Abstract
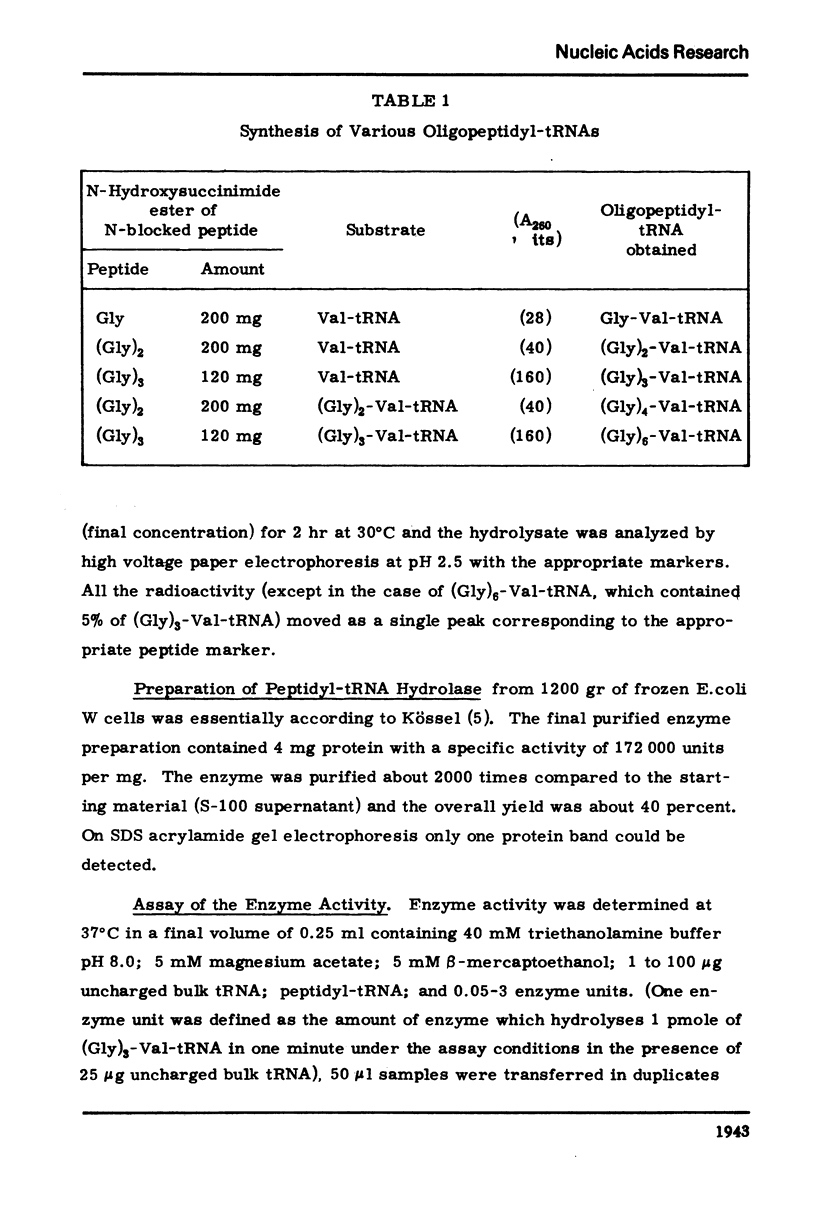
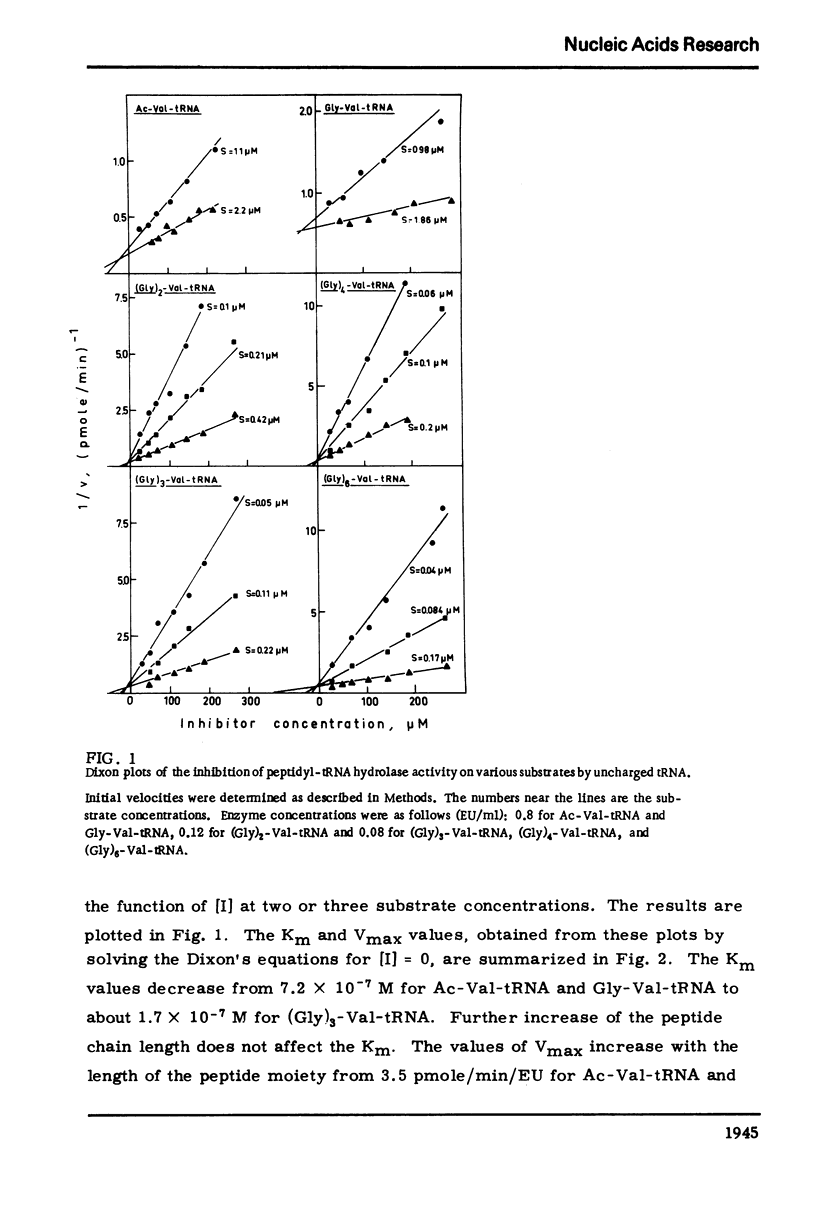
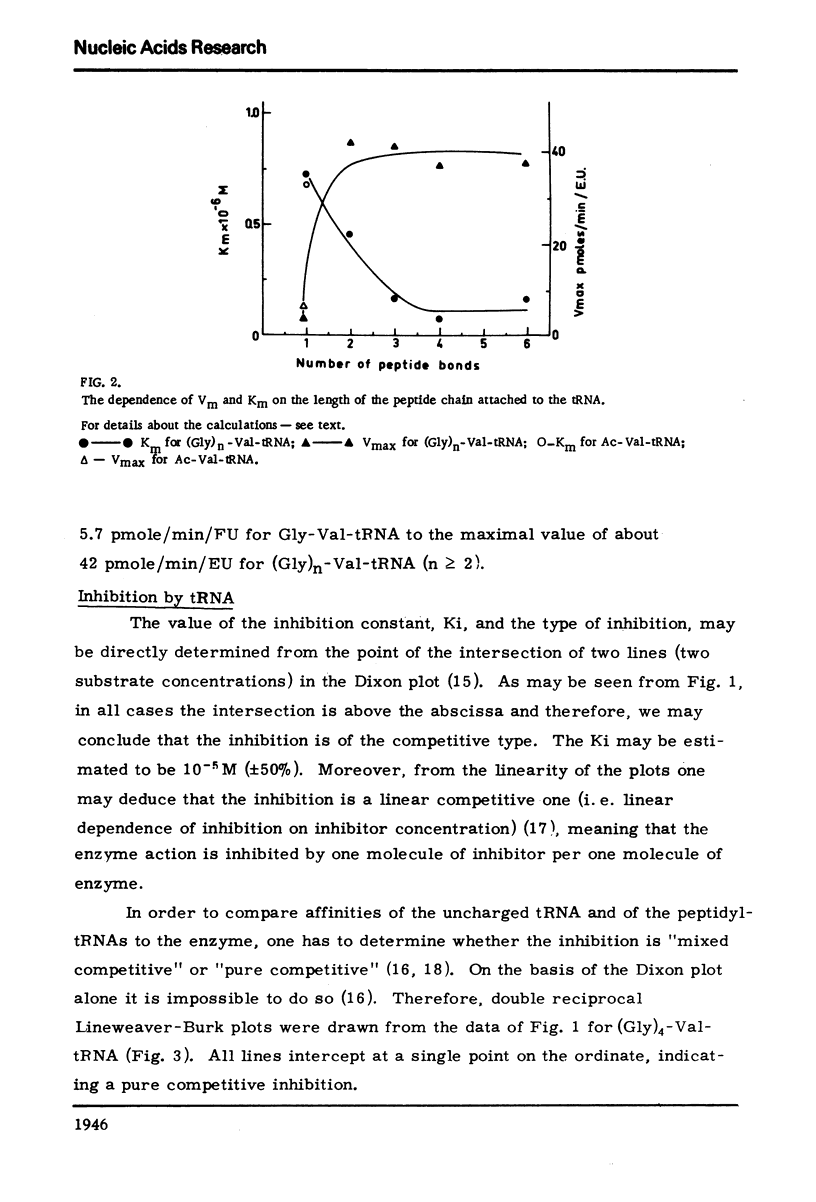
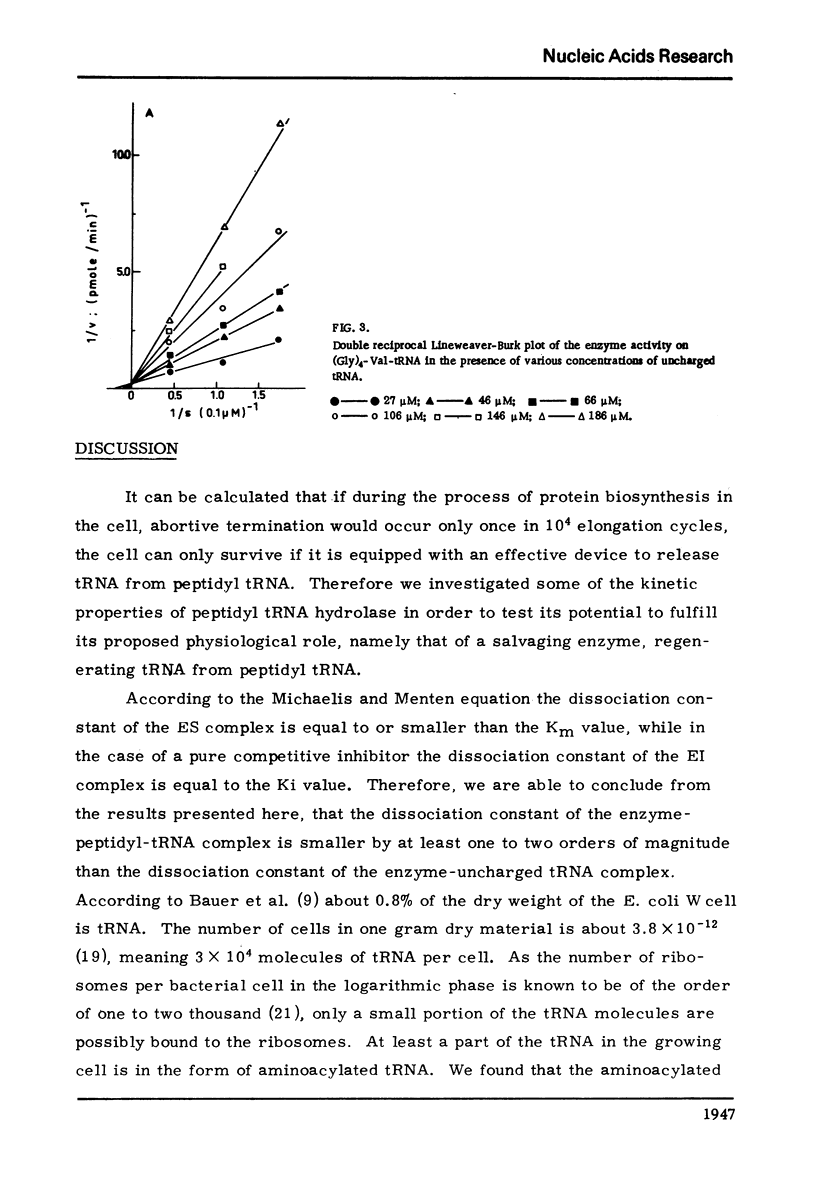
The dependence of the Vmax and Km on the length of the peptide moiety in the peptidyl-tRNA series (Gly)n-Val tRNA, was measured in the system peptidyl-tRNA hydrolase-peptidyl-tRNA. It was found that the Km value decreases from 7.2 X 10-7 M for Gly-Val-tRNA to 4.6 X 10-7 M FOR (Gly)2-Val-tRNA and to 1.7 X 10-7M for (Gly)3-Val-tRNA; further increase of the peptide chain is not followed by decrease of the Km. The Vmax values are 5.7 pmole/min/EU for Gly-Val-tRNA and 42 pmole/min/EU for (Gly)3-Val-tRNA. The enzyme activity is inhibited competitively by uncharged tRNA with a KI value of about 10-5M. The significance of these results described in this paper, in relation to the fact that peptides and peptide esters do not inhibit the enzyme activity, and in relation to the proposed physiological role of the enzyme, is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer S., Milner Y., Ziv E., Shiloach J., de Groot N., Lapidot Y. A modified procedure for the large scale preparation of tRNA from E. coli. Biotechnol Bioeng. 1973 Nov;15(6):1081–1088. doi: 10.1002/bit.260150607. [DOI] [PubMed] [Google Scholar]
