Abstract
ApoM is mainly associated with HDL. Nevertheless, we have consistently observed positive correlations of apoM with plasma LDL cholesterol in humans. Moreover, LDL receptor deficiency is associated with increased plasma apoM in mice. Here, we tested the idea that plasma apoM concentrations are affected by the rate of LDL receptor-mediated clearance of apoB-containing particles. We measured apoM in humans each carrying one of three different LDL receptor mutations (n = 9) or the apoB3500 mutation (n = 12). These carriers had increased plasma apoM (1.34 ± 0.13 µM, P = 0.003, and 1.23 ± 0.10 µM, P = 0.02, respectively) as compared with noncarriers (0.93 ± 0.04 µM). When we injected human apoM-containing HDL into Wt (n = 6) or LDL receptor-deficient mice (n = 6), the removal of HDL-associated human apoM was delayed in the LDL receptor-deficient mice. After 2 h, 54 ± 5% versus 90 ± 8% (P < 0.005) of the initial amounts of human apoM remained in the plasma of Wt and LDL receptor-deficient mice, respectively. Finally, we compared the turnover of radio-iodinated LDL and plasma apoM concentrations in 45 normocholesterolemic humans. There was a negative correlation between plasma apoM and the fractional catabolic rate of LDL (r = −0.38, P = 0.009). These data suggest that the plasma clearance of apoM, despite apoM primarily being associated with HDL, is influenced by LDL receptor-mediated clearance of apoB-containing particles.
Keywords: lipoprotein, low-density lipoprotein metabolism, apolipoprotein, familial hypercholesterolemia
HDL-associated apoM was recently shown to be a physiological carrier of sphingosine-1-phosphate (S1P) (1). S1P affects vascular integrity, and the S1P-dependent effects of HDL are dependent on apoM. Moreover, several studies have suggested that apoM can accelerate efflux of cholesterol from foam cells, delay oxidation of LDL, and increase production of pre-β-HDL, suggesting that apoM affects antiatherogenic functions of HDL (2–4). However, little is known about the plasma metabolism of apoM. ApoM is anchored in HDL via a retained hydrophobic signal peptide (5). Loss of the signal peptide abolishes apoM's binding to HDL, causing rapid clearance of the truncated apoM in the kidney. Even though >90% of plasma apoM resides in HDL plasma, apoM concentration has consistently been shown to be positively correlated with plasma LDL cholesterol in humans (6–8). Moreover, HDL-associated plasma apoM is increased 2-fold in Ldlr−/− mice lacking functional LDL receptors (9). These observations might reflect that plasma apoM is controlled by the rate of LDL receptor-mediated clearance of apoB-containing particles.
LDL receptor binding and internalization of LDL represent a major pathway controlling plasma LDL levels (10, 11), and the ligand binding domain of the LDL receptor, as well as the LDL receptor binding domain in apoB, have been extensively characterized (12, 13). The clinical diagnosis of familial hypercholesterolemia (FH) and impaired clearance of LDL can be caused by mutations in the LDLR and APOB genes (14). Genetic studies have identified multiple mutations in the LDL receptor causing FH, whereas the most important cause of FH related to the apoB gene is the R3500Q mutation resulting in impaired binding of apoB to the LDL receptor (15–17). The most frequent LDL receptor mutations in Danish FH patients are the W23X, W66G, and W556S mutations (18), accounting for approximately 45% of mutations in the LDL receptor.
In the present study, we have tested the hypothesis that the LDL receptor-mediated clearance of apoB-containing particles influences plasma apoM levels. Hence, we have examined A) whether carriers of mutations in the LDL receptor gene or apoB gene have increased plasma apoM levels, B) whether the clearance of apoM-containing HDL is delayed in mice lacking the LDL receptor, and C) whether plasma apoM concentrations correlate with in vivo LDL receptor-mediated clearance of apoB-containing particles estimated as the fractional catabolic rate of LDL in normocholesterolemic individuals.
METHODS
Subjects
Individuals with mutations in the LDLR [W23X (n = 2), W66G (n = 6), and W556S (n = 1)] or in APOB (R3500Q, n = 12) were identified by genotyping (16, 19) individuals from the prospective Copenhagen City Heart Study comprising individuals randomly selected on the basis of the Danish Central Population Register to reflect the adult general population (20, 21).
Controls (n = 42) matched on age and gender were collected from the general population, and did not have mutations in the LDLR or APOB. Two wild-type individuals were matched to each mutation carrier. At time of examination (1991–1994), plasma lipids, apoB, and apoA-I concentrations were measured as described previously (22). Samples were stored at −80°C. Plasma apoM was measured by ELISA (7). The study was approved by institutional review boards and a Danish ethical committee (no.KF-100.2039/91), and conducted according to the Declaration of Helsinki.
Random leftover aliquots of plasma with elevated LDL-cholesterol or elevated triglycerides that had been taken for diagnostic purposes at the Department of Clinical Biochemistry, Rigshospitalet were collected without knowledge of patient identity.
