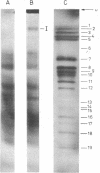
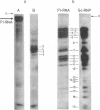
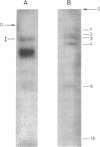
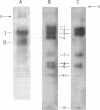
Abstract
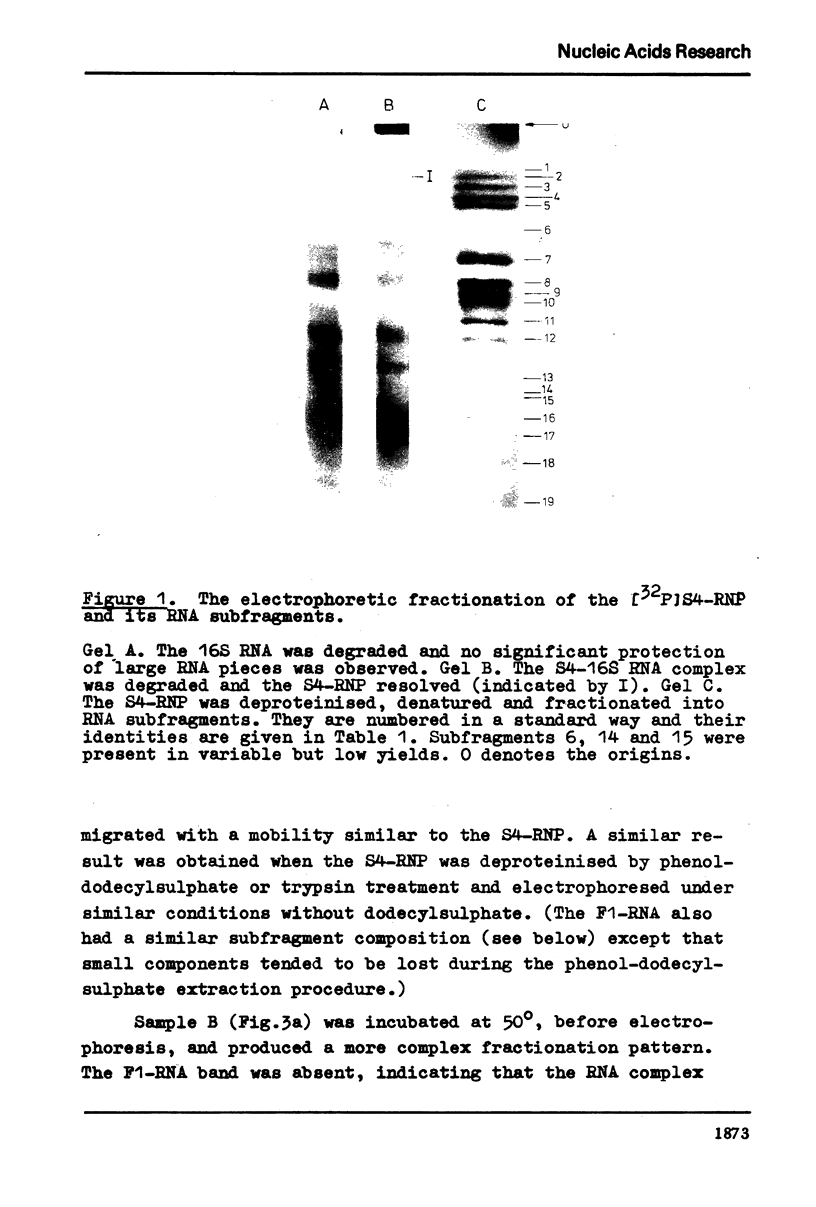
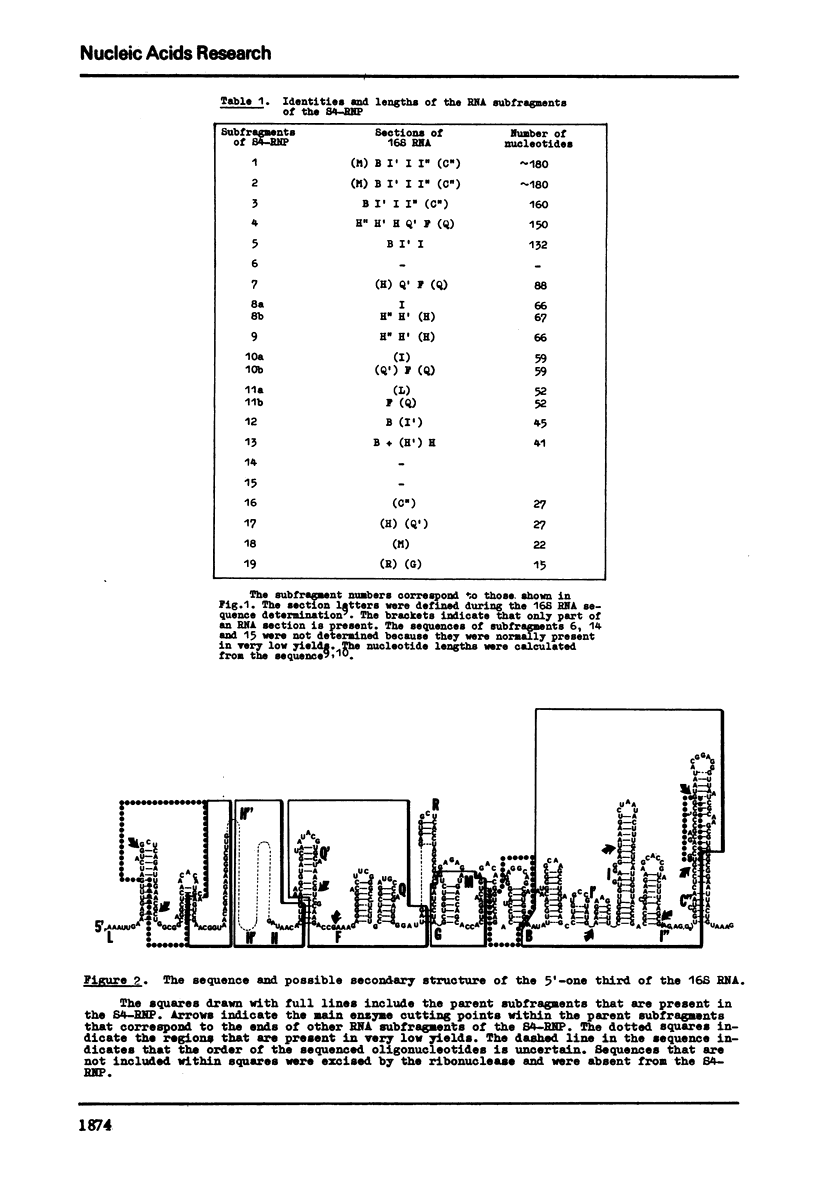
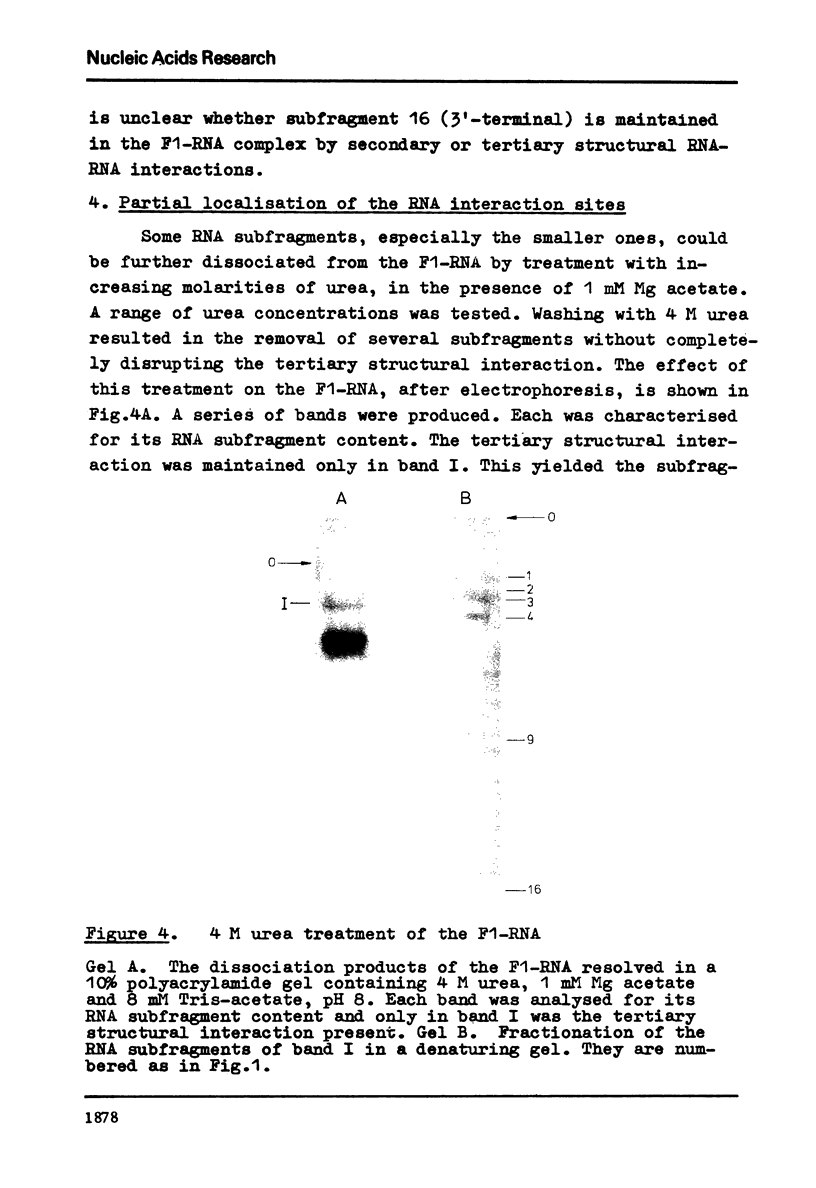
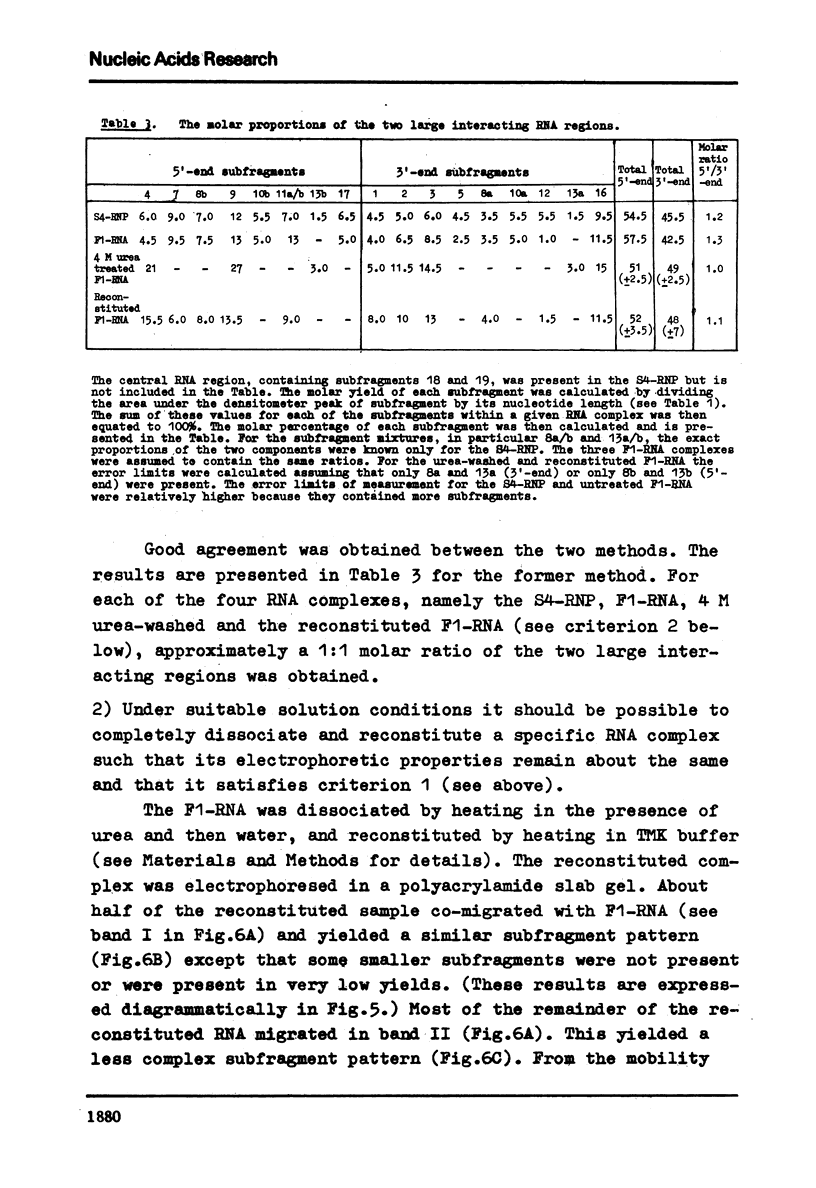
Evidence is presented for tertiary structural interaction(s) (interactions(s) between two regions of an RNA molecule that are widely separated in the RNA sequence) within the 5'-one third of the 16S ribosomal RNA of Escherichia coli that constitutes the binding site of protein S4. The two main interacting RNA regions were separated by about 120 nucleotides (sections Q to M) of the 16S RNA sequence. A second, smaller gap, of 13 nucleotides, occurred within section C". The two main interacting regions contain about 150 nucleotides (sections H" to Q) and 160 nucleotides (sections M to C"). They are folded back on one another and, especially in the presence of protein S4, are strongly protected against ribonuclease digestion. The intermediate region (sections Q to M), however, is relatively accessible to ribonucleases in the S4-RNP. By partial removal of subfragments from the RNA complex it was possible to localise the two main interacting sites within sections H" - H and sections I" - C". Three main criteria for the specificity of the RNA-RNA interactions were invoked and satisfied. The possibility of other tertiary structural RNA-RNA interactions occurring in other regions of the 16S RNA is discussed. Finally, all the structural information on the S4-RNP is summarised and a tentative model is proposed.