Abstract
Transintestinal cholesterol efflux (TICE) provides an attractive target to increase body cholesterol excretion. At present, the cholesterol donor responsible for direct delivery of plasma cholesterol to the intestine is unknown. In this study, we investigated the role of HDL in TICE. ATP-binding cassette protein A1 deficient (Abca1−/−) mice that lack HDL and wild-type (WT) mice were intravenously injected with chylomicron-like emulsion particles that contained radiolabeled cholesterol that is liberated in the liver and partly reenters the circulation. Both groups secreted radiolabeled cholesterol from plasma into intestinal lumen and TICE was unaltered between the two mouse models. To further investigate the role of HDL, we injected HDL with radiolabeled cholesterol in WT mice and Abca1−/−×Sr-b1−/− mice that lack HDL and are also unable to clear HDL via the liver. The intestines of both mice were unable to take up and secrete radiolabeled cholesterol from HDL via TICE. Although a generally accepted major player in the hepatobiliary route-based cholesterol excretion, HDL plays no significant role in TICE in mice.
Keywords: intestine, apolipoproteins, reverse cholesterol transport, neutral sterols, bile
Elevated circulating cholesterol levels are an important risk factor for atherosclerosis that is characterized by the accumulation of LDL-cholesterol in macrophages of the arterial wall (1). Statin treatment reduces LDL-cholesterol levels and is currently the most potent cholesterol-lowering therapy available (2). In the search for new therapeutic approaches, the reverse cholesterol transport (RCT) pathway has gained much attention during the last decade (3, 4). RCT via the commonly known hepatobiliary route describes the transport of peripheral cholesterol back to the liver for biliary secretion. In this pathway, HDL and apoAI, the major apolipoprotein of HDL, play a profound role. ApoAI and HDL take up cellular cholesterol from peripheral tissues, including macrophages in the arterial wall, via the cholesterol transporters ABC A1 and G1, respectively (3). In a scavenger receptor class BI (SR-BI)-dependent manner, HDL-cholesterol is delivered to the liver from where cholesterol can reenter the circulation via VLDL production or can be excreted via bile.
Recently, we reported on an additional nonhepatobiliary-related route for RCT, the trans-intestinal cholesterol efflux (TICE) pathway (5). This pathway describes the transport of cholesterol from blood to the intestinal lumen directly via enterocytes. TICE, which is predominantly exerted at the proximal part of small intestine, can contribute up to 70% of the daily total body neutral sterol secretion in mice. The activity of this pathway has been confirmed by Temel et al. (6) who demonstrated in elegant studies that acute biliary diversion does not influence intestinal cholesterol excretion significantly. An important issue that remains to be resolved for TICE is the identification of the donor particle that delivers the circulating cholesterol to the enterocyte. The intestine assembles lipids absorbed from the gut into chylomicrons and secretes these lipoprotein particles into lymph for distribution over the body (7). In addition, the intestine is an important contributor to circulating HDL levels (8). However, little is known about uptake of circulating lipoproteins by the intestine from the basolateral side. Interestingly, the rate of TICE was two-fold higher in SR-BI-deficient mice with increased HDL levels as related to an impaired delivery of HDL-cholesterol to the liver (9). It is thus conceivable that HDL is utilized for RCT via the hepatobiliary route as well as TICE.
In this study, we investigated basolateral uptake of cholesterol by the intestine and the potential role for HDL in TICE. We evaluated plasma kinetics, liver uptake, and biliary and intestinal secretion of radiolabeled cholesterol, which was injected via chylomicron-like particles in mice with normal and disturbed HDL metabolism. Results reported herein indicate that TICE is not mediated significantly via HDL particles.
METHODS
Animals
Male wild-type (WT) mice (C57Bl/6Jico; Charles River, L'Arbresle Cedex, France) as well as the Abca1−/− (10), Sr-bI−/− (11), and Abca1−/−×Sr-bI−/− mice (12), together with their WT littermates, received standard mouse chow diet [CRE(E), 3% (w/w) fat, no cholesterol added; Special Diet Services, Witham, UK]. Food and water were supplied ad libitum. The mice were maintained on a 12 h light/12 h dark cycle. All experiments were performed according to the Directive 2101/63/EU of the European Parliament and approved by the local Ethical Committees for Animal Experiments
Preparation of chylomicron-like emulsion particles
Chylomicron-like emulsion particles were prepared as described (13). Briefly, a mixture of 100 mg of total lipid was dispersed in NaCl buffer of density 1.10 g/ml. The lipid mixture consisted of egg yolk phosphatidylcholine (Lipoid, Ludwigshafen, Germany), triolein, L-α-lysophosphatidylcholine, cholesteryl oleate, and cholesterol (all from Sigma-Aldrich, The Netherlands) and at a weight ratio of 22.7: 70.0: 2.3: 3.0: 2.0. In addition, 50 µCi [14C]cholesterol and/or 160 µCi [3H]cholesteryl oleate ([3H]CO) (Amersham, The Netherlands) was added to the lipid mixture. The lipid mixture was sonicated using a Soniprep 150 (MSE Scientific Instruments, Crawley, UK) at 10 µm output (30 min at 54°C) under a constant stream of argon. Emulsion particles with an average size of 80 nm were collected after ultracentrifugation by aspiration.
