Abstract
The title compound, C12H16N2O2, crystallized from toluene with two independent molecules in the asymmetric unit. The dihedral angles between the amide groups and the benzene ring are 60.87 (11) and 54.08 (11)° in one independent molecule and 60.13 (11) and 64.64 (11) in the other. The crystal structure features weak C—H⋯O hydrogen bonds and C—H⋯π interactions.
Related literature
For related structures, see: Altamura et al. (2005 ▶); Anderson et al. (2004 ▶); Clayden et al. (2001 ▶); Comins et al. (1998 ▶); Sakamoto et al. (2004 ▶). 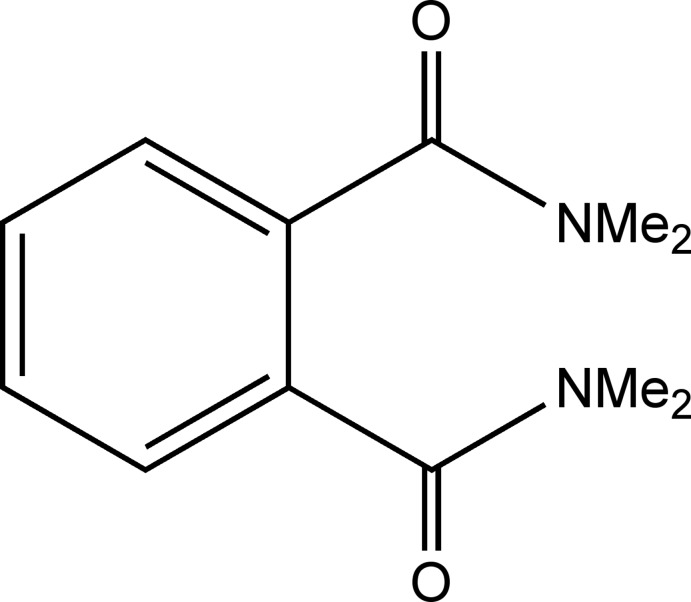
Experimental
Crystal data
C12H16N2O2
M r = 220.27
Monoclinic,
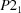
a = 6.7337 (6) Å
b = 18.1230 (14) Å
c = 9.8216 (8) Å
β = 104.918 (3)°
V = 1158.18 (17) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 100 K
0.56 × 0.52 × 0.33 mm
Data collection
Bruker APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2002 ▶) T min = 0.925, T max = 0.972
8205 measured reflections
2739 independent reflections
2414 reflections with I > 2σ(I)
R int = 0.045
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.124
S = 1.06
2739 reflections
297 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.34 e Å−3
Data collection: APEX2 (Bruker, 2006 ▶); cell refinement: SAINT (Bruker, 2006 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ATOMS (Dowty, 1995 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812035167/bq2372sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812035167/bq2372Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812035167/bq2372Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C2–C7 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O3i | 0.93 | 2.60 | 3.518 (3) | 170 |
| C5—H5⋯O4ii | 0.93 | 2.58 | 3.141 (4) | 119 |
| C6—H6⋯O4ii | 0.93 | 2.51 | 3.105 (4) | 122 |
| C18—H18⋯O2iii | 0.93 | 2.49 | 3.329 (3) | 150 |
| C24—H24A⋯O2iii | 0.96 | 2.56 | 3.224 (4) | 127 |
| C16—H16⋯Cg1 | 0.93 | 2.97 | 3.810 (4) | 124 |
Symmetry codes: (i) 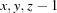 ; (ii)
; (ii) 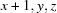 ; (iii)
; (iii) 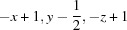 .
.
Acknowledgments
The authors thank the MESRS (Algeria) for financial support.
