Abstract
Our objective was to determine whether the endothelial nitric oxide synthase (eNOS) Glu298Asp polymorphism influences vascular response to raised NEFA enriched with saturated fatty acids (SFA) or long-chain (LC) n-3 polyunsaturated fatty acids (PUFA). Subjects were prospectively recruited for genotype (Glu298, n = 30 and Asp298, n = 29; balanced for age and gender) consumed SFA on two occasions, with and without the substitution of 0.07 g fat/kg body weight with LC n-3 PUFA, and with heparin infusion to elevate NEFA. Endothelial function was measured before and after NEFA elevation (240 min), with blood samples taken every 30 min. Flow-mediated dilation (FMD) decreased following SFA alone and increased following SFA+LC n-3 PUFA. There were 2-fold differences in the change in FMD response to the different fat loads between the Asp298 and Glu298 genotypes (P = 0.002) and between genders (P < 0.02). Sodium nitroprusside-induced reactivity, measured by laser Doppler imaging with iontophoresis, was significantly greater with SFA+LC n-3 PUFA in all female subjects (P < 0.001) but not in males. Elevated NEFA influences both endothelial-dependent and endothelial-independent vasodilation during the postprandial phase. Effects of fat composition appear to be genotype and gender dependent, with the greatest difference in vasodilatory response to the two fat loads seen in the Asp298 females.
Keywords: genetics, flow-mediated dilatation, omega-3 fatty acids, vascular biology
Evidence is now emerging that mechanisms involved in the control of vascular tone are influenced by dietary factors, with dietary fat composition considered to be an important modulator. There is reasonably consistent evidence for adverse effects of high-fat meals and diets containing saturated fatty acids (SFA) on vascular function (1–5); conversely, intake of long-chain (LC) n-3 polyunsaturated fatty acids (PUFA) appears to improve endothelial function (6–11). Although beneficial effects of LC n-3 PUFA are more consistently reported in chronic supplementation studies (6, 9, 10) and when nonesterified fatty acids (NEFA) are elevated postprandially (11), there is greater variability in the beneficial effects of LC n-3 PUFA reported from acute meal studies (11).
Vascular dysfunction, characterized by a decrease in vascular reactivity, has been recognized as an early modifiable step in the development of atherosclerosis. The key component in the regulation of vascular homeostasis is nitric oxide (NO), a potent vasodilator produced by endothelial nitric oxide synthase (eNOS).While a number of mechanisms may be responsible for mediating dietary effects, the final common pathway appears to be via fatty acid-dependent alterations in either the production or stability of NO, or the action of NO on the smooth muscle layer (8, 12–14), with eNOS acting as a key molecular locus (13). Identifying the molecular loci associated with variable responses to diet is important in identifying subgroups of the population who may be particularly responsive, and it will also clarify mechanisms underlying potentially powerful protective effects of diet.
Previous diet-genotype studies suggest the eNOS Glu298Asp polymorphism may be of particular interest with respect to effects of LC n-3 PUFA. Individuals homozygous for the minor (Asp298) allele [approximately 10% of Caucasians (15–17)] have been reported to have a 30% higher risk of coronary heart disease (CHD) (17). In this genotypic group, endothelial-dependent vasodilation performed after an overnight fast has been positively correlated with plasma LC n-3 PUFA levels (15), and LC n-3 PUFA supplementation for 12 weeks was shown to lead to greater reductions in plasma triacylglycerol (TAG) concentrations compared with the wild-type Glu298 homozygotes (18).
Although these data are suggestive of greater phenotypic responsiveness to the effects of LC n-3 PUFA in subjects carrying the Asp298 allele, use of retrospective genotyping in these types of studies offers risk of statistical bias and confounding due to comparisons between poorly matched groups and lack of power in the minor allele groups (19). In the present study, we have taken a prospective approach to recruitment of subjects according to eNOS Glu298Asp polymorphism to ensure equal numbers of subjects in each homozygous group (Glu298 and Asp298) and to match groups for the most important potential confounders [age, gender, blood pressure (BP), and body mass index (BMI)]. Because gender is an important determinant of variability in endothelial response, with higher flow-mediated dilatation (FMD) in females attributed to smaller vessel size as well as the vascular effects of estrogen in premenopausal women (20), the balance of the groups by gender was considered of particular importance. To investigate whether polymorphisms in the eNOS gene are a determinant of differences in vascular response to oral fat loads, we administered the fat loads (SFA, with or without LC n-3 PUFA) along with heparin, which raises levels of NEFA to those observed in obese or overweight subjects and subjects with type 2 diabetes; such levels are also commonly seen in healthy individuals consuming high-fat meals (11).
Here we report a 2-fold effect of both gender and the Glu298Asp eNOS polymorphism on endothelial responses to acute elevation of NEFA enriched with SFA or SFA+LC n-3 PUFA. These findings provide a basis for positive dietary advice to Asp298 females who appear to be particularly responsive to the benefits of LC n-3PUFA.The findings also shed light on potential mechanisms underlying the vascular effects of these fatty acids.
METHODS
Subjects
To achieve the required number of subjects homozygous for the minor (Asp298) and common (Glu298) alleles (n = 30 each group), a total of n = 370 healthy nonsmoking individuals ages 18–65 years and BMI 18–32 kg/m2 were prospectively genotyped for the eNOS Glu298Asp polymorphism. Screening yielded 185 potentially suitable subjects, of which 35 were Asp298 and 150 were Glu298 homozygotes. Exclusion criteria included any history of cardiovascular disease or any metabolic disorders, or taking any medication or dietary supplements that may affect blood clotting, lipids, or blood pressure. Of 35 potentially suitable Asp 298 subjects on screening, 3 were unable to participate and 2 were unsuitable according to exclusion criteria. These 30 subjects were matched for age, gender, BMI, and blood pressure with 30 Glu298 subjects. One Asp298 subject subsequently withdrew from the study, resulting in Asp298, n = 29 and Glu298, n = 30 (Table 1). Subjects taking oral contraceptives (OC) were required to remain on the same prescription throughout the study. The two genotypic groups were not matched for fasting blood lipids because these have been reported to differ in Asp and Glu homozygotes (21), which may reflect the phenotypic trait of interest, i.e., responsiveness to dietary fatty acids. All blood lipid values were within normal ranges, including high-density lipoprotein cholesterol (HDL-C) values; HDL-C was lower in Arg298 than in Glu298 subjects, with no other significant differences in blood lipids evident between the groups. Fasting blood lipids values were generally similar between genders with mean (± SEM) values for male and females, respectively, of total cholesterol (TC), 4.4 ± 0.2 and 4.7 ± 0.1 mmol/l [nonsignificant (NS)]; low-density lipoprotein cholesterol (LDL-C), 2.7 ± 0.1 and 2.6 ± 0.1 (NS); and TAG, 1.1 ± 0.1 and 1.0 ± 0.1 mmol/l (NS). HDL-C values were lower in males (1.3 ± 0.1) than in females (1.7 ± 0.1 mmol/l; P = 0.01).