Preparation radiolabeled HDL
Human HDL was isolated from blood of healthy subjects by differential ultracentrifugation as described by Redgrave et al. (14) and dialyzed against PBS with 1 mM EDTA. HDL (1.063 < d <1.21 g/ml) was labeled with [3H]CO via exchange from donor particles as reported previously (15). Donor particles were formed by sonication of egg yolk phosphatidylcholine supplemented with 50 µCi of [3H]CO using the Soniprep 150 at 12 µm output (40 min at 52°C) under a constant stream of argon in a 0.1 M KCl, 10 mM Tris, 1 mM EDTA. 0.025% NaN3 buffer, pH 8.0. Donor particles with a density of 1.03 g/ml were isolated after density gradient centrifugation. HDL was radiolabeled by incubation with donor particles (mass ratio of HDL protein/particle phospholipid = 8:1) in the presence of human lipoprotein-deficient serum (1:1, v/v), as a source of cholesteryl ester transfer protein (CETP), for 8 h at 37°C in a shaking-water bath under argon. Ethylmercurithiosalicylate (thimerosal; 20 mM) was added to inhibit phospholipid transfer and lecithin:cholesterol acyltransferase activity. Radiolabeled HDL was then isolated by density gradient ultracentrifugation.
Intestine perfusions, liver uptake, and plasma decay
Mice (2–4 months old, n = 5) were anesthetized by intraperitoneal injection of a mixture of 7 ml fluanisone (17.5 mg), fentanyl citrate (0.55 mg), and midazolam (8.75 mg) per kg body weight. Continous anesthesia was monitored by tail pinching. Radiolabeled chylomicron-like particles (2 µCi [3H]CO and 0.5 µCi [14C]cholesterol/mouse) or HDL (0.4 µCi [3H]CO/mouse) were administered by tail vein injection. Plasma samples were collected at the indicated time points after injection through tail-bleeding. After 1 h, bile was collected via bile cannulation for 15 min and the proximal small intestines (first 10 cm) were perfused with a bile salt-phospholipid mixture that was composed of 10 mM taurocholate and 2 mM phosphatidylcholine (PC) and at a fixed flow rate of 3 ml/hr, as described previously (5). This mixture of 10 mM taurocholate and 2 mM PC was shown earlier to yield optimal rates of cholesterol secretion (5). At the end of the perfusion period of 90 min, blood was collected by cardiac puncture. Liver was snap-frozen in liquid N2. The total amount of radioactivity in the plasma was calculated based on the estimated total plasma volume (4.5% of body weight) (16, 17). To determine liver uptake, the livers were weighed and tissue samples were dissolved in Soluene-350 (PerkinElmer, Waltham, MA). To determine intestinal uptake, the perfused intestinal segments were harvested; the mucosa was scraped, snap-frozen in liquid N2, and lipids were extracted according to Bligh and Dyer (18). For all samples radioactivity was determined using a liquid scintillation counter.
TLC
To determine the distribution of 3H and/or 14C over free cholesterol and cholesteryl esters in homogenized samples of snap-frozen livers and plasma samples, lipids were extracted according to Bligh and Dyer (18). After reconstitution of lipid films in chloroform, free cholesterol and cholesteryl esters were separated by spotting the samples on silica gel 60 TLC plates (Merck, Darmstadt, Germany). Subsequently, the sterols were identified by iodine staining and the distribution of radioactivity was quantitated using a liquid scintillation counter.
Cholesterol measurements
Biliary and perfusate cholesterol were measured by a fluorescent method as described previously (19). Total cholesterol in plasma was determined using cholesterol RTU kit from Biomerieux (Marcy l'Etoile, France).
Statistical analysis
All results are presented as mean ± SD. Group means for TICE, as depicted in the figures, were calculated by averaging the outcomes of all mice that got the same treatment. The values for the individual mice, used for the calculation of the group mean, were obtained by averaging the data of the last three perfusion time points. Differences between different groups were determined by one-way ANOVA. Outcomes of P < 0.05 were considered to be significant.