supplementary crystallographic information
Comment
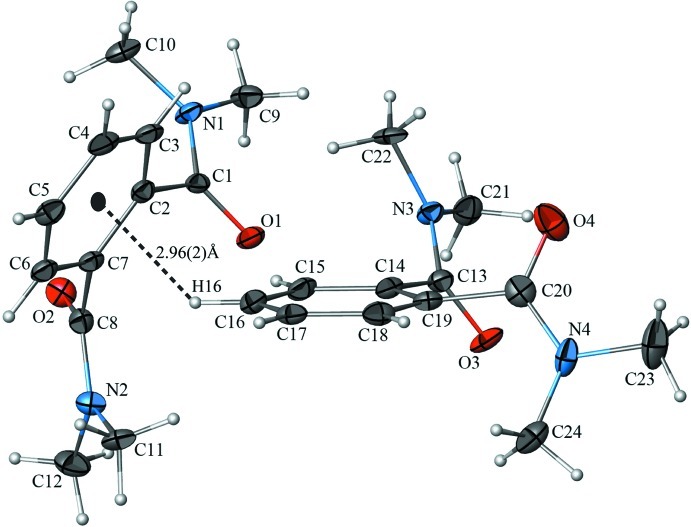
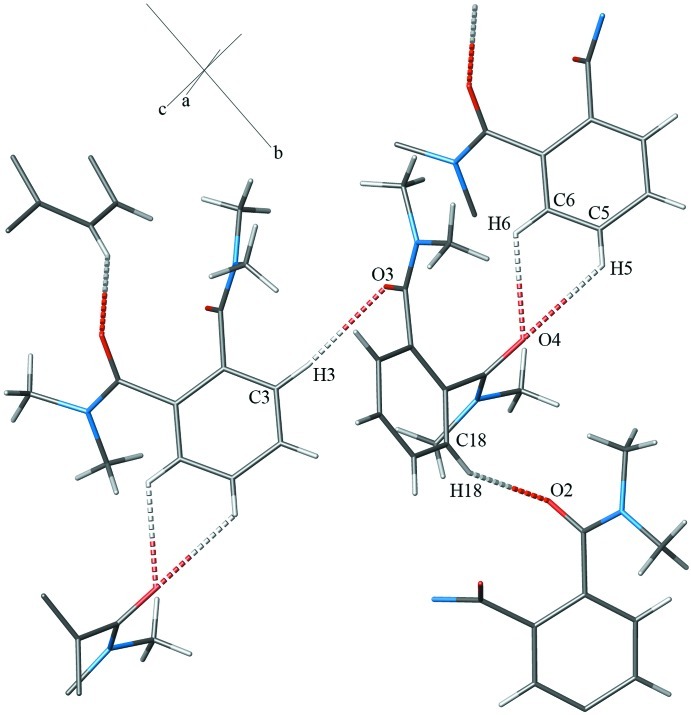
The molecular structure of (I) is composed of a two crystallographically independent molecules (IA and IB). A displacement ellipsoid plot of the two independent molecules of (I) is shown in Fig. 1. Bond lengths and angles in (I) are normal, and values for the two independent molecules agree with each other. The crystal structure of (I) is stabilized by intermolecular weak hydrogen-bonds type C—H···O (Fig. 2) and C—H··· π interactions, the latter interaction is observed between centroid of benzene ring (C2—C7) (IA) and hydrogen atom H16 of benzene ring (C14—C19) of the adjacent molecule (IB), with a Cg···H16 distance of 2.96 (2) Å (Fig. 1), resulting in the formation of infinite three-dimensional network reinforcing a cohesion of structure. Hydrogen-bonding parameters are listed in Table 1.
Experimental
To a suspension of phthalic acid (1 g, 6.02 mmol) in anhydrous toluene (10 ml) trimethlphosphine (6.02 mmol) was added and the mixture kept under reflux for 45 min. The resulting cloudy solution was allowed to cool to room temperature and a saturated aqueous solution of NaHCO3 (5 ml) was added. The layers were separated and the aqueous phase was extracted with methylene chloride (4.5 ml). The organic solutions were combined together, dried over anhydrous MgSO4 and concentrated to dryness, obtaining a white solid. Colorless single crystals suitable for X-ray diffraction analysis were obtained by dissolving the corresponding compound in toluene solution and letting it for slow evaporation at room temperature (Yield: 1.30 g, 92%).
Refinement
All non-hydrogen atoms were refined with anisotropic atomic displacement parameters. All H atoms were placed at calculated positions and treated as riding on their parent atoms with C—H = 0.93–0.96 Å, and Uiso (H) = 1.5Ueq(C) for methyl H atoms and 1.2Ueq(C) for the others. The absolute structure parameter is meaningless because the compound is a weak anomalous scatterer. So, the absolute structure parameter is removed from the CIF.
