Abstract
Atherosclerosis is a chronic inflammatory vascular disease. Toll-like receptors (TLRs) are major initiators of inflammation. TLR2 promotes atherosclerosis in LDL receptor (LDLr)-deficient mice fed a high-fat diet (HFD). TLR2 forms heterodimers with TLR1 or TLR6 to enable inflammatory responses in the presence of distinct ligands. Here we asked whether TLR1 and/or TLR6 are required. We studied atherosclerotic disease using either TLR1- or TLR6-deficient mice. Deficiency of TLR1 or TLR6 did not diminish HFD-driven disease. When HFD-fed LDLr-deficient mice were challenged with Pam3 or MALP2, specific exogenous ligands of TLR2/1 or TLR2/6, respectively, atherosclerotic lesions developed with remarkable intensity in the abdominal segment of the descending aorta. In contrast to atherosclerosis induced by the endogenous agonists, these lesions were diminished by deficiency of either TLR1 or TLR6. The endogenous ligand(s) that arise from consumption of a HFD and promote disease via TLR2 are unknown. Either TLR1 or TLR6 are redundant for this endogenous ligand detection, or they are both irrelevant to endogenous ligand detection. However, the exogenous ligands Pam3 and MALP2 promote severe abdominal atherosclerosis in the descending aorta that is dependent on TLR1 and TLR6, respectively.
Keywords: inflammation, lipids, macrophages, receptors, agonists
Atherosclerosis is a chronic inflammatory disease in which immune and metabolic factors interact to initiate and propagate arterial lesions (1). An understanding of the role of toll-like receptors (TLRs) in atherosclerosis could clarify the progression of this complex disease (2). TLRs, which are expressed on most cells in the body, are critical pathogen recognition receptors in innate immunity. They recognize and bind diverse molecules representative of multiple types of foreign microbes (3). These microbially derived molecules are considered “exogenous” TLR ligands; they include lipids, carbohydrates, nucleic acids, and proteins. TLRs also recognize host-derived ligands, referred to as “endogenous” TLR ligands because they arise from damaged tissue self molecules including breakdown products of oxidation and tissue degradation (4). Thus TLRs are involved in innate immune responses beyond anti-microbial responses, including roles in homeostatic mechanisms that resolve sterile injury.
We focused our atherosclerosis studies on TLR2 because in vitro experiments with cultured human coronary artery endothelial cells (ECs) show that cells exposed to an approximation of the disturbed flow conditions prevalent at sites of lesion development exhibit upregulated responsiveness to TLR2 agonists (5). This is due to an enhanced expression of TLR2 in ECs in response to disturbed flow.
We reported that a global deficiency of TLR2 in LDL receptor (LDLr)-deficient (LDLr−/−) mice fed a high-fat diet (HFD) for 10 or 14 weeks is atheroprotective (6). The deletion of TLR2 in these hyperlipidemic mice results in a 50% reduction in lesion severity in both the aorta and the heart sinus. EC expression of TLR2 is increased by consumption of a HFD, and this expression, which is observed within 2 weeks in the lesion-prone lesser curvature of the aortic arch (rather than the lesion-resistant greater curvature), correlates with intimal leukocyte accumulation (7). The endogenous agonist that binds TLR2 on EC and promotes increased TLR2 expression, EC activation, and leukocyte accumulation is unknown, but its presence is increased in LDLr−/− mice fed a HFD (6, 7). The identification of this unknown endogenous agonist is an ongoing project.
In additional studies, we administered a synthetic TLR2/1 agonist, Pam3, to the hyperlipidemic LDLr−/− mice to determine the atherosclerotic effects of systemic or whole-body TLR2 activation (6, 7). After 12 weeks of HFD consumption and weekly intraperitoneal (i.p.) injection of a vehicle control or Pam3, the Pam3-exposed mice exhibit a striking dose-dependent increase in lesion severity. Aortic lesion areas are increased 82% and 400% in the heart sinus and the en face aorta, respectively, in mice repeatedly exposed to Pam3 (6). Importantly, no accelerated disease is observed in TLR2+/+LDLr−/− mice receiving vehicle control or TLR2−/−LDLr−/− mice receiving Pam3, indicating absolute specificity for TLR2. Moreover, leukocyte TLR2 expression is sufficient for this exacerbated disease pathology in response to Pam3, whereas EC expression is not required (6).