RESULTS
Cholesterol introduced via chylomicron-like emulsion particles is secreted via TICE
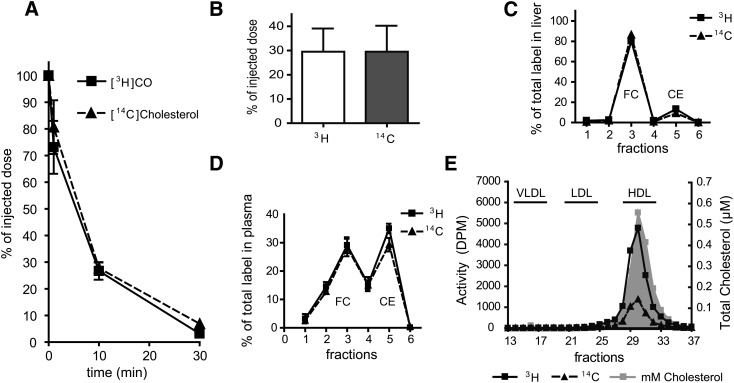
In our attempt to identify the cholesterol donor for TICE, we first investigated the fate of [3H]CO and [14C]cholesterol contained in chylomicron-like emulsion particles upon administration to WT mice by intravenous injection. Both [3H]CO and [14C]cholesterol were cleared rapidly from the circulation (t1/2= 6–7 min) (Fig. 1A). In previous reports we have shown that, in rats and mice, the majority of the injected dose (>60%) is taken up by the liver within 30 min and that no direct uptake is observed by the intestine (13). After 2.5 h, 30% of the injected dose was still present in the liver, which was similar to our previous observation and earlier reports (14, 20). Interestingly, this was observed for both isotopes (Fig. 1B), suggesting that no exchange occurs of free cholesterol (and CO) from the emulsion particles to their environment in plasma before uptake by the liver.
Fig. 1.
Plasma decay, liver uptake, and distribution of radiolabeled cholesterol from chylomicron-like emulsion particles in WT mice. After tail vein injection of emulsion particles containing [14C]cholesterol and [3H]CO into WT mice (n = 5), plasma samples were obtained at the indicated times and plasma decay was determined (A). After intestine perfusions, the remaining activity of both radiolabels in the liver was determined as % of injected dose (B). Liver samples were analyzed for distribution of 14C and 3H over free (FC) and esterified cholesterol (CE) via TLC (C). Lipoproteins in pooled plasma samples obtained at 2.5 h after injection were separated by FPLC and in the obtained fractions both total plasma cholesterol and radioactivity were determined (D). Free cholesterol and cholesteryl esters in plasma were separated by TLC and 14C and 3H radioactivity was measured in the different fractions (E). Values are means ± SD.
To analyze the distribution of both the 3H and the 14C radiolabel between its free and esterified form in the liver, TLC was performed on extracted lipids of liver homogenates (Fig. 1C). Although the introduced [14C]cholesterol remained largely in the free form, the majority of introduced [3H]cholesteryl oleate was hydrolyzed and also present as free cholesterol. In plasma of these mice, cholesteryl esters made up about 75% of the total circulating cholesterol. Analysis of plasma 2.5 h after injection showed that the 3H activity in the circulation (9.1 ± 1.5% of the injected dose) was evenly distributed over free and esterified cholesterol (Fig. 1D). A similar distribution was found for plasma 14C activity (9.7 ± 1.6% of injected dose). These data illustrate the reutilization of the radiolabeled cholesterol after specific uptake by the liver as demonstrated before (21–23). In addition, lipoprotein analysis of these plasma samples by fast protein liquid chromatography demonstrated that, like the total plasma cholesterol, both radiolabels were mostly associated with the HDL fraction (Fig. 1E).
To determine TICE and biliary cholesterol secretion, we performed intestine perfusions and bile cannulations, starting at 1 h after injection, for a period of 90 min. Table 1 shows that secretion of cholesterol via TICE was 2.5-fold higher than the biliary cholesterol secretion. This finding confirmed our earlier observation that TICE plays a very prominent role in fecal neutral sterol excretion in mice (5, 20). A substantial amount of the intravenously injected radiolabeled cholesterol taken up by the liver is cleared via biliary cholesterol secretion. In addition, we also demonstrate with our intestine perfusions that radiolabeled cholesterol is secreted into the intestinal lumen (Table 1). In all measured compartments, the activity ratio between 3H and 14C remained approximately 4:1 (data not shown). Although these data confirm direct secretion of cholesterol from blood into the intestinal lumen via TICE, they do not discriminate between different potential cholesterol donors and previous work already excluded direct uptake of our radiolabeled emulsion particles by the intestine (20).
TABLE 1.