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Fuller W., Hodgson A., Prutton I. Molecular conformations and structure transitions of RNA complementary helices and their possible biological significance. Nature. 1968 Nov 9;220(5167):561–564. doi: 10.1038/220561a0. [DOI] [PubMed] [Google Scholar]
- Aubert M., Scott J. F., Reynier M., Monier R. Rearrangement of the conformation of Escherichia coli 5S RNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):292–299. doi: 10.1073/pnas.61.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Sriwidada J., Fellner P., Crichton R. The identification of the RNA binding site for a 50 S ribosomal protein by a new technique. FEBS Lett. 1973 Sep 15;35(2):265–272. doi: 10.1016/0014-5793(73)80301-1. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Nucleotide sequences from the low molecular weight ribosomal RNA of Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):337–353. doi: 10.1016/s0022-2836(67)80109-8. [DOI] [PubMed] [Google Scholar]
- Cammack K. A., Miller D. S., Grinstead K. H. Physical properties of ribosomal ribonucleic acid isolated from bacteria deficient in ribonuclease I. Biochem J. 1970 May;117(4):745–755. doi: 10.1042/bj1170745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 2. Nucleotide sequences of products from partial enzymatic hydrolysis. Biochimie. 1972;54(7):901–967. doi: 10.1016/s0300-9084(72)80007-5. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 1. Nucleotide sequence analysis of T 1 and pancreatic ribonuclease digestion products. Biochimie. 