LDL turnover study in humans
Individuals (n = 45) were selected from The Copenhagen City Heart Study and reexamined between 2002 and 2005 (23–25). LDL (density 1.019–1.050 g/ml to exclude lipoprotein [a]) was isolated from each individual and labeled as previously described with either 18.5 MBq 125I or 131I per 5 mg LDL protein before reinjection into the same individuals (26). Blood samples were taken at 1, 2, 4, 6, and 8 h and daily for the ensuing 8 days (23). Radioactivity was measured in total plasma after precipitation with trichloracetic acid (26). Fractional catabolic rate (pools per day) of LDL after injection was calculated as described (24, 27). Individuals with mutations in the LDLR gene (W23X, W66G, W556S) and APOB gene (R3500Q, R3531C, E4154K) affecting the fractional catabolic rate of LDL were excluded (25). Ten individuals with APOB mutations [R3611Q (n = 4), T2488T (n = 5), P2712L (n = 1)] with no known effects on the fractional catabolic rate of LDL were included (25). Samples taken prior to injection of labeled LDL (2002–2005) were stored at –80°C and used for measurement of apoM (7).
Distribution of apoM between lipoproteins
Two individuals heterozygous for a mutation (N1800H and R2144X) in the ABCA1 gene were identified (21). Aliquots (250 µl) of plasma from the two carriers, a plasma pool from 5 individuals with elevated plasma triglycerides (2.4–4.6 mM and LDL <2.5 mM), a plasma pool from 9 individuals with elevated plasma LDL (3.8–4.4 mM and triglyceride <1.4 mM), or a plasma pool from 10 individuals with normal plasma triglyceride (1.2 mM) and LDL (2.5 mM) were subjected to fast-protein liquid chromatography (FPLC) analyses on a Superose 6 column (3). The concentration of cholesterol and triglyceride in each fraction was measured, and fractions containing VLDL, LDL, or HDL were pooled. Fifteen microliters of the pools were loaded on a 12% SDS-PAGE gel prior to Western blotting with a monoclonal mouse anti-human apoM antibody (EPR2904, GeneTex; Labinova, Denmark) or a polyclonal rabbit anti-human apoA-I antibody (Q492; Dako, Denmark).
Mice
Human apoM transgenic (ApoM-TgH) and Ldlr−/− mice were housed at the Panum Institute (University of Copenhagen, Denmark) in a temperature-controlled facility with a 12-h dark/light cycle and fed standard chow. ApoM-TgH mice were backcrossed more than seven times on a C57B6/j background (9).
Blood samples were drawn from the venous plexus in the orbital cavity into Na2ETDA tubes and kept on ice. Plasma was isolated by centrifugation at 3,000 rpm for 10 min at 4°C and was used the same day for injection into recipient mice or stored at −80°C for human apoM analysis.
Liver biopsies were collected from Wt, Ldlr−/−, and Apoe−/− mice housed in Leuven, Belgium (28). Biopsies were stored at −80°C until extraction of RNA. Mouse apoM mRNA was measured with real-time RT-PCR (2). All procedures were approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark, and the Institutional Animal Care and Research Advisory Committee of the KU Leuven.
Metabolism of apoM-containing HDL in mice
To compare clearance of apoM-containing HDL in Wt and Ldlr−/− mice, plasma from nine ApoM-TgH mice was pooled and injected (110 μl/mouse) intravenously into Wt (n = 6) or Ldlr−/− mice (n = 6). Blood samples were taken 5, 30, 60, and 120 min after injection, and human apoM in plasma was measured by ELISA (using two monoclonal antibodies against human apoM) which has no cross-reactivity with mouse apoM (7). Plasma taken after 120 min was pooled and used for FPLC analyses on a Superose 6 column. Human apoM in FPLC fractions was measured with ELISA (7). Additional data from this study have been published previously (9).
Statistics
Medians were compared between groups by a Kruskal-Wallis ANOVA, with the Mann-Whitey U test as a posthoc test. Association between compounds was analyzed using the Spearman rank-based correlation. Comparisons at different time points in mice studies were done by Student's t-test.
RESULTS
Plasma apoM is increased in carriers with mutations in the APOB or LDLR genes
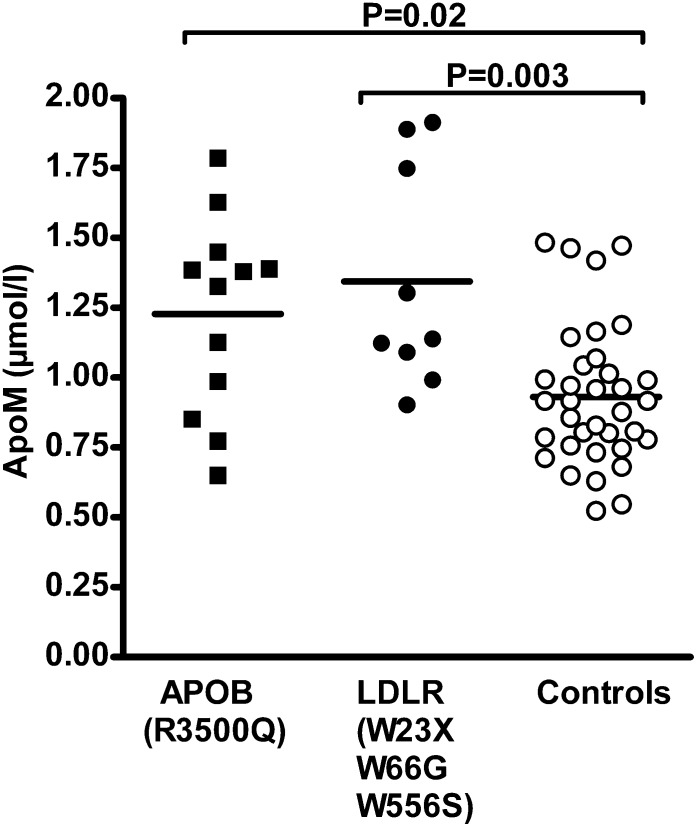
Carriers of mutations in APOB [R3500Q (n = 12)] or LDLR [W23X (n = 2), W66G (n = 6), and W556S (n = 1)] were identified by genetic screening of ∼9,257 Danish individuals from the general population (16, 19). As compared with age- and gender-matched controls, the plasma total and LDL cholesterol and apoB concentrations were increased in both groups of carriers (Table 1). APOB R3500Q carriers also had significantly reduced plasma HDL cholesterol levels (Table 1). The plasma apoM concentration was increased in both carriers of mutations in the LDLR [1.14 (interquartile range: 1.04–1.82) μM, P = 0.003] and APOB R3500Q mutation [1.35 (interquartile range: 0.92–1.42) μM, P = 0.02] as compared with noncarriers [0.92 (interquartile range: 0.76–1.04) µM] (Fig. 1). This result suggests that delayed clearance of plasma LDL due to mutations in either LDLR or APOB causes increased plasma apoM.