The specificity of TLR2 is fairly broad; some of this breadth derives from its association with either TLR1 or TLR6. Heterodimer TLR2/6 binds bis-acylated lipopeptides such as MALP2, whereas the TLR2/1 heterodimer binds tris-acylated lipopeptides such as Pam3. Both the specific loci of the lipopeptides and the lipopeptide moieties are key (8, 9). It is unknown whether TLR2 homodimers are formed or signal. TLR2 and TLR6 can associate with other surface receptors (such as CD36) to activate cells (10). Because TLR2 is proatherogenic, we sought to determine which of its two known TLR signaling partners, TLR1 and TLR6, are in involved in promoting lesion progression in response to cell activation via either endogenous or exogenous agonists.
METHODS
LDLr−/− mice backcrossed onto a C57BL/6 background were purchased from the Jackson Laboratory and bred in-house. TLR1−/− and TLR6−/− mice were kindly provided by Shizuo Akira (Osaka University, Japan). The TLR1−/− and TLR6−/− mice were backcrossed into a C57BL/6 background, and after seven generations, double-mutant mice (TLR1−/−LDLr−/− and TLR6−/−LDLr−/−) were generated by crossing TLR1−/− or TLR6−/− mice with LDLr−/−mice. TLR1 and TLR6 genotyping was performed via PCR. In brief, DNA from tail clippings was amplified by 33 cycles of 45 s at 94°C, 45 s at 65°C, and 2 min extension at 72°C using specific primer pairs. The primers used for TLR1 DNA detection were designed to bind in the regions just upstream and within the neomycin (NEO) insert cassette inserted into the TLR1 gene. The TLR1 forward primer (5′-GATGGTGACAGTCAGCAGAACAGTATC) and reverse primer (5′- AAGGTGATCTTGTGCCACCCAACAGTC) and NEO cassette (5′- ATCGCCTTCTATCGCCTTCTTGACGAG) were synthesized by Operon. The primers used for TLR6 DNA detection also bound in the regions just upstream and within the NEO cassette inserted into the TLR6 gene. The TLR6 forward primer (5′- GAAATGTAAATGAGCTTGGGGATGGCG) and reverse primer (5′- TTATCAGAACTCACCAGAGGTCCAACC) and NEO cassette (5′- ATCGCCTTCTATCGCCTTCTTGACGAG) were synthesized by Operon. PCR products were separated in 3% agarose gels and visualized with ethidium bromide. LDLr−/−genotyping was performed as previously described (6, 7).
Mice were weaned at 4 weeks and fed ad libitum a standard mouse chow diet (Harlan Teklad 7019). All mice were housed five per cage in autoclaved, filter-top cages with autoclaved water and kept on a 12 h light/dark cycle. Animal care and use for all procedures was done in accordance with guidelines approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Mouse cohorts selected for study were between 8 and 10 weeks old when they were first fed the HFD, which contained 1.25% cholesterol, 15.8% fat, and no cholate (Harlan Teklad 94059). Mouse cohort sizes are indicated in the figures. The HFD was fed for 10 weeks unless otherwise noted. The TLR2/1 agonist Pam3 was obtained from Invivogen and was diluted in saline. Mice were injected intraperitoneally with 0.05 ml of either saline or 1 mg/ml Pam3. The TLR2/6 agonist, MALP2, was obtained from ENZO Life Sciences and was stored at −80°C in 50 mM octyl glucoside in PBS. Mice were injected intraperitoneally with 0.05 ml of either 50 mM octyl glucoside in PBS or 10 μg of MALP2.
Mice were fasted periodically and weighed, and venous blood was drawn from the retro-orbital sinus. Plasma was isolated, and total cholesterol levels were measured by a colorimetric enzymatic method (Thermo Electron Corp.). Plasma IL-12/IL-23p40 was measured with an ELISA from R and D Systems (DY2398).
At euthanasia, animals were perfused with PBS, followed by formal sucrose (4% paraformaldehyde and 5% sucrose in PBS, pH 7.4). For en face analysis, the entire mouse aorta was dissected from the proximal ascending aorta to the bifurcation of the iliac artery using a dissecting microscope. Adventitial fat was removed, and the aorta was opened longitudinally, pinned flat onto black dissecting wax, stained with Sudan IV, and photographed at a fixed magnification. The photographs were digitized, and total aortic areas and lesion areas were calculated using Adobe Photoshop version 7.0 Chromatica V and NIH Scion Image software (http://rsb.info.nih.gov/nih-image/Default.html). Results were reported as a percentage of the total aortic area that contained lesions (11).