TABLE 1.
Subject characteristics according to genotype
| Asp289 (n = 29) | Glu298 (n = 30) | P between Genotypes | |
| Female/male | 14/15 | 15/15 | |
| Post-menopausal | 1 | 1 | |
| Post-menopausal on HRT | 0 | 0 | |
| Premenopausal taking OC | 9 | 9 | |
| Caucasian ethnicity | 27 | 28 | |
| Age (y) | 28.5 ± 2.4 | 28.4 ± 2.2 | 0.955 |
| BMI (kg/m2) | 23.3 ± 0.7 | 23.2 ± 0.5 | 0.893 |
| Systolic blood pressure (mmHg) | 124.9 ± 1.8 | 122.6 ± 1.6 | 0.347 |
| Diastolic blood pressure (mmHg) | 76.8 ± 1.3 | 76.0 ± 1.7 | 0.694 |
| TC (mmol/l) | 4.5 ± 0.2 | 4.6 ± 0.1 | 0.562 |
| HDL-C (mmol/l) | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.008 |
| LDL-C (mmol/l) | 2.7 ± 0.1 | 2.5 ± 0.1 | 0.429 |
| TAG (mmol/l) | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.911 |
| FMD (%) | 6.0 ± 0.7 | 5.4 ± 0.5 | 0.446 |
| LDI-Ach AUC (AU) | 1520 ± 251 | 1225 ± 231 | 0.390 |
| LDI-SNP AUC (AU) | 1506 ± 253 | 1025 ± 200 | 0.140 |
Bold text indicates significant P-values.
Study design and protocol
The design was a single-blind crossover in which subjects attended the Hugh Sinclair Unit of Human Nutrition on two occasions separated by four weeks for females (to control for potential effects of the menstrual cycle on endothelial function) and at least one week for males. The protocol for acutely elevating NEFA of specific fatty acid types was based on work of Beysen et al. (22), which we have previously shown to achieve the level of NEFA elevation required to increase arterial stiffness (7, 11). Subjects were randomly assigned to receive test drinks rich in either SFA (0.52 g/kg body weight) or a combination of SFA (0.45 g/kg body weight) and LC n-3 PUFA (0.07 g/kg body weight) each day. A 70 kg individual would therefore receive 36.4 g palm stearin or 31.5 g palm stearin and 4.9 g of fish oil concentrate, which contained 3.8 g docosahexaenoic acid (DHA) and 0.4 g eicosapentaenoic acid (EPA) (equivalent to 1.5 times a standard 140 g portion of oily fish). The composition of the two test drinks is shown in supplementary Table I. Test drinks were prepared as previously described (11, and they were divided into a bolus drink of 66 g given at time 0, and eight smaller volumes of 22 g given at 30 min intervals for a period of 240 min. The drinks were identical in protein (7.7 g) and carbohydrate (18.7 g) content.
Subjects arrived at the unit after an overnight fast, having been asked to refrain from strenuous exercise and alcohol for 24 h. To standardize food intake on the day preceding each study day, subjects were asked to record their food and beverage consumption for the 24 h before the first visit and to replicate this intake in the 24 h before the second visit. In addition, a standard low-fat ready-meal (<5 g fat) was provided to all subjects for consumption on the evening preceding each study day. After resting for 30 min in the supine position in a quiet air-conditioned room (22–24°C), baseline vascular reactivity measurements were performed on one arm. An intravenous cannula was then inserted in the wrist of the opposite arm for venous blood sampling before the main test drink was consumed (0 min). Every 30 min, a smaller test drink was given, and a blood sample collected into K3 EDTA or serum tubes. Samples were centrifuged at 1,700 g for 10 min at 4°C in a bench-top centrifuge and stored at −20°C until analysis. At 60 min, a second cannula was inserted into the antecubital vein in the sampling arm, and a bolus of heparin (500 IU) was administered, followed by a continuous infusion of heparin (0.4 IU/kg body weight/min) until the end of the 240 min study day. At 180 min, the vascular reactivity measurements were repeated, and at 240 min, the final blood sample was taken.
All subjects provided written consent, and the project was reviewed by the University of Reading Research Ethics committee and given a favorable ethical opinion for conduct. The study was conducted in the Hugh Sinclair Unit of Human Nutrition at the University of Reading, UK, and is registered at ClinicalTrials.gov (CMW-BB/E021816/1).
Vascular reactivity measurements
For each set of vascular measurements, FMD was measured first, using the nondominant arm, with Laser Doppler imaging with iontophoresis (LDI) also performed on the nondominant arm but commencing 30 min after the FMD measurement was initiated. Full details of the vascular reactivity measurements used have been reported previously (11).
Flow-mediated dilatation.
Briefly, FMD was measured by trained researchers using an ATL Ultrasound HDI5000 broadband ultrasound system (Philips Ultrasound, Bothell, Washington), with analysis of the arterial diameter and Doppler-derived velocity performed using MIA-llc software (Medical Imaging Applications, Coralville, IA) by a single researcher blinded to subject genotype. Preocclusion images were taken for 1 min, after which a blood pressure cuff was inflated to 220 mmHg to occlude blood flow. After 5 min of occlusion, the pressure was rapidly released, allowing reactive hyperemia to occur; measurement collection continued for 5 min post release. FMD response was calculated using change from preocclusion diameter to the peak diameter that occurred post release, divided by baseline, and reported as a percentage value (% FMD).
Laser Doppler imaging.
Acetylcholine-induced (endothelial dependent) and sodium nitroprusside-induced (endothelial independent) reactivity were determined simultaneously using LDI. A Moor LDI2-VR laser Doppler imager and a MIC2 iontophoresis controller (Moor Instruments, Axminster, UK) transdermally delivered the 1% acetylcholine and 1% sodium nitroprusside solutions into separate, adjacent wells as the current increased from 0 to 20 μÅ over approximately 20 min. The area under the flux versus time curve [arbitrary units (AU)] was calculated as an indicator of microvascular response due to acetylcholine (LDI-Ach) or sodium nitroprusside (LDI-SNP).