Secretion of radiolabeled cholesterol from chylomicron-like emulsion particles into intestine via TICE vs hepatobiliary pathway
| TICE |
Hepatobiliary Pathway |
|||
| nmol cholesterol/min.100g body weight | 3.0 ± 1.1 |
1.2 ± 0.4 |
||
| Isotope | 3H | 14C | 3H | 14C |
| Specific activity (dpm/nmol cholesterol) | 126 ± 32 | 22 ± 6 | 3167 ± 883 | 701 ± 200 |
Secretion of cholesterol from chylomicron-like emulsion particles via TICE is relatively unaffected in mice that lack HDL or HDL uptake
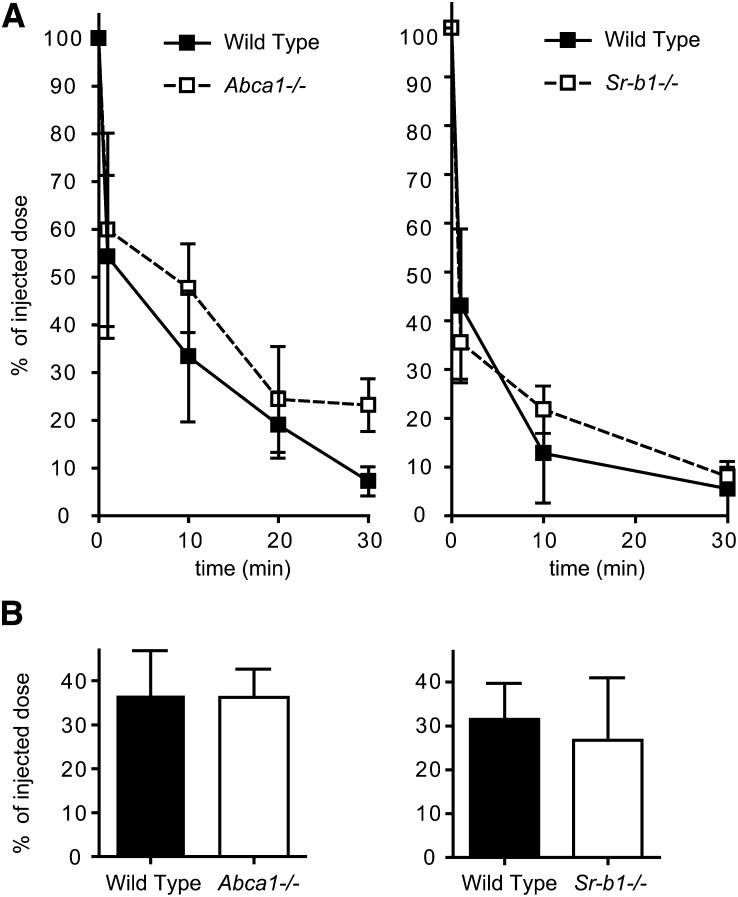
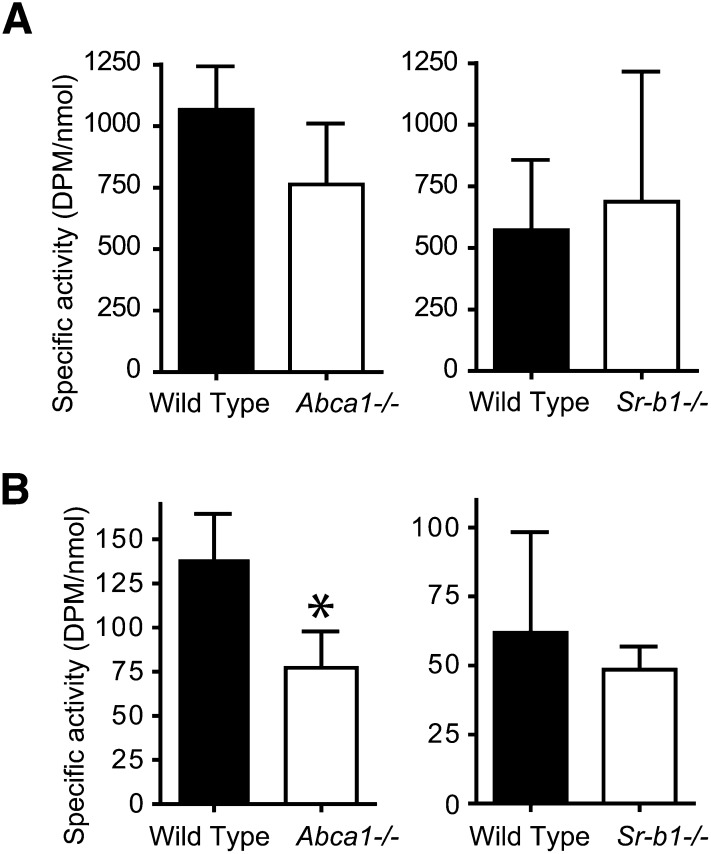
In a previous report, we showed that Abca1−/− mice with almost no HDL levels show unaltered fecal neutral sterol excretion compared with their WT littermates (24). To investigate whether TICE is also unaltered in the absence of HDL, we investigated the transport of radiolabeled cholesterol from chylomicron-like emulsion particles into the intestinal lumen. To this end, we injected [3H]CO-labeled particles into Abca1−/− mice and their WT littermates. Clearance of 3H-activity from plasma and hepatic uptake of 3H-activity in Abca1−/− mice was similar to that in WT littermates (Fig. 2A, B; left panels). Interestingly, despite the absence of HDL, specific activity of biliary cholesterol secretion was not significantly altered (Fig. 3A; left panel), in line with the unaltered biliary cholesterol secretion previously reported by our group (24).