As a second assessment of atherosclerosis, lesions of the aortic root (aortic sinus) were analyzed by a modification of current methods of heart valve lesion analysis (6). Utilizing stereological principles, lesion volume was estimated across a fixed distance of the aortic sinus. Our preliminary data demonstrated that lesion volume estimation, compared with lesion cross-sectional area estimation, was a more-conservative and robust method for estimating aortic sinus atherosclerosis in mice. After overnight fixation of hearts in 4% paraformaldehyde and 5% sucrose, they were cut at an angle approximately 100–120° (clockwise) from the long axis of the heart and embedded in OCT (Tissue-Tek). Frozen hearts were sectioned on a Leica cryostat, with 10 μm sections collected from the beginning of the aortic sinus (defined as when a valve leaflet became visible) to 500 μm below the beginning of the sinus. For hearts cut at an angle that resulted in valve leaflets not appearing in the same section (due to poor section angle), the lagging leaflet was used to determine the 500 μm distance. Sections were collected in duplicate at 50 μm intervals. Sections were stained with Oil Red O, counterstained with Gill hematoxylin 1 (Fischer Scientific International), photographed, and digitized for lesion analysis. Scoring of valve lesion areas was done for each of the three valve cusps individually. Only lesion areas found within the valve cusp were measured. Lesion volume estimation was determined from a 1 in 10 sampling rate; hence, valve cusps spaced at 140 μm were used to determine the lesion volume for a total of four sections analyzed per valve cusp. Lesion volume was calculated from an integration of the measured cross-sectional areas. Prediction of the coefficient of error (CE) in approximating lesion volume was computed using the Cavalieri estimator derived from a covariogram analysis of an ordered set of estimates of cross-sectional areas. This yielded CE values of less than 10% that were acceptable for a stereological computation of lesion volume.
All scatter plots are expressed with the mean ± SE. Atherosclerosis data were analyzed by the unpaired Student's t-test. One-factor ANOVA or the nonparametric Kruskal-Wallis ANOVA on ranks was used to determine whether differences among multiple (greater than 2) groups existed with the treatment. For each data set, a normality test (P > 0.05) and an equal variance test (P > 0.05) were performed to identify normally distributed data. If either test failed, the nonparametric test was performed. Body weight and total plasma cholesterol changes over time were analyzed utilizing 2-factor repeated measures ANOVA. The analysis of factor level effects was done by the Holm-Sidak test of pairwise multiple comparisons. All statistical analysis was done with the use of SigmaStat 3.00 (SPSS, Inc.). A value of P < 0.05 was considered significant.
RESULTS
Endogenous TLR agonists
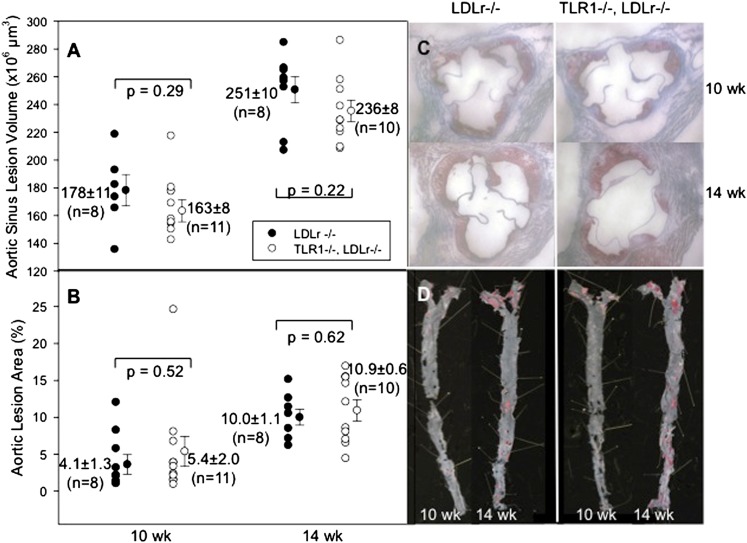
To determine the role of TLR1 in atherosclerosis driven by endogenous agonists, TLR1−/− mice were crossed with LDLr−/−mice to generate double-knockout TLR1−/−LDLr−/− mice. Male mice were fed the HFD for 10 and 14 weeks. Plasma cholesterol levels (see supplementary Fig. IA) and body weights (see supplementary Fig. IB) were statistically identical in the two cohorts except that the plasma cholesterol level in the TLR1−/−LDLr−/− cohort was diminished at 14 weeks. The heart sinus lesion volumes and percent aortic en face lesion areas revealed no significant differences between genotypes at 10 or 14 weeks of HFD feeding (Fig. 1A, B). This shows that increased HFD-induced atherosclerosis progression was not dependent on TLR1.