Blood sample analysis
The buffy coat layer was isolated from 10 ml of blood collected into an EDTA vacutainer, and the DNA was isolated using the Qiagen DNA Blood Mini Kit (Qiagen, Crawley, West Sussex, UK). The Glu298Asp polymorphism (rs1799983) was determined using an Assay-on-Demand SNP genotyping assay (Applied Biosystems, Warrington, Cheshire, UK).
An automated clinical chemistry analyzer (ILAB 600) was used to determine plasma TAG and NEFA using kits supplied by Instrumentation Laboratory (Warrington, UK) and Alpha Laboratories (Eastleigh, UK), respectively. An ELISA (R and D systems Europe, Abingdon, UK) was used to measure serum plasma endothelin-1 (ET-1). Total serum nitrates and nitrites (NOx) were measured using a NOx quantification kit (Actif Motif, Rixen Sort, Belgium).
Assessment of habitual dietary intake
Subjects completed a food frequency questionnaire (FFQ) based on that used by Caslake et al. in the FINGEN study (23), which uses a 10-point scale for assessing the frequency of intake of major food groups, plus eight additional questions about fish consumption (analysis provided in supplementary Table II).
Plasma fatty acid profiles
The NEFA fatty acids were extracted from the fasting (0 min) and 240 min plasma samples, and converted into fatty acid methyl esters (FAME) (7). The fasting samples from both study days were pooled to ensure a sufficient yield of lipid to assess habitual dietary fatty acid intakes between the groups. The fatty acid profiles measured at 240 min were used to confirm the expected differences in LC n-3 PUFA compositions following consumption of the different test drinks. Gas chromatography was used to analyze the FAME, which were identified by comparison of retention times against a known standard (Suplelco 37 component FAME mix; Supelco, Dorset, UK). The percentage weight of fatty acids was calculated for total SFA, total MUFA, total PUFA, n-6 PUFA, and LC n-3 PUFA.
Statistical analysis
Power calculations were performed for the primary outcome: change in FMD response according to the two genotypes. At 95% power and 5% significance, the minimum number of subjects in each genotype required to detect a difference of 1.5% in FMD response between the two genotype groups to the two oral fat loads was calculated to be 22; additional subjects were recruited into each genotype group (n = 30) to allow for possible dropouts due to the intensive nature of the protocol.
All statistical analysis was performed using SPSS 17.0, and results are given as mean ± SEM. The data were checked for normality and transformed if necessary. Paired and independent t-tests were used to compare results between study days and genotypes or genders, respectively. Repeated measures ANOVA were performed using a mixed-model approach, applying Bonferroni correction to control for multiple comparisons. Spearman's correlation coefficients (ρ) and Pearson's correlation coefficients (r) were used to determine relationships between variables of interest. Values of P ≤ 0.05 were taken as significant.
RESULTS
A total of n = 59 participants completed the two study visits (n = 29 Asp298 and n = 30 Glu298); there were no significant between study day differences in any of the baseline (fasting) measures prior to consumption of the oral fat loads. To determine whether there were differences in baseline measures of vascular function or habitual food intake according to gender or eNOS genotype group that might confound the findings, these fasting data were averaged for the two visits (Tables 2 and 3; supplementary Table II).
TABLE 2.
Subject-matched characteristics and fatty acid composition (% main classes) of fasting plasma NEFA and phosphatidyl choline according to genotype and gender
| Females (mean ± SEM) |
Males (mean ± SEM) |
|||||
| Asp289 (n = 14) | Glu298 (n = 15) | Asp289 (n = 15) | Glu298 (n = 15) | P between Genotypes | P between Genders | |
| Age (y) | 27.6 ± 3.07 | 27.1 ± 2.98 | 28.5 ± 3.54 | 28.4 ± 3.46 | 0.995 | 0.734 |
| BMI (kg/m2) | 22.4 ± 0.2 | 22.5 ± 0.2 | 24.1 ± 0.2 | 23.8 ± 0.2 | 0.893 | 0.069 |
| Blood pressure | ||||||
| Systolic (mmHg) | 118.6 ± 0.5 | 118.2 ± 0.5 | 129.7 ± 0.5 | 127.0 ± 0.6 | 0.514 | <0.001 |
| Diastolic (mmHg) | 77.2 ± 0.6 | 76.3 ± 0.6 | 76.1 ± 0.6 | 75.6 ± 0.7 | 0.746 | 0.699 |
| Baseline plasma NEFA composition | ||||||
| SFA (%) | 38.5 ± 0.8 | 39.3 ± 1.1 | 38.6 ± 0.8 | 37.9 ± 0.8 | 0.983 | 0.438 |
| MUFA (%) | 37.1 ± 1.4 | 35.3 ± 2.5 | 37.3 ± 1.2 | 38.2 ± 0.8 | 0.783 | 0.336 |
| n-6 PUFA (%) | 12.9 ± 0.5 | 13.9 ± 0.8 | 12.2 ± 0.4** | 14.0 ± 0.5 | 0.019 | 0.681 |
| LC n-3 PUFA (%) | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 0.945 | 0.810 |
| Baseline plasma PC composition | ||||||
| SFA (%) | 40.8 ± 0.4 | 39.9 ± 0.4 | 39.8 ± 0.5 | 39.0 ± 0.6 | 0.080 | 0.048 |
| MUFA (%) | 10.7 ± 0.5 | 9.7 ± 0.3 | 35.3 ± 0.4 | 10.3 ± 0.3 | 0.323 | 0.790 |
| n-6 PUFA (%) | 30.0 ± 0.5 | 28.8 ± 0.4 | 29.1 ± 0.5 | 28.3 ± 0.5 | 0.316 | 0.693 |
| LC n-3 PUFA (%) | 3.9 ± 0.4 | 4.3 ± 0.3 | 4.5 ± 0.3 | 4.1 ± 0.2 | 0.966 | 0.623 |
Statistical significance of difference between genotypes within a gender: **P ≤ 0.01. Bold text indicates significant P-values.
TABLE 3.