Fig. 2.
Plasma decay and liver uptake of radiolabeled cholesterol from chylomicron-like emulsion particles in Abca1−/− and Sr-b1−/− mice. After tail vein injection of emulsion particles containing [3H]CO into Abca1−/− (left panel; open squares) and Sr-b1−/− (right panel; open squares) mice and their WT littermates (solid squares)(n = 5), plasma samples were obtained at the indicated times and plasma decay was determined (A). 2.5 h after injection, the percentage of the injected dose of 3H-cholesterol present in the liver was determined (B). Values are means ± SD.
Fig. 3.
Biliary cholesterol secretion and TICE of radiolabeled cholesterol from chylomicron-like emulsion particles in Abca1−/− and Sr-b1−/− mice. One hour after injection of emulsion particles containing [3H]CO, bile cannulations (A) and intestine perfusions were performed (B). Cholesterol and 3H radioactivity were measured in the collected bile samples and perfusate. Specific radioactivities for cholesterol are expressed as dpm/nmol cholesterol. Values are means ± SD. * indicates a significant difference between knockout mouse model and WT littermates (P < 0.05).
Intestine perfusions performed on the Abca1−/− mice showed that TICE was not significantly changed. As monitored by the appearance of unlabeled cholesterol in the perfusate samples, the rate of TICE in mice lacking HDL was 1.4 ± 0.5 nmol/min.100g body weight compared with 1.9 ± 0.6 nmol/min.100g body weight in their WT littermates. Furthermore, despite the absence of HDL in Abca1−/− mice, transintestinal secretion of radiolabeled cholesterol introduced via chylomicron-like emulsion particles was still 60% compared with WT littermates (Fig. 3B; left panel). Mice with a disrupted Sr-b1 gene show elevated HDL and have increased TICE (9). To investigate whether the increased TICE is mirrored by increased flux of 3H-cholesterol from chylomicron-like emulsion particles to the intestinal lumen, Sr-b1−/− mice and their wild-type littermates were injected with [3H]CO-labeled particles. As shown in Fig. 2A (right panel), the label was cleared rapidly from the circulation and there was no difference between WT and knockout mice. Interestingly, specific activity of cholesterol was unaltered both in bile and intestinal perfusate derived from Sr-b1−/− mice (Fig. 3).
Cholesterol introduced via HDL is not secreted via TICE
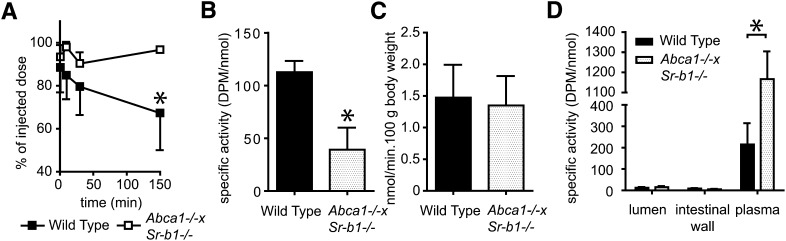
The results with Abca1−/− and Sr-b1−/− single knockout mice are clearly not conclusive with respect to the role of HDL as a cholesterol donor for TICE. We therefore tested the role of HDL directly in Abca1 and Sr-b1 double-deficient mice that lack HDL and are also defective in clearing HDL-cholesterol via the liver (12). To test whether or not HDL can function as a cholesterol donor for TICE, we introduced [3H]CO incorporated into HDL. As anticipated, the clearance of [3H]CO containing HDL from the circulation was attenuated in Abca1−/−×Sr-b1−/− mice (Fig. 4A). In addition, the lowered hepatic uptake of [3H]CO is reflected by the reduced specific activity of cholesterol secreted into bile (Fig. 4B). However, biliary cholesterol secretion was unaltered in the Abca1−/−×Sr-b1−/− mice (12). Whereas Sr-b1−/− mice were characterized by an elevated cholesterol secretion by the intestine (9), the rate of TICE (in nmol/min.100g body weight) in the double knockout mice was similar compared with their WT littermates (Fig. 4C). Interestingly, HDL-derived cholesterol was hardly secreted by the intestine in both Abca1−/−×Sr-b1−/− and WT mice, indicating that HDL is not taken up by the intestine and, concomitantly, hardly any radiolabeled cholesterol incorporated in the injected HDL could be secreted via TICE (Fig. 4D).
Fig. 4.
Plasma decay, biliary cholesterol secretion, and TICE of radiolabeled cholesterol from HDL in Abca1−/−×Sr-b1−/− mice. After injection of [3H]CO-labeled HDL into Abca1−/−×Sr-b1−/− mice and their WT littermates (n = 4), plasma samples were obtained at indicated time points and plasma decay was determined (A). At 1 h after injection, bile cannulations and intestine perfusions were performed to determine biliary cholesterol secretion (B) and TICE (C, D). Both total cholesterol and 3H activity were measured in bile, plasma, perfused intestine, and perfusate and the specific radioactivities are expressed as dpm/nmol cholesterol. Values are means ± SD. * indicates a significant difference between Abca−/− and WT littermates (P < 0.05).