1972;54(7):853–900. doi: 10.1016/s0300-9084(72)80006-3. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Stöffler G., Wittmann H. G. Ribosomal proteins. Isolation of the proteins from 30S ribosomal subunits of Escherichia coli. Eur J Biochem. 1971 Nov 11;23(1):7–11. doi: 10.1111/j.1432-1033.1971.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. The assembly of the 30S ribosomal subunit of Escherichia coli. J Supramol Struct. 1974;2(2-4):178–188. doi: 10.1002/jss.400020212. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A., Zimmermann R. A. Characterization of fragments of 16 S ribonucleic acid protected against pancreatic ribonuclease digestion by ribosomal protein S4. J Biol Chem. 1975 Jun 10;250(11):4100–4112. [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Muto A., Ehresmann C., Fellner P., Zimmermann R. A. RNA-protein interactions in the ribosome. I. Characterization and ribonuclease digestion of 16 S RNA-ribosomal protein complexes. J Mol Biol. 1974 Jun 25;86(2):411–432. doi: 10.1016/0022-2836(74)90028-x. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Sloof P., Sival J., Borst P. Gel electrophoresis of RNA under denaturing conditions. Biochim Biophys Acta. 1973 Oct 26;324(3):320–333. doi: 10.1016/0005-2787(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Kurland C. G. Molecular interactions of ribosomal components. 3. Isolation of the RNA binding site for a ribosomal protein. Mol Gen Genet. 1972;114(4):350–357. doi: 10.1007/BF00267503. [DOI] [PubMed] [Google Scholar]