TABLE 1.
Characteristics of individuals with functional mutations in APOB (R3500Q) or LDLR (W23X, W66G, W556S)
| APOB (R3500Q) | LDLR (W23X, W66G, W556S) | Non-carrier Controls | P | |
| Number | 12 | 9 | 42 | |
| Age (year) | 53.5 (42–60.5) | 53 (44–61) | 53 (44–61) | NS |
| Gender (female/male) | 4/8 | 6/3 | 20/22 | |
| P-cholesterol (mM) | 6.8 (6.3–8.9)a | 8.05 (6.85–8.95)b | 5.8 (5.1–7.1) | 0.004 |
| P-LDL (mM) | 4.71 (3.8–6.5)a | 5.82 (4.85–6.99)b | 3.52 (2.97–4.51) | 0.0006 |
| P-HDL (mM) | 1.1 (0.8–1.4)a | 1.38 (1.25–1.6) | 1.4 (1.3–1.75) | 0.047 |
| P-triglyceride (mM) | 1.79 (0.98–2.76) | 1.08 (0.83–1.53) | 1.41 (1.03–2.45) | NS |
| P-apoB (mg/dl) | 111 (98–133.5)c | 119 (103–142)c | 83.5 (70–96.5) | P < 0.0001 |
| P-apoA-I (mg/dl) | 117 (105–147) | 120 (107.5–171.5) | 137.5 (119.5–152.5) | NS |
Individuals with functional mutations in APOB (R3500Q) or LDLR (W23X, W66G, W556S) were each matched with two controls on age and gender. Values are medians and interquartile range. Median values between groups are compared with a Kruskal-Wallis variance test, followed by Mann-Whitey U test as a posthoc test. P, plasma.
P < 0.05 compared with the control group.
P < 0.005 compared with the control group.
P < 0.0005 compared with the control group.
Fig. 1.
Plasma apoM concentration in individuals with mutations in the genes encoding APOB (R3500Q) or the LDLR (W23X, W66G, W556S). Lines indicate mean values. Each dot represents value from a single individual. P values from Mann-Whitney U test.
Clearance of apoM-containing HDL in Ldlr−/− mice
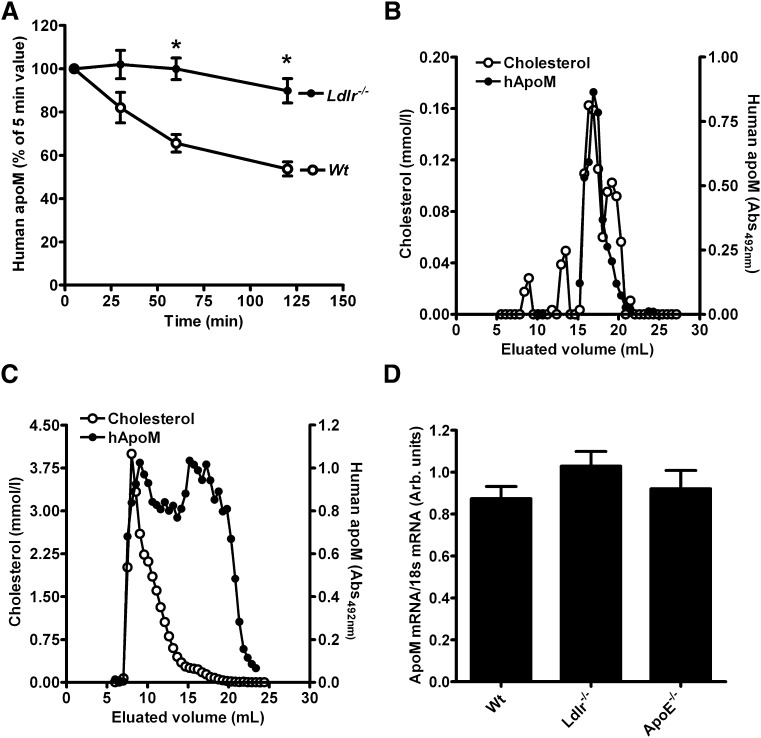
Principally, the previously observed ∼2-fold increased plasma apoM in Ldlr−/− mice (9) could reflect decreased clearance or increased production of apoM. To assess whether the clearance of HDL-associated apoM is affected by the LDL receptor, we injected plasma from human apoM-transgenic mice [where the human apoM is associated with HDL (2)] into Ldlr−/− or Wt mice and measured human plasma apoM with a human apoM-specific ELISA. The clearance of human apoM was markedly slower in Ldlr−/− than in Wt mice (Fig. 2A), suggesting that the LDL receptor-mediated clearance of apoB-containing particles affects the plasma clearance of apoM.