Mean measures of vascular function after an overnight fast (time 0) on the two study days
| Females (mean ± SEM) | Males (mean ± SEM) | |||||
| Asp289 (n = 14) | Glu298 (n = 15) | Asp289 (n = 15) | Glu298 (n = 15) | P between Genotypes | P between Genders | |
| FMD parameters | ||||||
| Pre-occlusion flow (ml/min) | 10.7 ± 0.8 | 10.1 ± 0.9 | 20.2 ± 2.1 | 18.1 ± 1.3 | 0.245 | <0.001 |
| Pre-occlusion diameter (mm) | 3.2 ± 0.1 | 3.3 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 0.465 | <0.001 |
| Post occlusion change in diameter (mm) | 0.24 ± 0.2 | 0.22 ± 0.2 | 0.19 ± 0.3 | 0.19 ± 0.2 | 0.672 | 0.019 |
| FMD (%) | 7.6 ± 1.0 | 6.4 ± 0.7 | 4.4 ± 0.7 | 4.3 ± 0.5 | 0.446 | 0.001 |
| LDI AUC | ||||||
| LDI-Ach (AU) | 2485 ± 364 | 2047 ± 367 | 2382 ± 361 | 2375 ± 300 | 0.521 | 0.747 |
| LDI-SNP (AU) | 2106 ± 308 | 1608 ± 246 | 2942 ± 379 | 2404 ± 298 | 0.116 | 0.012 |
| Circulating measures of endothelial function | ||||||
| ET-1 (ng/ml) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.978 | 0.105 |
| NOx (μM) | 25 ± 2 | 24 ± 1 | 20 ± 1 | 23 ± 2 | 0.332 | 0.067 |
Data are the mean values of the time 0 measures for the two study days. Bold text indicates significant P-values.
Baseline data
Table 2 shows the subject characteristics for the main potential confounders by gender and genotype, which demonstrate the successful matching of the two genotypic groups. The fatty acid distribution of the plasma NEFA and PC at baseline, which provides an assessment of habitual fatty acid intake, are shown in Table 2. There were no differences between genotypes or genders for SFA, MUFA, or LC n-3 PUFA, but male Asp298 had significantly lower proportions of n-6 PUFA in NEFA and PC than did their Glu298 counterparts (P = 0.018). The FFQ showed no significant differences in the habitual consumption of the major food groups, including foods and beverages known to affect vascular function (oily fish, flavonoid-rich fruits or vegetables, tea, coffee, wine) by genotype or gender (supplementary Table II).
Values for the mean measures of vascular function undertaken after an overnight fast prior to each study day (time 0) were not significantly different at baseline between genotypes (Table 3), and none of the baseline vascular reactivity parameters varied significantly between study days. There were a number of differences between genders, which were generally in agreement with previous reports (20).
A positive correlation was found for Asp298 females between the percentage of NEFA LC n-3 PUFA at baseline and their FMD response at baseline (r = 0.905, P < 0.000). This strong positive correlation remained after removal of a single outlying observation (r = 0.776, P = 0.002). No significant correlation between NEFA composition and any measure of vascular function was seen for Glu298 females or males of either genotype (supplementary Table III).
Postprandial changes in response to the fat loads
Vascular reactivity.
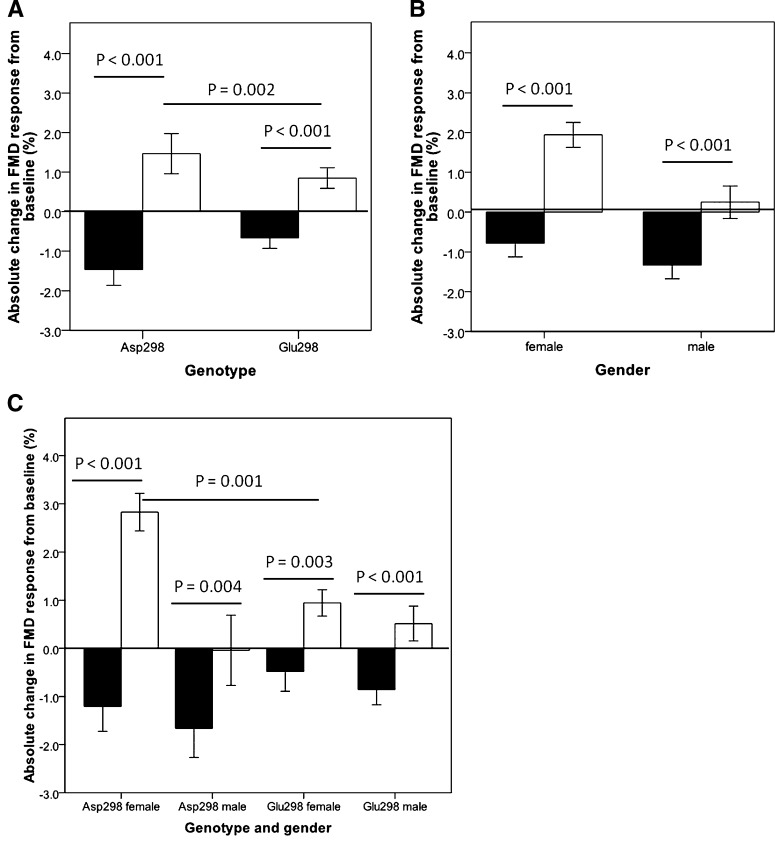
There were no significant differences in velocity, flow, or shear rate at baseline (time 0), at the end of the 240 min study day, or the postprandial change (240–0 mins) for either fat type (supplementary Table IV). The effect of the different test drinks on FMD response is presented in Fig. 1 as absolute change from baseline (ΔFMD response), split by genotype (Fig. 1A), gender (Fig. 1B), and genotype by gender (Fig. 1C). The difference in FMD response between the two fat loads was significant when data was analyzed by genotype (P < 0.002) or gender (P ≤ 0.02). Females had higher increases in FMD than did males for the SFA+LC n-3 PUFA study day (P = 0.002; Fig. 1B), but the decrease in FMD following the SFA test drinks was similar in both genders (P = 0.38).
Fig. 1.
Absolute change in FMD response from overnight fasting value (time = 0) to the postprandial measurement (time = 240 min) following consumption of SFA (black bars) or SFA+LC n-3 PUFA (white bars) test drinks. Effect of (A) Glu298Asp polymorphism, (B) gender, and (C) Glu298Asp polymorphism and gender. Data are presented as mean ± SEM. In (A), n = 29 Asp298, n = 30 Glu298. In (B), n = 29 females, n = 30 males. In (C), n = 14Asp298 females, n = 15 Glu298 females, n = 15 Asp298 males, and n = 15 Glu298 males.