- Bauer S., Shiloach J. Maximal exponential growth rate and yield of E. coli obtainable in a bench-scale fermentor. Biotechnol Bioeng. 1974 Jul;16(7):933–941. doi: 10.1002/bit.260160707. [DOI] [PubMed] [Google Scholar]
- Butterworth P. J. The use of Dixon plots to study enzyme inhibition. Biochim Biophys Acta. 1972 Dec 7;289(2):251–253. doi: 10.1016/0005-2744(72)90074-5. [DOI] [PubMed] [Google Scholar]
- Chapeville F., Yot P., Paulin D. Enzymatic hydrolysis of N-acyl-aminoacyl transfer RNAs. Cold Spring Harb Symp Quant Biol. 1969;34:493–498. doi: 10.1101/sqb.1969.034.01.055. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Kretchmer N., Greenberg R. E., Hurwitz R., Chapeville F. Enzymatic hydrolysis of N-substituted aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2079–2086. doi: 10.1073/pnas.58.5.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot N., Groner Y., Lapidot Y. Peptidyl-tRNA. VII. Substrate specificity of peptidyl-tRNA hydrolase. Biochim Biophys Acta. 1969 Aug 20;186(2):286–296. doi: 10.1016/0005-2787(69)90006-9. [DOI] [PubMed] [Google Scholar]
