitive performance when open-label memantine was added to stable-dose cholinesterase inhibitor treatment. There was an increase in mI and a decrease in NAA/mI over the 24-week phase of combination therapy as compared with the 24-week phase of cholinesterase inhibitor monotherapy. The clinical significance of these findings is questionable in the absence of concomitant changes in the other metabolite ratios. Given the unidirectional crossover design of the study, this tendency towards worsening mI and NAA/mI during the second phase of the study could be due to the natural history of disease progression rather than an effect of medication.
Our inability to demonstrate a differential effect of memantine does not necessarily exclude the utility of 1H MRS as a surrogate longitudinal biomarker in clinical trials of AD, as memantine has not been proven to have any clinical benefit in patients with mild AD [20, 21]. An open-label study of donepezil found that lower baseline parietal NAA/Cr predicted a clinical response to donepezil over 3 months, and that changes in parietal NAA/Cr correlated with changes in ADAS-Cog scores [36]. A non-randomized controlled study of rivastigmine found that changes in metabolite ratios in the frontal cortex correlated with changes on clinical scales [37]. A randomized rater-blinded parallel-group 6-month trial of memantine versus donepezil in mild to moderate AD patients found no significant differences between memantine and donepezil in clinical scales or metabolite levels, although changes in posterior cingulate region NAA/Cr significantly correlated with changes in ADAS-Cog scores [38]. A recent double-blind placebo-controlled pilot study of memantine treatment in mild to moderate AD patients also failed to demonstrate a benefit of memantine on the inferior parietal region NAA/Cr ratio, or any secondary outcome measures [39].
1H MRS can reproducibly distinguish between relatively early AD patients and normal subjects. Further studies will be needed to replicate whether changes in precuneus/posterior cingulate metabolite ratios reliably correlate with measures of function, and whether they may yet prove useful as longitudinal biomarkers in clinical trials of disease-modifying therapies. As previously suggested [40], the precuneus and posterior cingulate region NAA/mI ratio may be a particularly sensitive spectroscopic surrogate outcome parameter for validation in future studies.
Acknowledgement
This research study was supported by an unrestricted grant from Forest Research Institute, a subsidiary of Forest Labs, Inc.
References
- 1.Danielsen E, Ross B. New York: Marcel Dekker; 1999. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. [Google Scholar]
- 2.Watanabe T, Shiino A, Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is superior to relative ratio to discriminate Alzheimer's disease from Binswanger's disease. Dement Geriatr Cogn Disord. 2008;26:89–100. doi: 10.1159/000144044. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Shiino A, Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is useful to differentiate amnesic mild cognitive impairment from Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord. 2010;30:71–77. doi: 10.1159/000318750. [DOI] [PubMed] [Google Scholar]
- 4.Rose SE, de Zubicaray GI, Wang D, et al. A 1H MRS study of probable Alzheimer's disease and normal aging: implications for longitudinal monitoring of dementia progression. Magn Reson Imaging. 1999;17:291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 5.Adalsteinsson E, Sullivan EV, Kleinhans N, et al. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer's disease. Lancet. 2000;355:1696–1697. doi: 10.1016/s0140-6736(00)02246-7. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Alexander GE, Chang L, et al. Brain metabolite concentration and dementia severity in Alzheimer's disease: a (1)H MRS study. Neurology. 2001;57:626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56:592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- 8.Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64:282–289. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer's disease predicted by brain magnetic resonance spectroscopy. Am J Psychiatry. 2005;162:667–675. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- 10.Metastasio A, Rinaldi P, Tarducci R, et al. Conversion of MCI to dementia: role of proton magnetic resonance spectroscopy. Neurobiol Aging. 2006;27:926–932. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Modrego PJ, Fayed N. Longitudinal magnetic resonance spectroscopy as marker of cognitive deterioration in mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2011;26:631–636. doi: 10.1177/1533317511433809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catani M, Cherubini A, Howard R. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12:2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhoff DJ, MacKay S, Constans JM. Axonal injury and membrane alterations in Alzheimer's disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 15.Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007;28:1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HS, Pellegrini JW, Aggarwal SK, et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid(1-40). Brain Res. 2002;958:210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 18.Reisberg B, Doody R, Stöffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 19.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Porsteinsson AP, Grossberg GT, Mintzer J, et al. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 21.Schneider LS, Dagerman KS, Higgins JPT, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68:991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 24.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 25.Reitan RM. Validity of the Trail Making test as an indicator of brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 26.Spreen O, Benton AL. Victoria: University of Victoria; 1977. Neurosensory Center Comprehensive Examination for Aphasia (revised) [Google Scholar]
- 27.Wechsler D. San Antonio: The Psychological Corporation; 1987. Wechsler Memory Scale – Revised. [Google Scholar]
- 28.Wechsler D. New York: The Psychological Corporation; 1981. Wechsler Adult Intelligence Scale – Revised. [Google Scholar]
- 29.Rey A. L'examen psychologique dans le cas d'encéphalopathie traumatique (in French). Arch Psychol (Genève) 1941;28:286–340. [Google Scholar]
- 30.Benton A, Hannay HJ, Varney NR. Visual perception of line direction in patients with unilateral brain disease. Neurology. 1975;25:907–910. doi: 10.1212/wnl.25.10.907. [DOI] [PubMed] [Google Scholar]
- 31.Buschke H. Selective reminding for analysis of memory and learning. J Verb Learn Verb Behav. 1973;12:543–550. [Google Scholar]
- 32.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 33.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 34.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 35.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessen F, Traeber F, Freymann K, et al. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology. 2006;67:528–530. doi: 10.1212/01.wnl.0000228218.68451.31. [DOI] [PubMed] [Google Scholar]
- 37.Modrego PJ, Pina MA, Fayed N, Diaz M. Changes in metabolite ratios after treatment with rivastigmine in patients with Alzheimer's disease: a nonrandomised controlled trial with proton magnetic resonance spectroscopy. CNS Drugs. 2006;20:867–877. doi: 10.2165/00023210-200620100-00006. [DOI] [PubMed] [Google Scholar]
- 38.Modrego PJ, Fayed N, Errea JM, et al. Memantine versus donepezil in mild to moderate Alzheimer's disease: a randomized trial with magnetic resonance spectroscopy. Eur J Neurol. 2010;17:405–412. doi: 10.1111/j.1468-1331.2009.02816.x. [DOI] [PubMed] [Google Scholar]
- 39.Ashford JW, Adamson M, Beale T, et al. MR spectroscopy for assessment of memantine treatment in mild to moderate Alzheimer dementia. J Alzheimers Dis. 2011;26:331–336. doi: 10.3233/JAD-2011-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer's disease. J Int Neuropsychol Soc. 2002;8:934–942. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]