Figures
Fig. 1.
View of the two molecules in the asymmetric unit of (I) showing the numbering schemes; displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Partial view of the crystal structure of (I) showing the non standard hydrogen bonds.
Crystal data
| C12H16N2O2 | F(000) = 472 |
| Mr = 220.27 | Dx = 1.263 Mg m−3 |
| Monoclinic, P21 | Melting point: 118 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.7337 (6) Å | Cell parameters from 2761 reflections |
| b = 18.1230 (14) Å | θ = 2.4–26.8° |
| c = 9.8216 (8) Å | µ = 0.09 mm−1 |
| β = 104.918 (3)° | T = 100 K |
| V = 1158.18 (17) Å3 | Needles, colourless |
| Z = 4 | 0.56 × 0.52 × 0.33 mm |
Data collection
| Bruker APEXII diffractometer | 2739 independent reflections |
| Graphite monochromator | 2414 reflections with I > 2σ(I) |
| Detector resolution: 18.4 pixels mm-1 | Rint = 0.045 |
| CCD rotation images, thin slices scans | θmax = 27.5°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2002) | h = −8→8 |
| Tmin = 0.925, Tmax = 0.972 | k = −23→20 |
| 8205 measured reflections | l = −11→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.124 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0785P)2] where P = (Fo2 + 2Fc2)/3 |
| 2739 reflections | (Δ/σ)max < 0.001 |
| 297 parameters | Δρmax = 0.32 e Å−3 |
| 1 restraint | Δρmin = −0.34 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.7861 (3) | 0.24084 (11) | −0.0065 (2) | 0.0216 (6) | |
| O2 | 0.8041 (3) | 0.28421 (11) | 0.3142 (2) | 0.0235 (6) | |
| N1 | 0.4493 (3) | 0.26282 (13) | −0.0175 (2) | 0.0195 (7) | |
| N2 | 1.0989 (4) | 0.21659 (13) | 0.3653 (2) | 0.0220 (7) | |
| C1 | 0.6269 (4) | 0.22638 (15) | 0.0296 (3) | 0.0171 (7) | |
| C2 | 0.6276 (4) | 0.16096 (14) | 0.1249 (3) | 0.0168 (7) | |
| C3 | 0.5027 (4) | 0.10057 (15) | 0.0751 (3) | 0.0189 (7) | |
| C4 | 0.5113 (4) | 0.03795 (16) | 0.1583 (3) | 0.0235 (8) | |
| C5 | 0.6447 (4) | 0.03548 (15) | 0.2915 (3) | 0.0211 (8) | |
| C6 | 0.7738 (4) | 0.09499 (15) | 0.3414 (3) | 0.0200 (7) | |
| C7 | 0.7675 (4) | 0.15789 (14) | 0.2577 (3) | 0.0171 (7) | |
| C8 | 0.8934 (4) | 0.22495 (16) | 0.3135 (3) | 0.0189 (7) | |
| C9 | 0.4327 (5) | 0.32006 (16) | −0.1231 (3) | 0.0244 (8) | |
| C10 | 0.2644 (4) | 0.25242 (16) | 0.0331 (3) | 0.0232 (8) | |
| C11 | 1.2112 (4) | 0.14818 (17) | 0.3610 (3) | 0.0261 (8) | |
| C12 | 1.2221 (5) | 0.27854 (19) | 0.4338 (3) | 0.0312 (9) | |
| O3 | 0.1819 (3) | 0.12996 (12) | 0.7353 (2) | 0.0259 (6) | |
| O4 | −0.0391 (4) | −0.02611 (13) | 0.5606 (2) | 0.0358 (7) | |