Fig. 2.
Plasma clearance of human apoM in mice. A: Plasma from ApoM-TgH mice containing human apoM in HDL (110 μl) was injected into Ldlr−/− mice (n = 6) or Wt mice (n = 6). Plasma samples were taken after 5, 30, 60, and 120 min for quantification of human apoM. Filled circles represent Ldlr−/− mice; open circles represent Wt mice. Bars indicate SEM values at each time point. * P < 0.005 on Student’s t-test. Plasma from Wt (B) and Ldlr−/− (C) mice 120 min after injection of human apoM-containing HDL was pooled and subjected to gel filtration. The content of cholesterol (open circles) and human apoM (closed circles) was measured in each fraction. D: Mouse apoM mRNA expression in liver tissue from Wt (n = 6), Ldlr−/− (n = 5), and Apoe−/− (n = 6) mice. Bars represent mean ± SEM.
On gel filtration analyses, the Ldlr−/− mice had increased LDL and VLDL cholesterol as compared with the Wt mice. At 120 min after injection of HDL-associated human apoM, the human apoM was also present in the VLDL/LDL fractions of the Ldlr−/− mice, but remained associated with HDL in Wt mice (Fig. 2B, C).
The apoM mRNA expression in the liver was similar in Ldlr−/−, Apoe−/−, and Wt mice (Fig. 2D).
LDL fractional catabolic rate and plasma apoM in humans
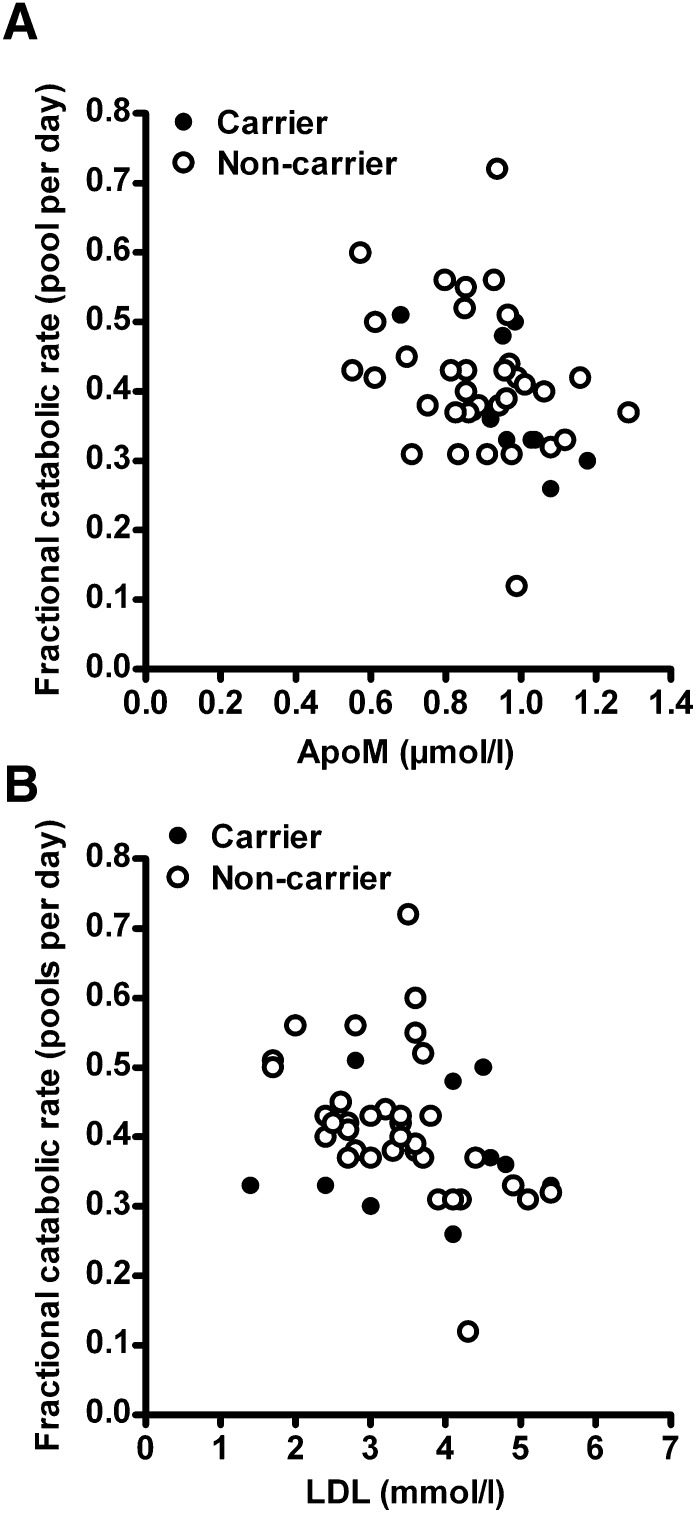
To further assess whether the clearance of apoB-containing particles affects plasma apoM levels in humans, we measured plasma apoM in 45 normocholesterolemic individuals without cholesterol-raising mutations in the LDLR or APOB genes (Table 2), where the efficiency of the clearance of apoB-containing particles was estimated as the fractional catabolic rate of LDL (23, 24, 27). Linear regression analysis showed a significant inverse relationship (r = −0.38, P = 0.009, n = 45) between the plasma apoM concentration and the fraction catabolic rate of LDL (Fig. 3A), even when analyzing carriers (r = −0.80, P = 0.007, n = 10) and noncarriers (r = −0.32, P = 0.02, n = 35) separately. This result further supports the notion that the LDL receptor-mediated clearance of apoB-containing particles can contribute to inter-individual variations in plasma apoM. There was a significant inverse relationship between the fractional catabolic rate and LDL cholesterol (r = −0.41, P = 0.005, n = 45, Fig. 3B).