Comparison of the FMD response with the two fat loads according to eNOS genotype (Fig. 1A) showed the direction and nature of the response to be similar for the Asp298 and Glu298 groups. However, the difference in the response to the two fat loads (ΔFMDSFA+LC n-3 PUFA – ΔFMDSFA) was approximately 2-fold greater in the Asp298 than in the Glu298 group (3.9% versus 1.4%, P = 0.002).
Fig. 1C shows the effect of genotype according to gender on FMD response to the two fat loads. Asp298 females showed the most marked difference in response to the two fat loads (ΔFMDSFA+LC n-3 PUFA – ΔFMDSFA) with a difference in FMD response (%) at the end of the two fat loads of 4.0% (SEM±0.6%), compared with a difference of 1.3% (SEM ± 0.4%) for the Glu298 females. For Asp298 males, the difference in response to the two fat loads was statistically significant (P = 0.004), but unlike the other groups, although the addition of LC n-3 PUFA attenuated the effect of SFA, it did not lead to an increase in FMD compared to the baseline measure.
Analysis of the endothelial-dependent ΔLDI-Ach responses to the two test drinks (Table 4) showed the only significant change occurred in Asp298 females following the SFA+LC n-3 PUFA test drink (P < 0.001), but there was a tendency for an increase in Asp298 males (P = 0.06). There was a 4-fold higher LDI-Ach response to the SFA+LC n-3 PUFA test drink in Asp298 than in Glu298 females (P = 0.05; Table 4). There were no significant differences in LDI-SNP response following the SFA test drink. Both Asp298 and Glu298 females showed significantly increased LDI-SNP responses following the SFA+LC n-3 PUFA test drink (P < 0.001 and P = 0.002, respectively). Although the mean response was 2-fold higher in the Asp298 than in the Glu298 females, the difference did not reach statistical significance (P = 0.07).
TABLE 4.
Postprandial changes in LDI and circulating measures of vascular function before (time 0) and 240 min following an oral fat load of either SFA or SFA + LC n-3 PUFA
| Females (mean ± SEM) |
Males (mean ± SEM) |
Differences between the Two Fat Loads |
||||
| Asp289 (n = 14) | Glu298 (n = 15) | Asp289 (n = 15) | Glu298 (n = 15) | P (genotype all) | P (gender all) | |
| LDI AUC | ||||||
| LDI-Ach (AU) SFA fat load | 596 ± 445 | 589 ± 347 | 524 ± 444 | 476 ± 460 | ||
| LDI-Ach (AU)SFA + LC n-3 PUFA fat load | 1296 ± 314* | 309 ± 339 | 878 ± 437 | 569 ± 520 | 0.408 | 0.951 |
| LDI-SNP (AU) SFA fat load | 393 ± 424 | 626 ± 396 | 271 ± 578 | 926 ± 469 | ||
| LDI-SNP (AU) SFA + LC n-3 PUFA fat load | 1453 ± 347 | 719 ± 190 | 557 ± 659 | 393 ± 362 | 0.118 | 0.180 |
| Circulating measures | ||||||
| ET-1 (ng/ml) SFA fat load | 0.01 ± 0.01 | −0.03 ± 0.05 | −0.10 ± 0.09 | 0.04 ± 0.10 | ||
| ET-1 (ng/ml) SFA +LC n3 PUFA fat load | −0.02 ± 0.08 | 0.01 ± 0.06 | 0.06 ± 0.05 | 0.02 ± 0.08 | 0.591 | 0.590 |
| NOx (μM) SFA fat load | −4.73 ± 1.18 | −3.01 ± 0.84 | −2.29 ± 1.0 | −4.59 ± 0.98 | ||
| NOx (μM) SFA +LC n3 PUFA fat load | −2.89 ± 1.14 | −3.86 ± 1.58 | −2.35 ± 0.81 | −2.37 ± 0.99 | 0.88 | 0.61 |
Statistical significance of difference between genotypes within a gender: *P ≤ 0.05.
Circulating NOx level decreased following both the fat loads and in all groups (P < 0.05; Table 4). There were no differences in responses of NOx or ET-1 to the two fat loads by gender or genotype. Males showed significantly higher ET-1 responses following both SFA and SFA+LC n-3 PUFA fat loads than did females (P = 0.013 and P = 0.037, respectively; Table 4). The ET-1 response to the SFA+LC n-3 PUFA fat load was significantly higher in Asp298 than in Glu298 males (P = 0.05; Table 4).
Plasma NEFA composition.
At the end of the SFA study day, the proportion of NEFA SFA had increased (from 38.6 to 46.3%, P < 0.001) and NEFA LC n-3 PUFA had declined [from 1.5 to 1.3%, P = 0.048; supplementary Table VI(i)]. This compared with a smaller increase in NEFA SFA on the SFA+LC n-3 PUFA study day (from 38.6 to 43.9%, P < 0.001; difference between study days, P < 0.001) and a large increase in LC n-3 PUFA (from 1.6 to 6.4%, P < 0.001; difference between study days, P < 0.001). There were no differences between genotypes or genders in the proportions of SFA, MUFA, n-6 PUFA, or LC n-3 PUFA in NEFA at the end of either study day.
Plasma NEFA and TAG responses.
Plasma NEFA levels declined following consumption of the bolus oral load but increased rapidly upon initiation of the heparin infusion (60 min) to reach approximately twice the baseline NEFA level [supplementary Table V(i)]. The incremental area under the curve (iAUC) was calculated between 60 min and 240 min to provide a summary of NEFA exposure during oral fat feeding plus heparin [supplementary Table VI(ii)]. There were no differences in the NEFA responses between the two fat loads, although the absolute iAUC NEFA60–240 levels for the males were higher than females for both fat types (SFA, P < 0.001; SFA+LC n-3 PUFA, P = 0.020), and on the SFA+LC n-3 PUFA day, the NEFA response was significantly lower in Asp298 than in Glu298 males (P < 0.05).