| N3 | −0.1580 (3) | 0.14947 (12) | 0.7148 (2) | 0.0186 (6) | |
| N4 | 0.2973 (4) | −0.05367 (13) | 0.6492 (3) | 0.0258 (8) | |
| C13 | 0.0169 (4) | 0.11065 (15) | 0.7583 (3) | 0.0188 (7) | |
| C14 | 0.0152 (4) | 0.04326 (15) | 0.8474 (3) | 0.0180 (7) | |
| C15 | −0.0313 (4) | 0.05018 (16) | 0.9770 (3) | 0.0209 (8) | |
| C16 | −0.0171 (5) | −0.01003 (17) | 1.0661 (3) | 0.0224 (8) | |
| C17 | 0.0435 (4) | −0.07820 (16) | 1.0259 (3) | 0.0209 (8) | |
| C18 | 0.0923 (4) | −0.08572 (14) | 0.8968 (3) | 0.0206 (7) | |
| C19 | 0.0795 (4) | −0.02558 (15) | 0.8073 (3) | 0.0189 (7) | |
| C20 | 0.1098 (5) | −0.03514 (15) | 0.6626 (3) | 0.0235 (8) | |
| C21 | −0.1553 (5) | 0.22148 (16) | 0.6495 (3) | 0.0244 (8) | |
| C22 | −0.3615 (4) | 0.12250 (17) | 0.7172 (3) | 0.0243 (8) | |
| C23 | 0.3187 (7) | −0.0733 (2) | 0.5096 (4) | 0.0426 (13) | |
| C24 | 0.4731 (5) | −0.06937 (18) | 0.7671 (3) | 0.0292 (9) | |
| H3 | 0.41290 | 0.10210 | −0.01430 | 0.0230* | |
| H4 | 0.42730 | −0.00230 | 0.12440 | 0.0280* | |
| H5 | 0.64820 | −0.00600 | 0.34780 | 0.0250* | |
| H6 | 0.86440 | 0.09290 | 0.43050 | 0.0240* | |
| H9A | 0.56090 | 0.32440 | −0.14820 | 0.0370* | |
| H9B | 0.40060 | 0.36620 | −0.08570 | 0.0370* | |
| H9C | 0.32570 | 0.30750 | −0.20520 | 0.0370* | |
| H10A | 0.29320 | 0.21810 | 0.11010 | 0.0350* | |
| H10B | 0.15540 | 0.23350 | −0.04200 | 0.0350* | |
| H10C | 0.22350 | 0.29890 | 0.06420 | 0.0350* | |
| H11A | 1.13770 | 0.11860 | 0.28320 | 0.0390* | |
| H11B | 1.22450 | 0.12150 | 0.44730 | 0.0390* | |
| H11C | 1.34530 | 0.15940 | 0.34950 | 0.0390* | |
| H12A | 1.13530 | 0.32080 | 0.43170 | 0.0470* | |
| H12B | 1.32500 | 0.28980 | 0.38510 | 0.0470* | |
| H12C | 1.28750 | 0.26590 | 0.52980 | 0.0470* | |
| H15 | −0.07230 | 0.09570 | 1.00410 | 0.0250* | |
| H16 | −0.04820 | −0.00480 | 1.15250 | 0.0270* | |
| H17 | 0.05160 | −0.11880 | 1.08500 | 0.0250* | |
| H18 | 0.13380 | −0.13130 | 0.87050 | 0.0250* | |
| H21A | −0.01970 | 0.23140 | 0.63960 | 0.0370* | |
| H21B | −0.19320 | 0.25870 | 0.70760 | 0.0370* | |
| H21C | −0.25120 | 0.22170 | 0.55830 | 0.0370* | |
| H22A | −0.34750 | 0.07910 | 0.77490 | 0.0360* | |
| H22B | −0.43820 | 0.11070 | 0.62300 | 0.0360* | |
| H22C | −0.43260 | 0.16000 | 0.75520 | 0.0360* | |
| H23A | 0.22120 | −0.04580 | 0.43950 | 0.0640* | |
| H23B | 0.29370 | −0.12510 | 0.49400 | 0.0640* | |
| H23C | 0.45540 | −0.06180 | 0.50360 | 0.0640* | |
| H24A | 0.47420 | −0.12090 | 0.79030 | 0.0440* | |