Fig. 1.
Effect of endogenous agonists in TLR1−/−LDLr−/− mice. A: Individual LDLr−/− mice (closed circles) and TLR1−/−LDLr−/− mice (open circles) were fed the HFD for 10 or 14 weeks. At harvest, the hearts were removed and sectioned and the lesion volumes were measured as described in Methods. B: The aortas were dissected, pinned, and stained, and the percent of the en face aortic surface that contained lesions was measured as described. C, D: Representative images of heart sections and aortic surfaces, respectively.
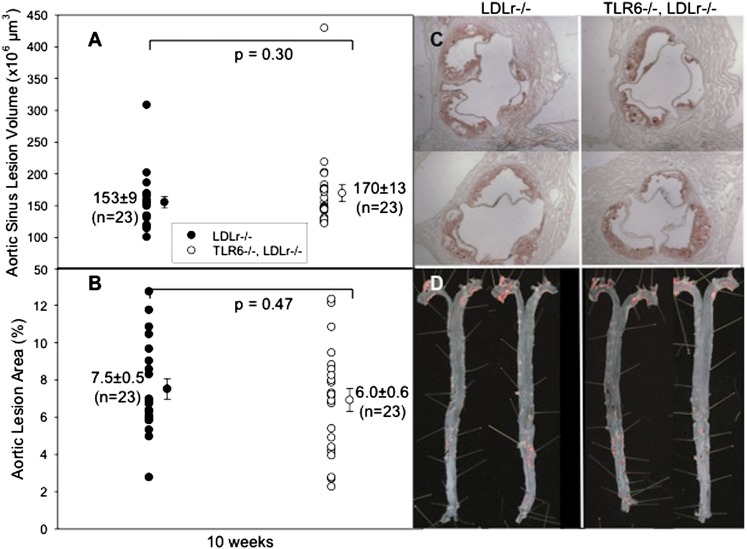
To determine the role of TLR6 in atherosclerosis driven by endogenous agonists, TLR6−/− mice were crossed with atherosclerosis-prone LDLr−/−mice to generate double-knockout TLR6−/−LDLr−/− mice. Male mice were fed a HFD for 10 weeks. Relative to LDLr−/− mice (n = 21), TLR6−/−LDLr−/− mice (n = 22) had significantly reduced total plasma cholesterol levels only at week 3, and both cohorts had similar body weights throughout the 10 weeks of HFD consumption (see supplementary Fig. IIA, B). Analysis of heart sinus lesion volumes and percent aortic en face lesion areas again revealed no difference between genotypes in disease severity (Fig. 2A, B).
Fig. 2.
Effect of endogenous agonists in TLR6−/−LDLr−/− mice. A: Individual LDLr−/− mice (closed circles) and TLR6−/−LDLr−/− mice (open circles) were fed the HFD for 10 weeks. At harvest, the hearts were removed and sectioned and the lesion volumes were measured as described in Methods. B: The aortas were dissected, pinned, and stained, and the percent of the en face aortic surface that contained lesions was measured as described. C, D: Representative images of heart sections and aortic surfaces, respectively.
Exogenous TLR agonists
These studies were designed to evaluate the impact on atherosclerosis of chronic exposure to exogenous TLR2/1 or TLR2/6 agonists. Male LDLr−/− mice or TLR1−/−LDLr−/− mice received weekly intraperitoneal injections of 50 μg of Pam3 while they were consuming the HFD. We previously reported increased levels of plasma SAA in LDLr−/− mice 24 h after initiation of the HFD and intraperitoneal Pam3 injections (6) to verify the systemic inflammatory response to Pam3. Throughout the study, the TLR1−/−LDLr−/− mice had significantly greater plasma cholesterol levels and greater weight gain than LDLr−/− mice (see supplementary Fig. IIIA, B).