Plasma TAG levels fell slightly but not significantly after initiation of the heparin infusion, then increased gradually during the remainder of the study day until levels returned to baseline [supplementary Table V(ii)]. The iAUC was calculated from 90 to 240 min, corresponding to the period when TAG levels began to increase [supplementary Table VI(iii)]. There were no differences between the TAG responses to the two fat loads for any of the groups. However, females had higher iAUC TAG90–240 than did males to the SFA fat load (P = 0.004 for SFA), with a similar trend for the SFA+LC n-3 PUFA (P = 0.094). The female Asp298 also had a higher iAUC TAG90–240 on the SFA+LC n-3 PUFA study day compared with their Glu298 counterparts (P < 0.05), with a tendency for a similar response on the SFA study day (P = 0.074).
Relationships between endothelial function with NEFA fatty acid composition, NEFA, and TAG responses following the fat loads.
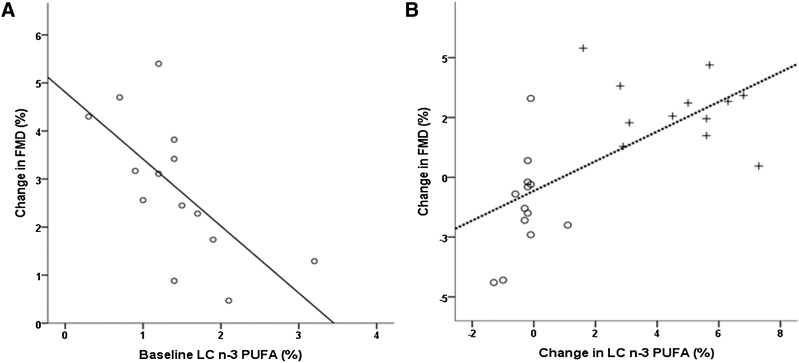
In Asp298 females, the baseline NEFA LC n-3 PUFA levels were negatively correlated with the ΔFMD for the SFA+LC n-3 PUFA study day (ρ = −0.668, P = 0.009; Fig. 2A), suggesting that in this group, the beneficial effects of LC n-3 PUFA on endothelial-dependent FMD were greater in subjects that had lower levels of NEFA LC n-3 PUFA at baseline.
Fig. 2.
(A) Correlation between absolute change in FMD response from overnight fasting value (time = 0) to the postprandial measurement (time = 240 min) following consumption of SFA+LC n-3 PUFA drinks and baseline NEFA LC n-3 in Asp298 females (ρ = −0.668, P = 0.009). (B) Absolute change in FMD response from overnight fasting value (time = 0) to the postprandial measurement (time = 240 min) and change in NEFA LC n-3 in Asp298 females following consumption of SFA (open circles) and SFA+LC n-3 PUFA (plus signs) drinks. (ρ = 0.664, P < 0.001)
There was a strong positive correlation for the whole group between ΔFMD and change in NEFA LC n-3 PUFA levels (ρ = 0.428, P < 0.001), which was strongest for the Asp298 females (ρ = 0.664, P < 0.001; Fig. 2B), with a weaker correlation in Glu298 subjects (females ρ = 0.458, P = 0.021; males ρ = 0.459, P = 0.014), and no correlation in Asp298 males (ρ = 0.258, P = 0.247).
DISCUSSION
The present study has demonstrated the beneficial postprandial actions of LC n-3 PUFA on endothelial function to be strongly influenced by a common polymorphism in the eNOS gene and by gender. These findings may be important in identifying population subgroups with greater responsiveness to the beneficial effects of these dietary fatty acids, and they may help delineate underlying mechanisms for the cardioprotective actions of LC n-3 PUFA.
This study observed a 2-fold greater FMD response following the addition of LC n-3 PUFA to the SFA fat load in female subjects, and in males and females homozygous for the Asp298 genotype. Female Asp298 subjects showed the greatest response to addition of LC n-3 PUFA to the standard SFA fat load, with a 3-fold greater FMD response and a 2-fold and 4-fold higher ΔLDI-SNP and ΔLDI-Ach response, than did the Glu298 females. Notably, although LC n-3 PUFA reversed the adverse effects of a SFA fat load on FMD in male Asp298 subjects, this was the only subgroup that failed to show a statistically significant improvement in postprandial FMD response. These findings suggest females with the Asp298 polymorphism may be particularly responsive to the vasomodulatory effects of LC n-3 PUFA. This is also supported by the observation that a positive correlation was found between the percentage of NEFA LC n-3 PUFA at baseline and the change in the FMD response from baseline to 240 min (ΔFMD), providing support to the findings of Leeson et al. (15) that this group shows greater responsiveness to environmental stimuli that influence endothelial function.
Measurement of FMD response in the present study relies on the endothelial production of NO in large vessels as stimulated by shear stress (24), whereas LDI measures the reactivity of the microcirculation. The LDI measures are of particular value in delineating potential mechanisms that may underlie these findings, as they provide data with respect to endothelial (NO)-independent (Ach stimulated) as well as endothelial (NO)-dependent (SNP stimulated) pathways involved in vasodilatation. An increase in the ΔLDI-Ach response was observed in the females when LC n-3 PUFA were added to the fat load, with the difference reaching significance in Asp298 females and near significance in the Asp298 males (P = 0.06). These findings are consistent with our findings for effects of LC n-3 PUFA on FMD and with our hypothesis that LC n-3 PUFA may enhance the signal to activation of eNOS and NO production, supporting the proposition that effects of LC n-3 PUFA operate via an endothelial-dependent mechanism. The Asp298 eNOS polymorphism has been reported to result in a functional change in the eNOS protein, which leads to a more unstable product (25) and which may therefore be more responsive to stimuli that enhance (e.g., LC n-3 PUFA) or attenuate (e.g., SFA) the signal activation pathway to eNOS.
In contrast to the above, we observed no effect of addition of LC n-3 PUFA on circulating NOx concentrations, which were reduced in the same manner by both fat loads. Although the sensitivity of the NOx assay may have been insufficient to detect small changes in NO production, it is unlikely this null finding is due to consumption of nitrate-containing foods (beetroot, cured meats, some cheeses) because food intake was standardized for 24 h prior to each study day. The lack of effect of the different fat loads on circulating NO therefore raises the possibility that the effects of LC n-3 PUFA on FMD and LDI-Ach may be explained instead by an endothelial-independent mechanism involving enhanced response of smooth muscle to NO. For the LDI-SNP response, SNP acts as an NO donor, and changes in this response reflect effects distal to the production of NO (endothelial-independent stimulation) (26). Significant increases in LDI-SNP responses were observed in both Asp298 and Glu298 females following LC n-3 PUFA, a finding that suggests a site of action of these fatty acids at a locus distal to the point of NO production and that appears to be more pronounced in females. This distal site could include a mechanism that protects NO from degradation or that enhances the response of smooth muscle to NO. For some time, there has been evidence to support the possibility that LC n-3 PUFA increase the responsiveness of the smooth muscle cell layer to NO (27, 28). However, these effects have been attributed to long-term changes in membrane fatty acid composition, which could not explain the acute effects we have observed here. Evidence showing that cytochrome P450 enzymes in endothelial cells convert EPA and DHA to novel and biopotent epoxides (29), which have vasodilatory effects on smooth muscle (30), raises the possibility that at least part of the effects we have observed are due to direct actions of locally produced epoxides in response to shear stress. Also of interest is the observation of effects of estrogen on the activity of endothelial cytochrome P450, which could accentuate local effects of LC n-3 PUFA via increased conversion to fatty acid epoxides (31), providing possible explanation for the greater responsiveness observed in premenopausal female than in male subjects.