DISCUSSION
The existence of TICE has been demonstrated in various studies in mouse models (5, 25) and earlier results indicate that this pathway is also present humans (26). The contribution of direct cholesterol secretion via the intestine to fecal sterol excretion is a novel concept that can advance the development of cholesterol-lowering therapies. However, the mechanism of TICE remains to be elucidated. An important question that needs to be answered is which donor particle delivers the cholesterol for secretion via TICE. In the RCT pathway via the hepatobiliary route, HDL plays an important role (27). In this report, we provide important evidence that HDL does not contribute significantly to TICE.
Our results from intestine perfusions show that upon injection of chylomicron-like emulsion particles, as a model of endogenously circulating triglyceride-rich lipoproteins (17), the incorporated radiolabeled cholesterol can be secreted directly into the intestinal lumen independent of the biliary route. It was shown previously that these chylomicron-like emulsion particles acquire lipoproteins in the circulation and are rapidly taken up rapid by the liver (13, 20) via apoE-selective receptors. Confirming the landmark study of Robins and Fasulo (28), that part of the in the liver-liberated cholesterol is preferentially secreted into bile and part of is resecreted into the circulation and is distributed over the lipoproteins including HDL, similar to the lipoprotein cholesterol profile of the mouse. A previous report from Post et al. (23) demonstrated this reutilization of cholesterol from chylomicron-like particles after uptake by the liver and ruled out direct transfer of radiolabeled cholesterol to HDL in plasma.
Because HDL plays an important role in RCT via the hepatobiliary route and contains the majority of circulating lipoprotein cholesterol in mice, we considered HDL a likely candidate to directly supply the intestine with cholesterol for TICE. Consistent with this hypothesis, in Sr-b1−/− mice that are characterized by elevated HDL-cholesterol levels, we found a two-fold increase in TICE (9); however, as demonstrated in the present study (Fig. 3B), specific activity in the perfusate was unaltered, which may be due to the increased pool of free cholesterol in the plasma (11). We now show also that in Abca1−/− mice without detectable HDL-cholesterol, TICE is not altered compared with their WT littermates. In fact, we still detected a significant secretion of radiolabeled cholesterol from injected chylomicron-like emulsion particles via TICE. The specific activity of the cholesterol secreted via TICE in Abca1−/− mice was approximately 60% of that found in their WT littermates. It cannot be excluded that the lack of TICE phenotype in Abca1−/− mice is due to compensatory mechanisms inducing peripheral cholesterol efflux in an HDL independent manner. In addition, possible decreased pheripheral cholesterol flux could be compensated for by increased cholesterol de novo synthesis in the intestine. HDL as a critical cholesterol donor for TICE was further refuted after we introduced [3H]CO-labeled HDL into mice. As expected, clearance of HDL via the liver was impaired in Abca1−/−×Sr-b1−/− mice due to Sr-b1 deficiency (11). Importantly, no uptake of HDL by the intestine was observed and hardly any radiolabeled cholesterol could be detected in the perfused intestinal lumen. Interestingly, no uptake and secretion of HDL-derived cholesterol could be detected in the WT littermates. These data suggest not only that TICE is not mediated via HDL, but also that little or no uptake of HDL by the intestine takes place, confirming earlier studies by Briand et al. (29). If HDL at a physiologic concentration is apparently not involved in TICE, it is interesting to speculate why we observed a two-fold increase of TICE in Sr-b1−/− mice in an earlier study (9). A closer look at the lipoprotein profile of these mice shows not only an increase of the HDL-peak but also a shift of HDL toward larger lipoprotein particles and an overlap with the LDL/IDL fractions (30). Furthermore, the abnormally large HDL particles that accumulate in SR-BI-knockout mice are enriched in apoE (11). It is thus possible that the severe enrichment of HDL with apoE caused the increase in TICE and may indicate that apoE may mediate TICE from lipoprotein remnants. Due to the extremely rapid metabolism of VLDL and LDL, we have not been able to delineate whether these particles specifically serve as substrate for TICE.
In an attempt to distinguish between the fate of free and esterified cholesterol, we incorporated both [14C]cholesterol and [3H]cholesteryl oleate into chylomicron-like emulsion particles. After clearance by the liver, [3H]cholesteryl oleate was mostly hydrolyzed into cholesterol and here, together with the [14C]cholesterol, it remained in the free form. Part of this hepatic radiolabeled cholesterol pool is resecreted into the circulation and distributed among the lipoproteins that circulate in WT mice. During the entire timeline from injection to secretion into the intestinal lumen, the ratio between the two isotopes (14C: 3H = 1: 4) remained similar. However, because both isotopes upon redistribution by the liver were then evenly distributed over free and esterified cholesterol, it was not possible to determine a possible preference by the intestine for either of the cholesterol forms.