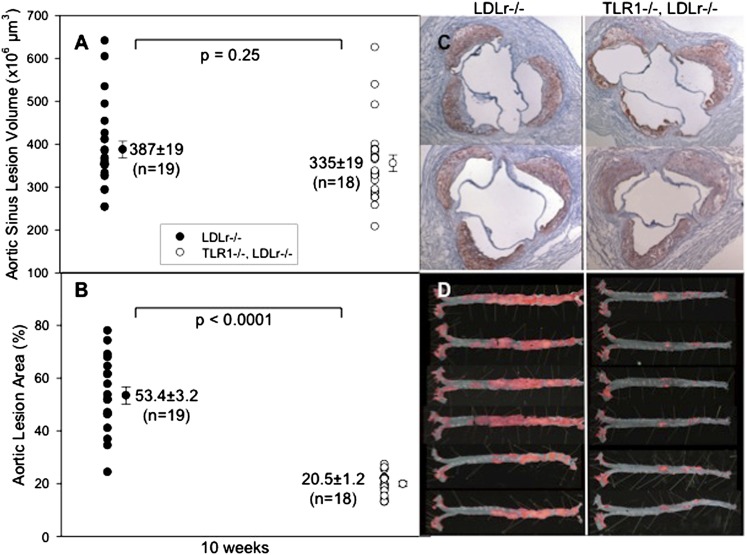
Although the heart sinus lesion volumes revealed no significant differences between LDLr−/− and TLR1−/−LDLr−/− mice (P = 0.25), the percent en face aortic lesion areas after 10 weeks of HFD feeding and Pam3 exposure were strikingly increased in LDLr−/− mice compared with TLR1−/−LDLr−/− mice (P < 0.0001) (Fig. 3A, B). We reported previously with LDLr−/− mice that saline exposure resulted in sporadic abdominal lesions (6). Here again, Pam3 administration triggered the growth of profuse abdominal atherosclerosis in the LDLr−/− mice (Fig. 3C). LDLr−/− mice deficient in TLR1 did not exhibit this same level of abdominal atherosclerosis. Also, this confirmed the specificity of Pam3 for TLR2/1-mediated cell activation.
Fig. 3.
Effect of an exogenous agonist in TLR1−/−LDLr−/− mice. A: Individual LDLr−/− mice (closed circles) and TLR1−/−LDLr−/− (open circles) were fed the HFD for 10 weeks and were injected intraperitoneally every week with 0.05 ml of 1 mg/ml Pam3. At harvest, the hearts were removed and sectioned and the lesion volumes were measured as described in Methods. B: The aortas were dissected, stained, and pinned, and the percent of the en face aortic surface that contained lesions was measured as described. C, D: Representative images of heart sections and aortic surfaces, respectively.
We next assessed the impact on atherosclerosis of chronic exposure to an exogenous TLR2/6 agonist. Male LDLr−/− mice or TLR6−/−LDLr−/− mice received weekly intraperitoneal injections of 10 μg of MALP2 (ENZO Life Sciences) or vehicle while they were fed the HFD. The TLR6−/−LDLr−/− mice exhibited greater plasma cholesterol levels but similar weight gain compared with LDLr−/− mice (see supplementary Fig. IVA, B).
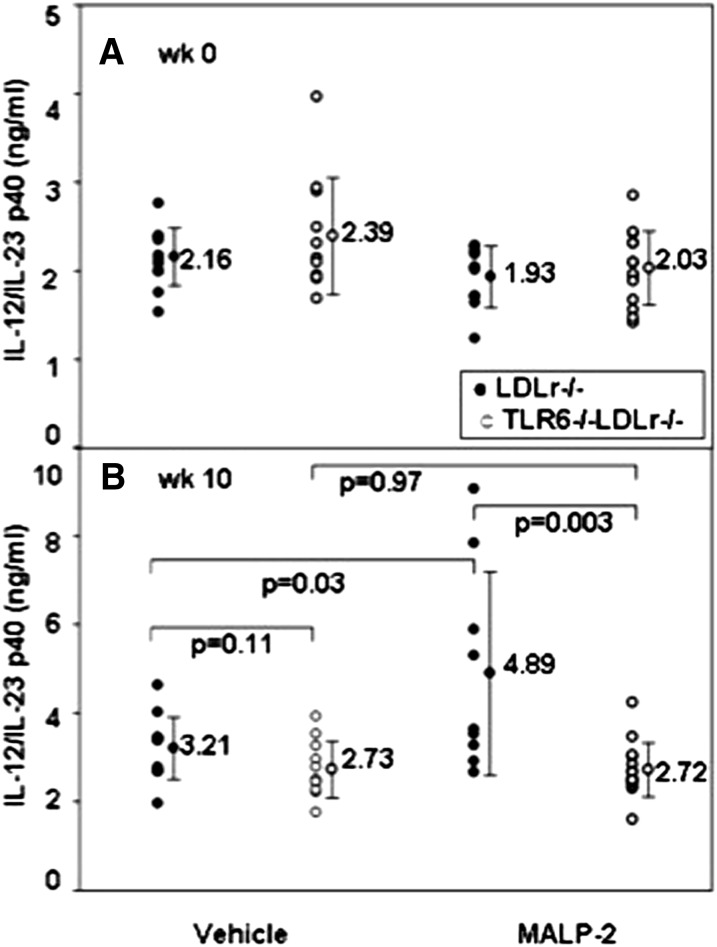
MALP2 induced a systemic inflammatory response in the LDLr−/− mice, as revealed by the plasma levels of the proinflammatory cytokine, IL-12/IL-23p40. There were no differences among the groups before MALP2 exposure. But, at 10 weeks, the LDLr−/− mice exposed to MALP2 had elevated levels of IL-12/IL-23p40 (Fig. 4).