| H24B | 0.46360 | −0.04050 | 0.84720 | 0.0440* | |
| H24C | 0.59760 | −0.05710 | 0.74170 | 0.0440* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0140 (10) | 0.0250 (10) | 0.0271 (10) | −0.0016 (8) | 0.0076 (8) | 0.0017 (8) |
| O2 | 0.0234 (11) | 0.0206 (9) | 0.0257 (10) | 0.0012 (9) | 0.0047 (9) | −0.0004 (8) |
| N1 | 0.0116 (11) | 0.0236 (12) | 0.0242 (11) | 0.0015 (10) | 0.0063 (9) | 0.0041 (9) |
| N2 | 0.0166 (12) | 0.0240 (11) | 0.0235 (11) | −0.0029 (10) | 0.0019 (10) | −0.0011 (9) |
| C1 | 0.0128 (12) | 0.0197 (12) | 0.0185 (12) | −0.0004 (11) | 0.0033 (10) | −0.0047 (10) |
| C2 | 0.0144 (12) | 0.0182 (11) | 0.0193 (12) | 0.0025 (10) | 0.0069 (10) | −0.0008 (10) |
| C3 | 0.0087 (12) | 0.0237 (12) | 0.0245 (13) | 0.0005 (11) | 0.0044 (10) | −0.0019 (11) |
| C4 | 0.0134 (13) | 0.0222 (13) | 0.0365 (15) | −0.0026 (11) | 0.0094 (12) | −0.0036 (12) |
| C5 | 0.0181 (14) | 0.0188 (12) | 0.0290 (14) | 0.0027 (11) | 0.0106 (12) | 0.0048 (11) |
| C6 | 0.0149 (13) | 0.0226 (12) | 0.0233 (13) | 0.0029 (11) | 0.0066 (11) | −0.0009 (10) |
| C7 | 0.0117 (12) | 0.0188 (12) | 0.0215 (12) | 0.0017 (11) | 0.0057 (10) | −0.0017 (10) |
| C8 | 0.0178 (13) | 0.0228 (12) | 0.0169 (12) | 0.0005 (12) | 0.0058 (10) | 0.0026 (10) |
| C9 | 0.0179 (14) | 0.0258 (13) | 0.0273 (14) | 0.0004 (12) | 0.0020 (12) | 0.0050 (11) |
| C10 | 0.0127 (13) | 0.0242 (13) | 0.0338 (15) | 0.0013 (11) | 0.0082 (12) | 0.0014 (12) |
| C11 | 0.0122 (13) | 0.0331 (15) | 0.0310 (15) | 0.0020 (13) | 0.0017 (12) | 0.0005 (12) |
| C12 | 0.0271 (17) | 0.0319 (15) | 0.0297 (15) | −0.0084 (15) | −0.0014 (13) | −0.0054 (13) |
| O3 | 0.0141 (10) | 0.0294 (10) | 0.0368 (11) | −0.0012 (9) | 0.0111 (9) | 0.0060 (9) |
| O4 | 0.0446 (15) | 0.0339 (11) | 0.0247 (11) | 0.0143 (12) | 0.0015 (10) | 0.0000 (9) |
| N3 | 0.0120 (11) | 0.0202 (11) | 0.0231 (11) | 0.0032 (9) | 0.0036 (9) | 0.0016 (9) |
| N4 | 0.0330 (15) | 0.0220 (11) | 0.0273 (13) | 0.0061 (11) | 0.0165 (12) | 0.0022 (9) |
| C13 | 0.0145 (13) | 0.0218 (12) | 0.0203 (12) | 0.0007 (11) | 0.0047 (10) | −0.0033 (10) |
| C14 | 0.0092 (12) | 0.0189 (12) | 0.0242 (13) | 0.0001 (10) | 0.0010 (10) | −0.0006 (10) |
| C15 | 0.0127 (13) | 0.0236 (12) | 0.0265 (14) | 0.0004 (11) | 0.0052 (11) | −0.0040 (11) |
| C16 | 0.0159 (14) | 0.0285 (14) | 0.0241 (13) | −0.0028 (12) | 0.0076 (12) | −0.0013 (11) |
| C17 | 0.0112 (13) | 0.0239 (13) | 0.0261 (14) | −0.0042 (11) | 0.0020 (11) | 0.0025 (11) |
| C18 | 0.0166 (13) | 0.0177 (12) | 0.0259 (13) | −0.0007 (11) | 0.0025 (11) | −0.0029 (10) |