Based on observational and epidemiological studies, increased HDL levels are considered to be clinically beneficial partly due to an improved RCT via the hepatobiliary route (31). However, our study indicates that HDL apparently does not play a role in TICE. When, as generally accepted, HDL is the primary acceptor for uptake of excess cholesterol from macrophages, TICE may play no role in the pathogenesis of atherosclerosis. A recent study by Temel et al. (6) disproves the selectivity of HDL in macrophage-specific RCT. When acutely bile diverted mice were intraperitoneally injected with cholesterol-loaded macrophages, the radiolabled cholesterol appeared undeterred in the intestinal lumen demonstrating that TICE can mediate macrophage specific RCT. In contrast, Nijstad et al. (32) reported that mdr2 knockout mice with abrogated biliary cholesterol secretion showed greatly reduced fecal excretion of macrophage derived radioactive cholesterol. Very recently, Zhao et al. (12) used the same methodology to determine RCT in the animal models used in the present study. Here, it was observed that RCT from WT macrophages was inhibited about 25% in both Abca1−/− mice and Abca1−/−×Sr-b1−/− mice. Hence, upon full disruption of hepatic HDL-mediated cholesterol uptake the majority of macrophage-specific RCT still continues. These data support the conclusions of Temel et al. but contrast to those of Nijstad et al. Taking all of the data together, we conclude that macrophage-derived cholesterol can be transported to the intestinal lumen both via bile and TICE. We hypothesize that HDL-mediated cholesterol transport preferentially takes the hepatobiliary route but when abrogated, the cholesterol can be resecreted from the liver and be excreted via TICE.
Limitations of this study
The integrative physiological approach used in this study does not allow a detailed probe into the mechanism by which cholesterol is donated to the enterocyte. We have attempted to set up a transcellular cholesterol transport study in cultured CaCo2 cells but failed to visualize a TICE-like transport (data not shown). The results reported here cannot be directly transplanted to the human situation. Mice lack CETP and have a much lower biliary cholesterol relative to bile salt secretion compared with humans (33). Lack of CETP can be overcome by carrying out future studies in CETP transgenic mice. Humanization of biliary lipid secretion is more difficult to realize.
Despite these limitations, we feel this data allows for the conclusion that direct secretion of cholesterol from blood through the intestine into the intestinal lumen of mice occurs independently of HDL and bile. However, the identity of the cholesterol donor responsible for delivery of cholesterol to the intestine for secretion via TICE remains to be elucidated. With the intestine being an accessible target, the identification of this step in the mechanism of TICE would benefit the development of novel and less invasive cholesterol-lowering therapies.
Footnotes
Abbreviations:
- CETP
- cholesteryl ester transfer protein
- [3H]CO
- [3H]cholesteryl oleate
- RCT
- reverse cholesterol transport
- SR
- scavenger receptor
- TICE
- transintestinal cholesterol efflux
- WT
- wild-type
This work was supported by the Dutch Digestive Foundation (MLDS grant 04-55; C.V.), The Netherlands Heart Foundation (grant 2001T4101; Y.Z.) and Top Institute Pharma grant T2-110. M.V.E. and P.C.N.R. are Established Investigators of The Netherlands Heart Foundation (grants 2007T056 and 2009T038, respectively).
REFERENCES
- 1.Ross R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340: 115–126 [DOI] [PubMed] [Google Scholar]
- 2.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278 [DOI] [PubMed] [Google Scholar]
- 3.Tall A. R. 2008. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 263: 256–273 [DOI] [PubMed] [Google Scholar]
- 4.Rader D. J. 2007. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat. Clin. Pract. Cardiovasc. Med. 4: 102–109 [DOI] [PubMed] [Google Scholar]
- 5.van der Velde A. E., Vrins C. L., Van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., Groen A. K. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975 [DOI] [PubMed] [Google Scholar]
- 6.Temel R. E., Sawyer J. K., Yu L., Lord C., Degirolamo C., McDaniel A., Marshall S., Wang N., Shah R., Rudel L. L., et al. 2010. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 12: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain M. M. 2000. A proposed model for the assembly of chylomicrons. Atherosclerosis. 148: 1–15 [DOI] [PubMed] [Google Scholar]
- 8.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velde A. E., Vrins C. L., Van den Oever K., Seemann I., Oude Elferink R. P., Van Eck M., Kuipers F., Groen A. K. 2008. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G203–G208 [DOI] [PubMed] [Google Scholar]
- 10.Hamon Y., Broccardo C., Chambenoit O., Luciani M. F., Toti F., Chaslin S., Freyssinet J. M., Devaux P. F., McNeish J., Marguet D., et al. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2: 399–406 [DOI] [PubMed] [Google Scholar]