Fig. 4.
Exogenous agonist plasma cytokines. IL-12/IL-23p40 levels were measured at week 0 (A) or week 10 (B) in plasma of LDLr−/− mice (closed circles) or TLR6−/−LDLr−/− mice (open circles) that were injected intraperitoneally every week with 10 μg of MALP2 in 50 mM octyl glucoside in PBS, while they consumed the HFD for 10 weeks.
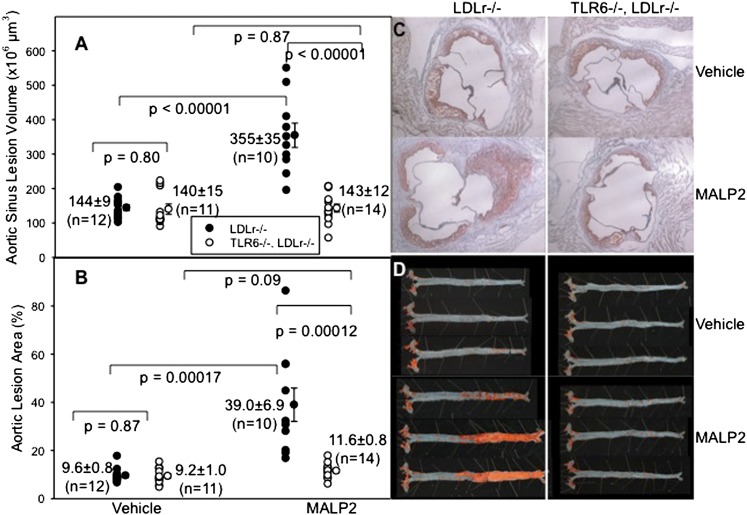
Although analysis of aortic atherosclerosis demonstrated no differences in the aortic sinus lesion volumes with weekly vehicle administration, MALP2 exposure of LDLr−/−mice induced a remarkable increase in heart valve lesions in LDLr−/− mice that was not observed in TLR6−/−LDLr−/− mice (Fig. 5A). Moreover, the en face lesions at 10 weeks of HFD feeding and MALP2 exposure were again strikingly different between LDLr−/− and TLR6−/−LDLr−/− mice exposed to MALP2 (40% vs. 9%, P < .0002). With both LDLr−/− mice and TLR6−/−LDLr−/− mice, vehicle exposure induced only sporadic abdominal lesions, whereas MALP2 administration in LDLr−/− mice triggered the growth of profuse abdominal atherosclerosis (Fig. 5B). Mice deficient in TLR6 (TLR6−/−LDLr−/− mice) exhibited no changes in aortic atherosclerosis and confirmed the specificity of MALP2 for TLR2/6-mediated cell activation.
Fig. 5.
Effect of an exogenous agonist in TLR6−/−LDLr−/− mice. A: Individual LDLr−/− mice (closed circles) and TLR6−/−LDLr−/− mice (open circles) were fed the HFD for 10 weeks and were injected intraperitoneally every week with 0.05 ml of either 50 mM octyl glucoside in PBS (vehicle) or 200 μg/ml of MALP2 in 50 mM octyl glucoside. At harvest, the hearts were removed and sectioned and the heart sinus lesion volumes were measured as described in Methods. B: The aortas were dissected, pinned, and stained with Sudan IV (red), and the percent of the en face aortic surface that contained lesions was measured as described. C, D: Representative images of heart sections and aortic surfaces, respectively.