There is no clear consensus as to the desirability or otherwise of providing fat loads either as a standard load or based on body weight, and we chose to provide our fat load based on body weight to take into account differences in body weight between male and female subjects. Although the incremental areas under the postprandial NEFA response curves were greater in males than in females, the incremental TAG response tended to be higher in females. However, these differences are unable to explain the effects of gender on vascular function that we have observed, and no correlations were observed between these incremental measures and any of the measures of vascular function, suggesting the provision of fat load based on body weight did not bias our observations.
Overall, our findings are consistent with differential effects of fatty acids on the vasculature and on both endothelial dependent and independent pathways. Beneficial (LC n-3 PUFA) and adverse (SFA) effects of fatty acids on the endothelial-dependent production of NO appear to be more pronounced in the Asp298 genotype, whereas direct vasodilatory effects of LC n-3 PUFA on smooth muscle may be more evident in female subjects.
The Asp298 group represents only 10% of the normal population; however, reports of their greater susceptibility to CVD (17, 32) and to preeclampsia of pregnancy in women (33) may justify advice for increased dietary intakes of LC n-3 PUFA in this genotypic group. Further verification of our findings would add strength to that advice. Notwithstanding the possibility of focused advice to this group, the relatively low dose of fish oils used here provided marked attenuation of the adverse effects of SFA on FMD response in all four genotype-gender groups, providing further evidence for the cardioprotective effects of this class of fatty acids. The power of these findings is increased by the identification of possible molecular loci that might explain potentiation of the actions of LC n-3 PUFA in Asp298 and female subjects.
Supplementary Material
Acknowledgments
The authors thank our volunteers for their participation, and Agnieszka Przemska, Dafni Vasilopoulou, Alice Turner, Petr Valasek, and Rada Mihaylova for their help during the study days and sample analysis.
Footnotes
Abbreviations:
- AU
- arbitrary perfusion unit
- BMI
- body mass index
- BP
- blood pressure
- CHD
- coronary heart disease
- DHA
- docosahexaenoic acid
- eNOS
- endothelial nitric oxide synthase
- EPA
- eicosapentaenoic acid
- ET-1
- endothelin-1
- FFQ
- food frequency questionnaire
- FMD
- flow-mediated dilation
- HDL-C
- high-density lipoprotein cholesterol
- HRT
- hormone replacement therapy
- iAUC
- incremental area under the curve
- LDI
- laser Doppler imaging with iontophoresis
- LDI-Ach
- LDI response to acetylcholine
- LDI-SNP
- LDI response to sodium nitroprusside
- LDL-C
- low-density lipoprotein cholesterol
- NO
- nitric oxide
- NOx
- total nitrite
- NS
- nonsignificant
- OC
- oral contraceptive
- PC
- phosphatidyl choline
- SFA
- saturated fatty acid
- TAG
- triacylglycerol
- TC
- total cholesterol
This work was supported by BBSRC Biotechnology and Biological Sciences Research Council (BB/E0221816/1), Unilever PLC, and FRST Foundation for Research, Science and Technology (New Zealand). The palm stearin and fish oil concentrate were kindly donated by Aarhuskarlshman, UK, and Croda Healthcare, UK, respectively.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of six tables.
REFERENCES
- 1.Vafeiadou K., Weech M., Sharma V., Yaqoob P., Todd S., Williams C. M., Jackson K. G., Lovegrove J. A. 2012. A review of the evidence for the effects of total dietary fat, SFA, MUFA and n-6 PUFA on vascular function, endothelial progenitor cells and microparticles. Br. J. Nutr. 107: 303–324 [DOI] [PubMed] [Google Scholar]
- 2.Nicholls S. J., Lundman P., Harmer J. A., Cutri B., Griffiths K. A., Rye K. A., Barter P. J., Celermajer D. S. 2006. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J. Am. Coll. Cardiol. 48: 715–720 [DOI] [PubMed] [Google Scholar]
- 3.Tentolouris N., Arapostathi C., Perrea D., Kyriaki D., Revenas C., Katsilambros N. 2008. Differential effects of two isoenergetic meals rich in saturated or monounsaturated fat on endothelial function in subjects with type 2 diabetes. Diabetes Care. 31: 2276–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuentes F., López-Miranda J., Sánchez E., Sánchez F., Paez J., Paz-Rojas E., Marín C., Gómez P., Jimenez-Perepérez J., Ordovás J. M., et al. 2001. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann. Intern. Med. 134: 1115–1119 [DOI] [PubMed] [Google Scholar]
- 5.Keogh J. B., Grieger J. A., Noakes M., Clifton P. M. 2005. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler. Thromb. Vasc. Biol. 25: 1274–1279 [DOI] [PubMed] [Google Scholar]
- 6.Rizza S., Tesauro M., Cardillo C., Galli A., Iantorno M., Gigli F., Sbraccia P., Federici M., Quon M. J., Lauro D. 2009. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 206: 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newens K. J., Thompson A. K., Jackson K. G., Wright J., Williams C. M. 2011. Acute effects of elevated NEFA on vascular function: a comparison of SFA and MUFA. Br. J. Nutr. 105: 1343–1351 [DOI] [PubMed] [Google Scholar]
- 8.Armah C. K., Jackson K. G., Doman I., James L., Cheghani F., Minihane A. M. 2008. Fish oil fatty acids improve postprandial vascular reactivity in healthy men. Clin. Sci. 114: 679–686 [DOI] [PubMed] [Google Scholar]
- 9.Khan F., Elherik K., Bolton-Smith C., Barr R., Hill A., Murrie I., Belch J. J. 2003. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc. Res. 59: 955–962 [DOI] [PubMed] [Google Scholar]