TABLE 2.
Characteristics of individuals prior to LDL turnover study
| Individuals | |
| Number | 45 |
| Gender (female/male) | 17/28 |
| P-cholesterol (mM) | 5.5 (4.7–6.4) |
| P-LDL (mM) | 3.4 (2.7–4.1) |
| P-HDL (mM) | 1.5 (1.3–1.9) |
| P-triglyceride (mM) | 0.94 (0.73–1.50) |
| P-apoB (mg/dl) | 111.5 (83.0–124.5) |
| P-apoE (mg/dl) | 33.4 (28.0–42.3) |
| P-apoM (µM) | 0.93 (0.82–0.99) |
Values are medians and interquartile range.
Fig. 3.
Scatter plot showing fractional catabolic rate of LDL versus (A) plasma apoM (r = −0.38, P = 0.009, n = 45) or (B) LDL (r = −0.41, P = 0.005, n = 45; r = 0.12, P = NS; and r = −0.55, P = 0.006) when testing carriers and noncarriers, respectively) in humans. Carriers of mutations in the APOB are shown as filled dots (n = 10); noncarriers are open dots (n = 35). Spearman's rank-based correlation coefficient was used for analysis.
Distribution of apoM between lipoproteins in humans with low HDL, high LDL, or high triglyceride
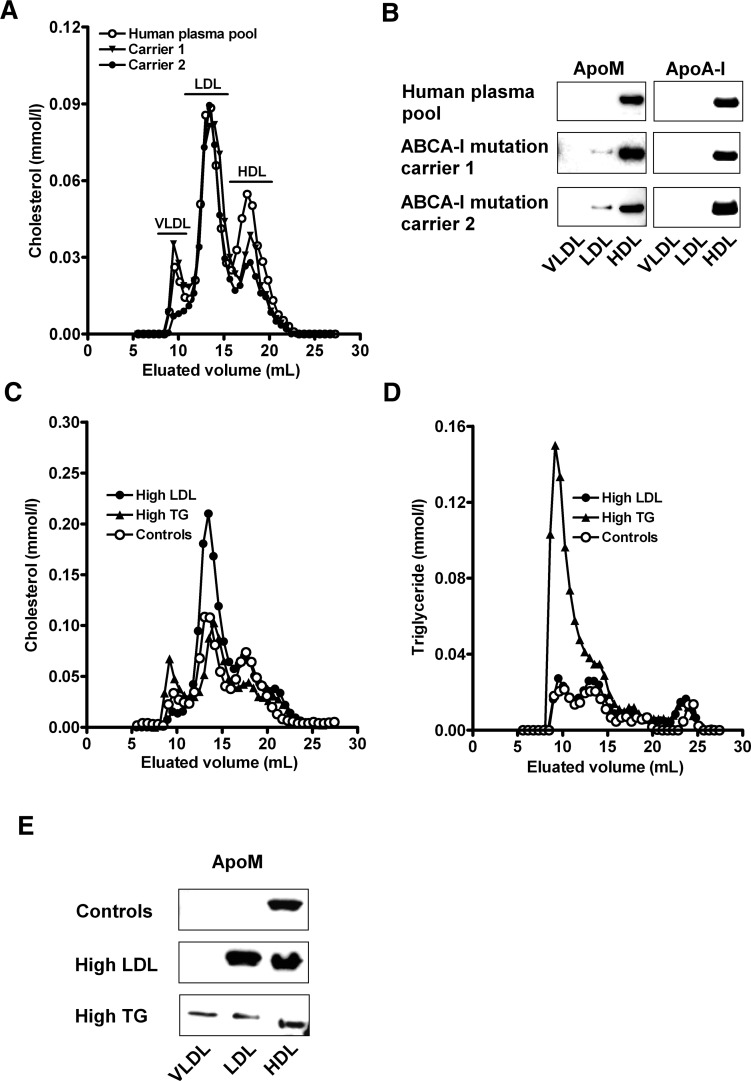
To study the effect of low HDL cholesterol, elevated LDL cholesterol, or elevated triglycerides on the distribution of apoM between the plasma lipoprotein fractions, we examined gel filtration profiles of plasma from two heterozygous carriers of mutations in the ABCA1 gene with low HDL cholesterol (∼0.7 mM in both carriers) (21), pooled plasma with elevated LDL cholesterol, and pooled plasma with elevated triglycerides.
ApoM was mainly present in apoA-I-containing HDL in normal individuals, whereas a significant fraction of the apoM was found in the LDL fraction in plasma with low HDL (Fig. 4A, B). Plasma with elevated LDL cholesterol also displayed apoM in the LDL fraction, whereas plasma with increased triglycerides displayed apoM in both the LDL and VLDL fractions (Fig. 4C, D, E). Hence, in human plasma, apoM is distributed between lipoprotein classes in proportion to their relative concentrations.
Fig. 4.
Effect of altered plasma lipoprotein levels on distribution of apoM between lipoproteins. Gel filtration chromatography profiles of cholesterol (A, C) and triglyceride (D) distribution between lipoproteins in plasma from healthy control individuals (A, C, D), plasma with low HDL cholesterol (A), and plasma with high LDL cholesterol or plasma triglycerides (C, D). Fractions representing HDL, LDL, and VLDL were pooled and used for Western blotting with antibodies against apoM (B, E) or apoA-I (B).