- 11.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. 1997. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA. 94: 12610–12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y., Pennings M., Vrins C. L., Calpe-Berdiel L., Hoekstra M., Kruijt J. K., Ottenhoff R., Hildebrand R. B., van der Sluis R., Jessup W., et al. 2011. Hypocholesterolemia, foam cell accumulation, but no atherosclerosis in mice lacking ABC-transporter A1 and scavenger receptor BI. Atherosclerosis. 218: 314–322 [DOI] [PubMed] [Google Scholar]
- 13.Rensen P. C., van Dijk M. C., Havenaar E. C., Bijsterbosch M. K., Kruijt J. K., Van Berkel T. J. 1995. Selective liver targeting of antivirals by recombinant chylomicrons–a new therapeutic approach to hepatitis B. Nat. Med. 1: 221–225 [DOI] [PubMed] [Google Scholar]
- 14.Mortimer B. C., Beveridge D. J., Martins I. J., Redgrave T. G. 1995. Intracellular localization and metabolism of chylomicron remnants in the livers of low density lipoprotein receptor-deficient mice and apoE-deficient mice. Evidence for slow metabolism via an alternative apoE-dependent pathway. J. Biol. Chem. 270: 28767–28776 [DOI] [PubMed] [Google Scholar]
- 15.Fluiter K., Vietsch H., Biessen E. A., Kostner G. M., Van Berkel T. J., Sattler W. 1996. Increased selective uptake in vivo and in vitro of oxidized cholesteryl esters from high-density lipoprotein by rat liver parenchymal cells. Biochem. J. 319: 471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz J., Qiu S. Q., Oesterle A., DeSilva H. V., Shafi S., Havel R. J. 1995. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 92: 4611–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rensen P. C., Herijgers N., Netscher M. H., Meskers S. C., Van E. M., Van Berkel T. J. 1997. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J. Lipid Res. 38: 1070–1084 [PubMed] [Google Scholar]
- 18.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 19.Huang H., Kauan J. W., Guilbault G. G. 1975. Fluorometric enzymatic determination of total cholesterol in serum. Clin. Chem. 21: 1605–1608 [PubMed] [Google Scholar]
- 20.van der Veen J. N., van Dijk T. H., Vrins C. L., Van Meer H., Havinga R., Bijsterveld K., Tietge U. J., Groen A. K., Kuipers F. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quarfordt S. H., Goodman D. S. 1967. Metabolism of doubly-labeled chylomicron cholesteryl esters in the rat. J. Lipid Res. 8: 264–273 [PubMed] [Google Scholar]
- 22.Thomas M. S., Rudel L. L. 1987. Intravascular metabolism of lipoprotein cholesteryl esters in African green monkeys: differential fate of doubly labeled cholesteryl oleate. J. Lipid Res. 28: 572–581 [PubMed] [Google Scholar]
- 23.Post S. M., Groenendijk M., van der Hoogt C. C., Fievet C., Luc G., Hoekstra M., Princen H. M., Staels B., Rensen P. C. 2006. Cholesterol 7alpha-hydroxylase deficiency in mice on an APOE*3-Leiden background increases hepatic ABCA1 mRNA expression and HDL-cholesterol. Arterioscler. Thromb. Vasc. Biol. 26: 2724–2730 [DOI] [PubMed] [Google Scholar]
- 24.Groen A. K., Bloks V. W., Bandsma R. H., Ottenhoff R., Chimini G., Kuipers F. 2001. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 108: 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown J. M., Bell T. A., III, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., et al. 2008. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 283: 10522–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmonds W. J., Hofmann A. F., Theodor E. 1967. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. J. Clin. Invest. 46: 874–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50(Suppl): S189–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robins S. J., Fasulo J. M. 1999. Delineation of a novel hepatic route for the selective transfer of unesterified sterols from high-density lipoproteins to bile: studies using the perfused rat liver. Hepatology. 29: 1541–1548 [DOI] [PubMed] [Google Scholar]
- 29.Briand F., Naik S. U., Fuki I., Millar J. S., Macphee C., Walker M., Billheimer J., Rothblat G., Rader D. J. 2009. Both the peroxisome proliferator-activated receptor (PPAR) delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal re-absorption of HDL-derived cholesterol. Clin. Transl. Sci. 2: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Eck M., Hoekstra M., Out R., Bos I. S., Kruijt J. K., Hildebrand R. B., Van Berkel T. J. 2008. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. J. Lipid Res. 49: 136–146 [DOI] [PubMed] [Google Scholar]
- 31.Chapman M. J. 2006. Therapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart disease. Pharmacol. Ther. 111: 893–908 [DOI] [PubMed] [Google Scholar]
- 32.Nijstad N., Gautier T., Briand F., Rader D. J., Tietge U. J. 2011. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 140: 1043–1051 [DOI] [PubMed] [Google Scholar]
- 33.Van der Wulp M. Y., Verkade H. J., Groen A. K. 2012. Regulation of cholesterol homeostasis. Mol. Cell Endocrinol. Epub ahead of print [DOI] [PubMed] [Google Scholar]