- 10.Goodfellow J., Bellamy M. F., Ramsey M. W., Jones C. J. H., Lewis M. J. 2000. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 35: 265–270 [DOI] [PubMed] [Google Scholar]
- 11.Newens K. J., Thompson A. K., Jackson K. G., Wright J., Williams C. M. 2011. Docosahexaenoic acid-rich fish oil reverses the detrimental effects of elevated saturated non-esterified fatty acids on postprandial vascular reactivity. Am. J. Clin. Nutr. 94: 742–748 [DOI] [PubMed] [Google Scholar]
- 12.Massaro M., Scoditti E., Carluccio M. A., De Caterina R. 2008. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot. Essent. Fatty Acids. 79: 109–115 [DOI] [PubMed] [Google Scholar]
- 13.Wang X. L., Zhang L., Youker K., Zhang M. X., Wang J., LeMaire S. A., Coselli J. S., Shen Y. H. 2006. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 55: 2301–2310 [DOI] [PubMed] [Google Scholar]
- 14.Xiao-Yun X., Zhuo-Xiong C., Min-Xiang L., Xingxuan H., Schuchman E. H., Feng L., Han-Song X., An-Hua L. 2009. Ceramide mediates inhibition of the AKT/eNOS signaling pathway by palmitate in human vascular endothelial cells. Med. Sci. Monit. 15: BR254–BR261 [PubMed] [Google Scholar]
- 15.Leeson C. P. M., Hingorani A. D., Mullen M. J., Jeerooburkhan N., Kattenhorn M., Cole T. J., Muller D. P., Lucas A., Humphries S. E., Deanfield J. E. 2002. Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ. Res. 90: 1153–1158 [DOI] [PubMed] [Google Scholar]
- 16.Rossi G. P., Taddei S., Virdis A., Cavallin M., Ghiadoni L., Favilla S., Versari D., Sudano I., Pessina A. C., Salvetti A. 2003. The T-786C and Glu298Asp polymorphisms of the endothelial nitric oxide gene affect the forearm blood flow responses of Caucasian hypertensive patients. J. Am. Coll. Cardiol. 41: 938–945 [DOI] [PubMed] [Google Scholar]
- 17.Casas J. P., Cavalleri G. L., Bautista L. E., Smeeth L., Humphries S. E., Hingorani A. D. 2006. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 164: 921–935 [DOI] [PubMed] [Google Scholar]
- 18.Ferguson J. F., Phillips C. M., McMonagle J., Perez-Martinez P., Shaw D. I., Lovegrove J. A., Helal O., Defoort C., Gjelstad I. M., Drevon C. A., et al. 2010. NOS3 gene polymorphisms are associated with risk markers of cardiovascular disease, and interact with omega-3 polyunsaturated fatty acids. Atherosclerosis. 211: 539–544 [DOI] [PubMed] [Google Scholar]
- 19.Williams C. M., Ordovas J. M., Lairon D., Hesketh J., Lietz G., Gibney M., van Ommen B. 2008. The challenges for molecular nutrition research 1: linking genotype to healthy nutrition. Genes Nutr. 3: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sader M. A., Celermajer D. S. 2002. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc. Res. 53: 597–604 [DOI] [PubMed] [Google Scholar]
- 21.Imamura A., Takahashi R., Murakami R., Kataoka H., Cheng X. W., Numaguchi Y., Murohara T., Okumura K. 2008. The effects of endothelial nitric oxide synthase gene polymorphisms on endothelial function and metabolic risk factors in healthy subjects: the significance of plasma adiponectin levels. Eur. J. Endocrinol. 158: 189–195 [DOI] [PubMed] [Google Scholar]
- 22.Beysen C., Karpe F., Fielding B. A., Clark A., Levy J. C., Frayn K. N. 2002. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia. 45: 1533–1541 [DOI] [PubMed] [Google Scholar]
- 23.Caslake M. J., Miles E. A., Kofler B. M., Lietz G., Curtis P., Armah C. K., Kimber A. C., Grew J. P., Farrell L., Stannard J., et al. 2008. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am. J. Clin. Nutr. 88: 618–629 [DOI] [PubMed] [Google Scholar]
- 24.Corretti M. C., Anderson T. J., Benjamin E. J., Celermajer D., Charbonneau F., Creager M. A., Deanfield J., Drexler H., Gerhard-Herman M., Herrington D., et al. 2002. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 39: 257–265 [DOI] [PubMed] [Google Scholar]
- 25.Tesauro M., Thompson W. C., Rogliani P., Qi L., Chaudhary P. P., Moss J. 2000. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc. Natl. Acad. Sci. USA. 97: 2832–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner J., Belch J. J. F., Khan F. 2008. Current concepts in assessment of microvascular endothelial function using laser doppler imaging and iontophoresis. Trends Cardiovasc. Med. 18: 109–116 [DOI] [PubMed] [Google Scholar]
- 27.Engler M. B., Ma Y-H., Engler M. M. 1999. Calcium-mediated mechanisms of eicosapentaenoic acid-induced relaxation in hypertensive rat aorta. Am. J. Hypertens. 12: 1225–1235 [DOI] [PubMed] [Google Scholar]
- 28.Chin J. P., Dart A. M. 1995. How do fish oils affect vascular function? Clin. Exp. Pharmacol. Physiol. 22: 71–81 [DOI] [PubMed] [Google Scholar]
- 29.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 285: 32720–32733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R. X., Chai Q., Lu T., Lee H. C. 2011. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc. Res. 90: 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang A., Sun D., Wu Z., Yan C., Carroll M. A., Jiang H., Falck J. R., Kaley G. 2004. Estrogen elicits cytochrome P450-mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ. Res. 94: 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odeberg J., Larsson C. A., Rastam L., Lindblad U. 2008. The Asp(298) allele of endothelial nitric oxide synthase is a riskfactor for myocardial infarction among patients with type 2 diabetes. BMC Cardiovasc. Disord. 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano N. C., Casas J. P., Díaz L. A., Páez C., Mesa C. M., Cifuentes R., Monterrosa A., Bautista A., Hawe E., Hingorani A. D., et al. 2004. Endothelial NO synthase genotype and risk of preeclampsia: a multicenter case-control study. Hypertension. 44: 702–707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.