- Schulte C., Garrett R. A. Optimal conditions for the interaction of ribosomal protein S8 and 16S RNA and studies on the reaction mechanism. Mol Gen Genet. 1972;119(4):345–355. doi: 10.1007/BF00272092. [DOI] [PubMed] [Google Scholar]
- Schulte C., Morrison C. A., Garrett R. A. Protein-ribonucleic acid interactions in Escherichia coli ribosomes. Solution studies on S4-16S ribonucleic acid and L24-23S ribonucleic acid binding. Biochemistry. 1974 Feb 26;13(5):1032–1037. doi: 10.1021/bi00702a031. [DOI] [PubMed] [Google Scholar]
- Schulte C., Schiltz E., Garrent R. The characterisation of a fragment of ribosomal protein S4 that is protected against trypsin digestion by 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Jun;2(6):931–941. doi: 10.1093/nar/2.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Garrett R., Ehresmann C., Stiegler P., Fellner P. An investigation of the 16-S RNA binding sites of ribosomal proteins S4, S8, S15, and S20 FROM Escherichia coli. Eur J Biochem. 1975 Feb 3;51(1):165–180. doi: 10.1111/j.1432-1033.1975.tb03917.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Mackie G. A., Muto A., Garrett R. A., Ungewickell E., Ehresmann C., Stiegler P., Ebel J. P., Fellner P. Location and characteristics of ribosomal protein binding sites in the 16S RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):279–302. doi: 10.1093/nar/2.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Muto A., Fellner P., Ehresmann C., Branlant C. Location of ribosomal protein binding sites on 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1282–1286. doi: 10.1073/pnas.69.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]