DISCUSSION
This study provides three independent sets of data suggesting that impairment of the LDL receptor-mediated clearance of apoB-containing lipoproteins increases plasma concentration of apoM due to delayed clearance. As such, the data provide a likely explanation for the positive association between plasma LDL cholesterol and plasma apoM in humans. Furthermore, the results are compatible with recent findings that use of statins lowers plasma apoM (29) and that plasma apoM concentrations are positively associated with plasma levels of the LDL receptor-degrading protease proprotein convertase subtilisin/kexin type 9 (30) in humans. Hence, the available data suggest a so-far-unknown link between LDL receptor-mediated clearance of apoB-containing lipoproteins and the metabolism of a mainly HDL-associated apolipoprotein.
Why is the clearance of HDL-associated apoM delayed when the LDL receptor-mediated clearance of apoB-containing lipoproteins is impaired? Theoretically, apoM itself or other HDL apolipoproteins (e.g., apoE) could be internalized by the LDL receptor. Even though apoM is a ligand for another member of the LDL receptor family (i.e., megalin), it does not bind LDL receptor-related protein 1 in surface plasmon resonance studies, and the apoM structure does not contain an LDL receptor binding domain, arguing against a direct binding of apoM to the LDL receptor (31, 32). ApoM-containing HDL can indeed contain apoE (2, 3), which is a known ligand for the LDL receptor. ApoE-deficient mice, however, do not have elevated plasma apoM, arguing against a major role of apoE in the clearance of plasma apoM (9). Further strong argumentation against the possibility that apoM-containing HDL is cleared by the LDL receptor pathway via direct binding of apoM or other HDL apolipoproteins comes from the present observation that carriers of the APOB R3500Q mutation have increased plasma apoM, even though these carriers are most likely to have intact and functional LDL receptors.
Another possible explanation for the present observations and the positive association between plasma LDL and apoM could be that apoM clearance is delayed by hypercholesterolemia irrespective of the underlying cause. However, despite marked hypercholesterolemia, apoE-deficient mice have normal plasma apoM and plasma apoM correlated with the fractional catabolic rate of LDL even in normocholesterolemic individuals, arguing against this proposition (9).
Instead, we suggest a model in which apoM rapidly exchanges between HDL and VLDL/LDL particles and is mainly cleared together with the apoB-containing lipoproteins. In support of such a model, we have observed an extremely rapid exchange of apoM between HDL and VLDL/LDL particles in vivo (9). Here we show the presence of human apoM in the VLDL/LDL-sized lipoproteins of Ldlr−/− mice at 120 min after injection of apoM-containing HDL. Hence, the distribution of apoM between lipoprotein classes in humans may to a large extent simply reflect the much higher molar plasma concentration of HDL particles (∼20 µM) than of LDL particles (∼1 µM). Accordingly, apoM, which normally is mainly associated with HDL (>90%) in human plasma, was present in LDL-sized lipoproteins in two individuals with low HDL cholesterol due to heterozygosity for mutations in the ABCA1 gene. Also, apoM was present in LDL and VLDL/LDL in plasma with elevated LDL-cholesterol and VLDL/LDL triglycerides, respectively. These results agree with data from Karuna et al. (33), who saw that apoM preferentially was bound in plasma LDL in individuals with low HDL due to LCAT deficiency, and a negative correlation between the HDL cholesterol concentration and the apoM content of apoB-containing lipoproteins. Moreover, in humans, a fatty meal causes a shift of apoM into triglyceride-rich (d < 1.006 g/ml) lipoproteins without affecting total apoM levels (34).
The apparent ability of apoM to exchange between plasma lipoprotein classes suggests that the VLDL/LDL pool of apoM will quickly be replenished from the HDL pool of apoM upon clearance of an apoM molecule together with the apoB-containing particle. This indicates that delayed clearance of LDL, irrespective of whether it is due to dysfunctional LDL receptors or apoB, will delay the clearance of apoM and hence is compatible with the observed increase of plasma apoM in carriers of both the APOB R3500Q and LDLR mutations. Moreover, the model is compatible with another observation, i.e., that human apoB-transgenic mice have ∼50% decreased plasma apoM (9). Hence, in the apoB-transgenic model, the LDL/HDL ratio is increased, which, according to the proposed model, would favor the presence of apoM in the LDL fraction. Because the turnover rate of LDL is higher than that of HDL in mice (35), this could lead to the observed reduction of plasma apoM levels in apoB-transgenic mice.
Plasma apoM is decreased in patients with metabolic syndrome (36), who often display reduced HDL and increased VLDL/IDL. However, at this stage, it is unknown whether the metabolic syndrome impairs apoM production in the liver or increases clearance of HDL-associated apoM by pathways other than the LDL receptor. This underscores that more data, e.g., from metabolic turnover studies with stable isotopes, are needed to fully understand how apoM is metabolized in humans, and that extrapolations from animals and extreme patient samples should be interpreted with caution.
ApoM has anti-atherogenic properties, such as accelerating pre-β-HDL formation, stimulating efflux of cholesterol from foam cells, delaying oxidation of LDL, and carrying S1P in HDL (1–3, 8). Accordingly, increased apoM protects against atherosclerosis in transgenic mice (2, 4). Nevertheless, in two human case-control studies, plasma apoM was not associated with cardiovascular risk (6). The presently suggested link between high plasma apoM and slow turnover of plasma LDL provides a possible explanation for these apparently contradictory findings. Increased residence time of LDL increases the propensity of LDL modification (e.g., by oxidation, carbamylation, or glycosylation), thereby probably increasing the atherogenicity of the plasma LDL particles (37). A high plasma apoM concentration could thus be associated with a more-atherogenic population of LDL particles, which might counteract the otherwise-beneficial effects of apoM with respect to development of atherosclerosis.