DISCUSSION
These studies were undertaken to identify which TLR2 coreceptors participated with TLR2 to promote atherosclerosis progression in mice. The endogenous agonist HFD studies were surprising, because TLR2 depletion is very atheroprotective (6, 7), and therefore, we anticipated that depletion of either TLR1 or TLR6 would also be atheroprotective toward endogenous agonists. However, deficiency of neither was protective. There are several hypotheses that would explain our observation that neither TLR1 nor TLR6 depletion was atheroprotective. First, TLR1 and TLR6 could be redundant with respect to activation by endogenous agonist(s). This could, theoretically, be tested in TLR1 and TLR6 doubly deficient mice. However TLR1 and TLR6 are very close together on mouse chromosome 5 (12), so close that doubly deficient mice cannot be obtained by simple cross breeding. Thus, an experiment with triple knock-out, LDLr−/−TLR1−/−TLR6−/− mice, is not currently feasible. A second hypothesis is that neither TLR1 nor TLR6 is required for TLR2-dependent activation by endogenous agonist(s). TLR2 is not known to function without a TLR signaling partner. Yet, perhaps in this context, TLR2 either does not require a partner, can function as a homodimer, or functions as a heterodimer with an as-yet-unidentified signaling partner.
These studies are relevant to the recent finding that TLR6, in conjunction with CD36 and TLR4, enabled macrophage responses to oxidized LDL (13). Because TLR6 deficiency did not diminish lesion progression in the absence of an exogenous agonist, our results indicate that macrophage recognition of endogenous oxidized LDL through TLR6 does not limit the lesion progression we observe in HFD-fed LDLr−/− mice. Furthermore, we observed previously that macrophage expression of TLR2 does not limit lesion formation mediated by endogenous agonist signaling via TLR2 (6).
With some exogenous agonists, CD36 is an important partner in TLR2-dependent ligand recognition (10). In the LDLr−/− model, CD36 deficiency is not atheroprotective, although it is atheroprotective in apoE−/− mice (14). Zhao et al. (14) have reported that levels of oxidized LDL are significantly higher in the plasma of ApoE−/− mice than they are in LDLr−/− mice. These several lines of evidence suggest that oxidized LDL is not the important driver of atherosclerosis in LDLr−/− mice that it is in apoE−/− mice. Identifying the endogenous TLR2 ligand that drives atherosclerosis in LDLr−/− mice is a current topic of investigation in our lab.
The exogenous agonist feeding studies were undertaken to understand how TLR1 and TLR6 participated in the TLR2-mediated acceleration of disease we had observed previously in the descending aorta, a pathology characterized by profuse abdominal lesions and minimal thoracic lesions (6). Although the current studies involved intraperitoneal administration of Pam3, previous experiments with intravenous administration of Pam3 reproduced these same severe abdominal lesions (unpublished observations). Thus, the severe abdominal pathology was not a result of the mode of Pam3 administration. This implicates a role for systemically activated bone marrow-derived cells in mediating the effects of intraperitoneally or intravenously injected Pam3.
A difference in the aortic sinus volumes of the mice exposed to the two different exogenous agonists was evident in these studies. MALP2 administration to LDLr−/−mice induced remarkable lesion volumes in heart valves in LDLr−/− mice that were not observed in TLR6−/−LDLr−/− mice (Fig. 5A). This was not the case in TLR1−/−LDLr−/− mice administered Pam3 (Fig. 3A). Pam3-injected TLR1−/−LDLr−/− mice were not protected against lesion formation in the heart sinus, whereas MALP2-injected LDLr−/−TLR6−/− mice were protected. We have no explanation for these results, but suspect they could be related to the specificity of the synthetic agonists. If MALP2 was specific for only TLR2/6 heterodimers, then a deficiency of TLR6 would provide protection in the heart sinus as well as the aorta. If Pam3 were not specific for only the TLR2/1 heterodimer, but also could activate TLR2 homodimers, then a deficiency of TLR1 might not provide protection in the heart. However, we are not aware of any data suggesting that Pam3 can stimulate TLR2 homodimers, or even that such homodimers exist in vivo.
It should be emphasized that Pam3- and MALP2-induced atherosclerosis in LDLr−/− mice is not a contrived model that is irrelevant to human atherosclerosis. If hyperlipidemia is combined with sources of inflammation that are external to the vessel wall, such as periodontal disease from Porphyromonas gingivalis infection (3) or some Firmicutes gut flora (15), which are the majority of microorganisms in the human body, then the combined risk factors can potentially exacerbate atherosclerosis. TLRs are sensors of multiple exogenous infectious agents, and many of these, such as LPS (Leptospira), zymosan, Staph. aureus, cytomegalovirus, Yersinia V antigen, Spirochetal OspA, Neisseria PorB, bacterial fimbrae, measles virus, herpes simplex virus, Mycobacterial and Brucella lipoproteins, are correlated with atherosclerosis risk (2, 16). When systemic TLR activation occurs with these multiple infectious agents over a lifetime, this activation (if chronic) can, when combined with hyperlipidemia, promote arterial inflammation (17, 18).