- Hänggi U. J., Zachau H. G. Partial nuclease digestion of transfer ribonucleic acids and aminoacylated transfer ribonucleic acids. Eur J Biochem. 1971 Feb;18(4):496–502. doi: 10.1111/j.1432-1033.1971.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Kössel H. Purification and properties of peptidyl-tRNA hydrolase from Escherichia coli. Biochim Biophys Acta. 1970 Mar 19;204(1):191–202. doi: 10.1016/0005-2787(70)90502-2. [DOI] [PubMed] [Google Scholar]
- Kössel H., RajBhandary U. L. Studies on polynucleotides. LXXXVI. Enzymic hydrolysis of N-acylaminoacyl-transfer RNA. J Mol Biol. 1968 Aug 14;35(3):539–560. doi: 10.1016/s0022-2836(68)80013-0. [DOI] [PubMed] [Google Scholar]
- Lapidot Y., Rappoport S. The synthesis of oligopeptidyl-tRNA. Methods Enzymol. 1974;29:688–695. doi: 10.1016/0076-6879(74)29061-x. [DOI] [PubMed] [Google Scholar]
- Menninger J. R., Mulholland M. C., Stirewalt W. S. Peptidyl-tRNA hydrolase and protein chain termination. Biochim Biophys Acta. 1970 Oct 15;217(2):496–511. doi: 10.1016/0005-2787(70)90547-2. [DOI] [PubMed] [Google Scholar]