Acknowledgments
The authors thank Charlotte Wandel, Karen Rasmussen, Christina Dam, Kurt Svarre Jensen, Mette Refstrup, and Leen Verbeek for technical assistance.
Footnotes
Abbreviations:
- FH
- familial hypercholesterolemia
- FPLC
- fast-protein liquid chromatography
- S1P
- sphingosine-1-phosphate
This work was supported by grants from the National Research Council (C.C., L.B.N.), Novo Nordisk Foundation (C.C., P.M.C.), Augustinus fonden (C.C.), Rigshospitalets Research Foundation (L.B.N.), the Research Foundation – Flanders (A.J.M.R.), the Swedish Research Council, Grant # 07143 (B.D.), and the Swedish Heart-Lung Foundation (B.D.).
REFERENCES
- 1.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnstrom J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 108: 9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christoffersen C., Jauhiainen M., Moser M., Porse B., Ehnholm C., Boesl M., Dahlback B., Nielsen L. B. 2008. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J. Biol. Chem. 283: 1839–1847 [DOI] [PubMed] [Google Scholar]
- 3.Christoffersen C., Nielsen L. B., Axler O., Andersson A., Johnsen A. H., Dahlback B. 2006. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 47: 1833–1843 [DOI] [PubMed] [Google Scholar]
- 4.Wolfrum C., Poy M. N., Stoffel M. 2005. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat. Med. 11: 418–422 [DOI] [PubMed] [Google Scholar]
- 5.Christoffersen C., Ahnstrom J., Axler O., Christensen E. I., Dahlback B., Nielsen L. B. 2008. The signal peptide anchors apolipoprotein M in plasma lipoproteins and prevents rapid clearance of apolipoprotein M from plasma. J. Biol. Chem. 283: 18765–18772 [DOI] [PubMed] [Google Scholar]
- 6.Ahnstrom J., Axler O., Jauhiainen M., Salomaa V., Havulinna A. S., Ehnholm C., Frikke-Schmidt R., Tybjaerg-Hansen A., Dahlback B. 2008. Levels of apolipoprotein M are not associated with the risk of coronary heart disease in two independent case-control studies. J. Lipid Res. 49: 1912–1917 [DOI] [PubMed] [Google Scholar]
- 7.Axler O., Ahnstrom J., Dahlback B. 2007. An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J. Lipid Res. 48: 1772–1780 [DOI] [PubMed] [Google Scholar]
- 8.Plomgaard P., Dullaart R. P., de Vries R., Groen A. K., Dahlbäck B., Nielsen L. B. 2009. Apolipoprotein M predicts pre-beta-HDL formation: studies in type 2 diabetic and nondiabetic subjects. J. Intern. Med. 266: 258–267 [DOI] [PubMed] [Google Scholar]
- 9.Christoffersen C., Pedersen T. X., Gordts P. L., Roebroek A. J., Dahlbäck B., Nielsen L. B. 2010. Opposing effects of apolipoprotein m on catabolism of apolipoprotein B-containing lipoproteins and atherosclerosis. Circ. Res. 106: 1624–1634 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein J. L., Brown M. S. 2009. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29: 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy S. M., Vega G. L., Bilheimer D. W. 1985. Kinetic mechanisms determining variability in low density lipoprotein levels and rise with age. Arteriosclerosis. 5: 623–630 [DOI] [PubMed] [Google Scholar]
- 12.Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47 [DOI] [PubMed] [Google Scholar]
- 13.Yang C. Y., Chen S. H., Gianturco S. H., Bradley W. A., Sparrow J. T., Tanimura M., Li W. H., Sparrow D. A., DeLoof H., Rosseneu M. 1986. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 323: 738–742 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein J. L., Hobbs H., Brown M. S.2001. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Disease. 8th edition. C. R. Scriver, A. L. Beaudet, W. S. Sly, and P. Valle, editors. McGraw-Hill, New York. 2863–2913.