| C19 | 0.0109 (13) | 0.0204 (12) | 0.0239 (13) | −0.0003 (11) | 0.0019 (10) | −0.0011 (10) |
| C20 | 0.0293 (17) | 0.0163 (12) | 0.0245 (14) | 0.0042 (12) | 0.0060 (13) | 0.0017 (10) |
| C21 | 0.0225 (15) | 0.0248 (13) | 0.0277 (14) | 0.0064 (13) | 0.0098 (12) | 0.0050 (12) |
| C22 | 0.0084 (13) | 0.0300 (14) | 0.0321 (15) | 0.0020 (12) | 0.0008 (11) | 0.0025 (12) |
| C23 | 0.064 (3) | 0.0364 (17) | 0.0380 (19) | 0.0132 (19) | 0.0323 (19) | 0.0050 (15) |
| C24 | 0.0218 (16) | 0.0261 (14) | 0.0431 (17) | −0.0013 (13) | 0.0145 (14) | −0.0019 (13) |
Geometric parameters (Å, º)
| O1—C1 | 1.241 (3) | C10—H10C | 0.9600 |
| O2—C8 | 1.232 (4) | C10—H10B | 0.9600 |
| O3—C13 | 1.240 (3) | C11—H11B | 0.9600 |
| O4—C20 | 1.232 (4) | C11—H11C | 0.9600 |
| N1—C10 | 1.467 (3) | C11—H11A | 0.9600 |
| N1—C1 | 1.340 (4) | C12—H12B | 0.9600 |
| N1—C9 | 1.451 (4) | C12—H12C | 0.9600 |
| N2—C11 | 1.459 (4) | C12—H12A | 0.9600 |
| N2—C8 | 1.354 (4) | C13—C14 | 1.504 (4) |
| N2—C12 | 1.454 (4) | C14—C15 | 1.393 (4) |
| N3—C13 | 1.344 (3) | C14—C19 | 1.410 (4) |
| N3—C22 | 1.461 (4) | C15—C16 | 1.387 (4) |
| N3—C21 | 1.456 (4) | C16—C17 | 1.390 (4) |
| N4—C23 | 1.459 (5) | C17—C18 | 1.396 (4) |
| N4—C24 | 1.455 (4) | C18—C19 | 1.389 (4) |
| N4—C20 | 1.345 (4) | C19—C20 | 1.497 (4) |
| C1—C2 | 1.510 (4) | C15—H15 | 0.9300 |
| C2—C7 | 1.400 (4) | C16—H16 | 0.9300 |
| C2—C3 | 1.390 (4) | C17—H17 | 0.9300 |
| C3—C4 | 1.391 (4) | C18—H18 | 0.9300 |
| C4—C5 | 1.384 (4) | C21—H21A | 0.9600 |
| C5—C6 | 1.393 (4) | C21—H21B | 0.9600 |
| C6—C7 | 1.400 (4) | C21—H21C | 0.9600 |
| C7—C8 | 1.502 (4) | C22—H22A | 0.9600 |
| C3—H3 | 0.9300 | C22—H22B | 0.9600 |
| C4—H4 | 0.9300 | C22—H22C | 0.9600 |
| C5—H5 | 0.9300 | C23—H23A | 0.9600 |
| C6—H6 | 0.9300 | C23—H23B | 0.9600 |
| C9—H9A | 0.9600 | C23—H23C | 0.9600 |
| C9—H9B | 0.9600 | C24—H24A | 0.9600 |
| C9—H9C | 0.9600 | C24—H24B | 0.9600 |
| C10—H10A | 0.9600 | C24—H24C | 0.9600 |
| O1···O2 | 3.218 (3) | C16···H4v | 2.9000 |
| O1···C8 | 3.052 (3) | C17···H4v | 2.8700 |
| O1···C10i | 3.150 (3) | C17···H10Cx | 2.8600 |
| O1···C22ii | 3.400 (4) | C18···H24B | 2.7900 |
| O1···C18iii | 3.355 (3) | C18···H10Cx | 3.0800 |
| O2···C24iii | 3.224 (4) | C18···H24A | 3.0800 |
| O2···C1 | 2.936 (3) | C19···H24B | 2.5300 |
| O2···C20iii | 3.322 (3) | C22···H15 | 3.0200 |
| O2···N4iii | 3.059 (3) | C24···H18 | 2.9500 |
| O2···C23iii | 3.329 (4) | C24···H22Ai | 2.9400 |
| O2···C18iii | 3.329 (3) | H3···C14vii | 2.8800 |