- Ninio J., Luzzati V., Yaniv M. Comparative small-angle x-ray scattering studies on unacylated, acylated and cross-linked Escherichia coli transfer RNA I Val . J Mol Biol. 1972 Nov 14;71(2):217–229. doi: 10.1016/0022-2836(72)90347-6. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J. Limitations in the use of Dixon plots to evaluate enzyme inhibition. Biochim Biophys Acta. 1972 Apr 7;268(1):1–3. doi: 10.1016/0005-2744(72)90189-1. [DOI] [PubMed] [Google Scholar]
- Rappoport S., Lapidot Y. The chemical preparation of acetylaminoacyl-tRNA. Methods Enzymol. 1974;29:685–688. doi: 10.1016/0076-6879(74)29060-8. [DOI] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. On the specificity and stability of an enzyme that hydrolyzes N-substituted aminoacyl-transfer RNA's. Proc Natl Acad Sci U S A. 1968 Oct;61(2):701–707. doi: 10.1073/pnas.61.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E., De Groot N., Lapidot Y. Peptidyl-tRNA. XII. Behaviour of peptidyl-tRNA-Phe from Escherichia coli on methylated albumin silicic acid column. Biochim Biophys Acta. 1971 Jan 1;228(1):135–140. [PubMed] [Google Scholar]