Acknowledgments
The authors thank Joan Lord for administrative help. This is manuscript #21087 from The Scripps Research Institute.
Footnotes
Abbreviations:
- CE
- coefficient of error
- EC
- endothelial cell
- HFD
- high-fat diet
- LDLr
- LDL receptor
- NEO
- neomycin
- TLR
- toll-like receptor
This work was supported by National Institutes of Health Grants HL-088093 and HL-108293. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures.
REFERENCES
- 1.Libby P., Okamoto Y., Rocha V. Z., Folco E. 2010. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 74: 213–220 [DOI] [PubMed] [Google Scholar]
- 2.Curtiss L. K., Tobias P. S. 2007. The toll of toll-like receptors, especially toll-like receptor 2, on murine atherosclerosis. Curr. Drug Targets. 8: 1230–1238 [DOI] [PubMed] [Google Scholar]
- 3.Hayashi C., Madrigal A. G., Liu X., Ukai T., Goswami S., Gudino C. V., Gibson F. C., III, Genco C. A. 2010. Pathogen-mediated inflammatory atherosclerosis is mediated in part via toll-like receptor 2-induced inflammatory responses. J. Innate Immun. 2: 334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin L. 2010. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10: 712–723 [DOI] [PubMed] [Google Scholar]
- 5.Dunzendorfer S., Lee H. K., Tobias P. S. 2004. Flow-dependent regulation of endothelial toll-like receptor 2 expression through inhibition of sp1 activity. Circ. Res. 95: 684–691 [DOI] [PubMed] [Google Scholar]
- 6.Mullick A. E., Tobias P. S., Curtiss L. K. 2005. Modulation of atherosclerosis in mice by toll-like receptor 2. J. Clin. Invest. 115: 3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullick A. E., Soldau K., Kiosses W. B., Bell T. A., III, Tobias P. S., Curtiss L. K. 2008. Increased endothelial expression of toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 205: 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omueti K. O., Beyer J. M., Johnson C. M., Lyle E. A., Tapping R. I. 2005. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J. Biol. Chem. 280: 36616–36625 [DOI] [PubMed] [Google Scholar]
- 9.Buwitt-Beckmann U., Heine H., Wiesmuller K. H., Jung G., Brock R., Ulmer A. J. 2005. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 272: 6354–6364 [DOI] [PubMed] [Google Scholar]
- 10.Hoebe K., Georgel P., Rutschmann S., Du X., Mudd S., Crozat K., Sovath S., Shamel L., Hartung T., Zahringer U., et al. 2005. Cd36 is a sensor of diacylglycerides. Nature. 433: 523–527 [DOI] [PubMed] [Google Scholar]
- 11.Schiller N. K., Black A. S., Bradshaw G. P., Bonnet D. J., Curtiss L. K. 2004. Participation of macrophages in atherosclerotic lesion morphology in LDLr−/− mice. J. Lipid Res. 45: 1398–1409 [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O., Sato S., Horiuchi T., Hoshino K., Takeda K., Dong Z., Modlin R. L., Akira S. 2002. Cutting edge: role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169: 10–14 [DOI] [PubMed] [Google Scholar]
- 13.Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., et al. 2010. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11: 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z., de Beer M. C., Cai L., Asmis R., de Beer F. C., de Villiers W. J., van der Westhuyzen D. R. 2005. Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler. Thromb. Vasc. Biol. 25: 168–173 [DOI] [PubMed] [Google Scholar]
- 15.He Z. Q., Zhen Y., Liang C., Wang H., Wu Z. G. 2008. Vicious cycle composed of gut flora and visceral fat. Med. Hypotheses. 70: 808–811 [DOI] [PubMed] [Google Scholar]
- 16.Ott S. J., El Mokhtari N. E., Musfeldt M., Hellmig S., Freitag S., Rehman A., Kuhbacher T., Nikolaus S., Namsolleck P., Blaut M., et al. 2006. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation, 113: 929–937 [DOI] [PubMed] [Google Scholar]
- 17.Henrichot E., Juge-Aubry C. E., Pernin A., Pache J. C., Velebit V., Dayer J. M., Meda P., Chizzolini C., Meier C. A. 2005. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler. Throm. Vasc. Biol. 25: 2594–2599 [DOI] [PubMed] [Google Scholar]
- 18.Mathieu P., Lemieux I., Després J. P. 2010. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 87: 407–416 [DOI] [PubMed] [Google Scholar]