- 15.Soria L. F., Ludwig E. H., Clarke H. R., Vega G. L., Grundy S. M., McCarthy B. J. 1989. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc. Natl. Acad. Sci. USA. 86: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tybjaerg-Hansen A., Steffensen R., Meinertz H., Schnohr P., Nordestgaard B. G. 1998. Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N. Engl. J. Med. 338: 1577–1584 [DOI] [PubMed] [Google Scholar]
- 17.Tybjaerg-Hansen A., Humphries S. E. 1992. Familial defective apolipoprotein B-100: a single mutation that causes hypercholesterolemia and premature coronary artery disease. Atherosclerosis. 96: 91–107 [DOI] [PubMed] [Google Scholar]
- 18.Jensen H. K., Jensen L. G., Meinertz H., Hansen P. S., Gregersen N., Faergeman O. 1999. Spectrum of LDL receptor gene mutations in Denmark: implications for molecular diagnostic strategy in heterozygous familial hypercholesterolemia. Atherosclerosis. 146: 337–344 [DOI] [PubMed] [Google Scholar]
- 19.Tybjaerg-Hansen A., Jensen H. K., Benn M., Steffensen R., Jensen G., Nordestgaard B. G. 2005. Phenotype of heterozygotes for low-density lipoprotein receptor mutations identified in different background populations. Arterioscler. Thromb. Vasc. Biol. 25: 211–215 [DOI] [PubMed] [Google Scholar]
- 20.Appleyard M., Tybjaerg-Hansen A., Jensen G., Schnohr P., Nyboe J. The Copenhagen City Heart study. Østerbroundersøgelsen. A book of tables with data from the first examination (1976–1978) and a five-year follow-up (1981–1983) 1989. Scand. J. Soc. Med. 170 (Suppl 41): 1–160 [PubMed] [Google Scholar]
- 21.Frikke-Schmidt R., Nordestgaard B. G., Stene M. C., Sethi A. A., Remaley A. T., Schnohr P., Grande P., Tybjaerg-Hansen A. 2008. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. J. Am. Med. Assoc. 299: 2524–2532 [DOI] [PubMed] [Google Scholar]
- 22.Schnohr P., Jensen G., Scharling H., Appleyard M. 2001;Eur Heart J. 3: H1–H83 [Google Scholar]
- 23.Benn M., Nordestgaard B. G., Jensen J. S., Tybjaerg-Hansen A. 2007. Polymorphisms in apolipoprotein B and risk of ischemic stroke. J. Clin. Endocrinol. Metab. 92: 3611–3617 [DOI] [PubMed] [Google Scholar]
- 24.Benn M., Nordestgaard B. G., Jensen J. S., Nilausen K., Meinertz H., Tybjaerg-Hansen A. 2005. Mutation in apolipoprotein B associated with hypobetalipoproteinemia despite decreased binding to the low density lipoprotein receptor. J. Biol. Chem. 280: 21052–21060 [DOI] [PubMed] [Google Scholar]
- 25.Benn M. 2009. Apolipoprotein B levels, APOB alleles, and risk of ischemic cardiovascular disease in the general population, a review. Atherosclerosis. 206: 17–30 [DOI] [PubMed] [Google Scholar]
- 26.Kornerup K., Nordestgaard B. G., Feldt-Rasmussen B., Borch-Johnsen K., Jensen K. S., Jensen J. S. 2002. Transvascular low-density lipoprotein transport in patients with diabetes mellitus (type 2): a noninvasive in vivo isotope technique. Arterioscler. Thromb. Vasc. Biol. 22: 1168–1174 [DOI] [PubMed] [Google Scholar]
- 27.Benn M., Nordestgaard B. G., Jensen J. S., Grande P., Sillesen H., Tybjaerg-Hansen A. 2005. Polymorphism in APOB associated with increased low-density lipoprotein levels in both genders in the general population. J. Clin. Endocrinol. Metab. 90: 5797–5803 [DOI] [PubMed] [Google Scholar]
- 28.Gordts P. L., Reekmans S., Lauwers A., Van Dongen A., Verbeek L., Roebroek A. J. 2009. Inactivation of the LRP1 intracellular NPxYxxL motif in LDLR-deficient mice enhances postprandial dyslipidemia and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29: 1258–1264 [DOI] [PubMed] [Google Scholar]
- 29.Kappelle P. J., Ahnström J., Dikkeschei B. D., de Vries R., Sluiter W. J., Wolffenbuttel B. H., van Tol A., Nielsen L. B., Dahlbäck B., Dullaart R. P. 2010. Plasma apolipoprotein M responses to statin and fibrate administration in type 2 diabetes mellitus. Atherosclerosis. 213: 247–250 [DOI] [PubMed] [Google Scholar]
- 30.Kappelle P. J., Lambert G., Dahlback B., Nielsen L. B., Dullaart R. P. 2011. Relationship of plasma apolipoprotein M with proprotein convertase subtilisin-kexin type 9 levels in non-diabetic subjects. Atherosclerosis. 214: 492–494 [DOI] [PubMed] [Google Scholar]
- 31.Duan J., Dahlback B., Villoutreix B. O. 2001. Proposed lipocalin fold for apolipoprotein M based on bioinformatics and site-directed mutagenesis. FEBS Lett. 499: 127–132 [DOI] [PubMed] [Google Scholar]
- 32.Faber K., Hvidberg V., Moestrup S. K., Dahlback B., Nielsen L. B. 2006. Megalin is a receptor for apolipoprotein M, and kidney-specific megalin-deficiency confers urinary excretion of apolipoprotein M. Mol. Endocrinol. 20: 212–218 [DOI] [PubMed] [Google Scholar]
- 33.Karuna R., Park R., Othman A., Holleboom A. G., Motazacker M. M., Sutter I., Kuivenhoven J. A., Rohrer L., Matile H., Hornemann T., et al. 2011. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 219: 855–863 [DOI] [PubMed] [Google Scholar]
- 34.Xu N., Dahlback B. 1999. A novel human apolipoprotein (apoM). J. Biol. Chem. 274: 31286–31290 [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. 1993. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dullaart R. P., Plomgaard P., de Vries R., Dahlbäck B., Nielsen L. B. 2009. Plasma apolipoprotein M is reduced in metabolic syndrome but does not predict intima media thickness. Clin. Chim. Acta. 406: 129–133 [DOI] [PubMed] [Google Scholar]
- 37.Kronenberg F., Ikewaki K., Schaefer J. R., Konig P., Dieplinger H. 2007. Kinetic studies of atherogenic lipoproteins in hemodialysis patients: do they tell us more about their pathology? Semin. Dial. 20: 554–560 [DOI] [PubMed] [Google Scholar]