| O2···O1 | 3.218 (3) | H3···O3vii | 2.6000 |
| O3···C20 | 3.085 (3) | H3···N1 | 2.9200 |
| O3···C22i | 3.128 (3) | H3···C10 | 2.9800 |
| O4···C13 | 3.111 (4) | H3···C13vii | 3.0100 |
| O4···C6iv | 3.105 (4) | H4···C16vii | 2.9000 |
| O4···C5iv | 3.141 (4) | H4···C17vii | 2.8700 |
| O1···H21Bii | 2.8700 | H5···O4i | 2.5800 |
| O1···H22Cii | 2.8300 | H5···H23C | 2.4600 |
| O1···H9A | 2.3300 | H6···C11 | 2.7800 |
| O1···H18iii | 2.6600 | H6···H11B | 2.4400 |
| O1···H10Bi | 2.6000 | H6···O4i | 2.5100 |
| O1···H15ii | 2.7900 | H6···N2 | 2.9100 |
| O2···H12A | 2.3300 | H9A···O1 | 2.3300 |
| O2···H24Aiii | 2.5600 | H9B···H10C | 2.4500 |
| O2···H18iii | 2.4900 | H9C···H10B | 2.5700 |
| O2···H21Ci | 2.7600 | H10A···H11Civ | 2.5200 |
| O2···H23Biii | 2.7100 | H10A···C11iv | 2.9500 |
| O3···H11Biv | 2.9200 | H10A···C2 | 2.4500 |
| O3···H21A | 2.3300 | H10A···C3 | 2.6300 |
| O3···H3v | 2.6000 | H10B···H9C | 2.5700 |
| O3···H22Ci | 2.6100 | H10B···C13vii | 2.9500 |
| O4···H6iv | 2.5100 | H10B···O1iv | 2.6000 |
| O4···H23A | 2.3800 | H10C···H9B | 2.4500 |
| O4···H5iv | 2.5800 | H10C···C17xi | 2.8600 |
| O4···H12Avi | 2.8500 | H10C···C18xi | 3.0800 |
| N4···O2vi | 3.059 (3) | H10C···H17xi | 2.5300 |
| N1···H3 | 2.9200 | H11A···C6 | 2.6900 |
| N2···H6 | 2.9100 | H11A···C7 | 2.5400 |
| N3···H15 | 2.9200 | H11B···O3i | 2.9200 |
| C1···O2 | 2.936 (3) | H11B···C6 | 2.9800 |
| C3···C15vii | 3.593 (4) | H11B···H6 | 2.4400 |
| C3···C10 | 3.159 (4) | H11B···H22Bix | 2.4800 |
| C4···C16vii | 3.546 (4) | H11C···H10Ai | 2.5200 |
| C5···O4i | 3.141 (4) | H11C···H12B | 2.4000 |
| C6···C11 | 3.058 (4) | H12A···O2 | 2.3300 |
| C6···O4i | 3.105 (4) | H12A···O4iii | 2.8500 |
| C8···O1 | 3.052 (3) | H12B···H11C | 2.4000 |
| C8···C21i | 3.400 (4) | H15···O1viii | 2.7900 |
| C10···O1iv | 3.150 (3) | H15···N3 | 2.9200 |
| C10···C3 | 3.159 (4) | H15···C2viii | 2.8500 |
| C11···C6 | 3.058 (4) | H15···C22 | 3.0200 |
| C13···O4 | 3.111 (4) | H15···H22A | 2.5400 |
| C15···C3v | 3.593 (4) | H16···C4viii | 3.0800 |
| C15···C22 | 3.202 (4) | H16···C5viii | 2.8500 |
| C16···C4v | 3.546 (4) | H16···C6viii | 3.0500 |
| C18···O1vi | 3.355 (3) | H17···H10Cx | 2.5300 |
| C18···C24 | 3.157 (4) | H18···C24 | 2.9500 |
| C18···O2vi | 3.329 (3) | H18···O1vi | 2.6600 |
| C20···O3 | 3.085 (3) | H18···O2vi | 2.4900 |
| C20···O2vi | 3.322 (3) | H18···C1vi | 3.0700 |
| C21···C8iv | 3.400 (4) | H21A···O3 | 2.3300 |
| C22···C15 | 3.202 (4) | H21A···C12iv | 3.0300 |
| C22···O3iv | 3.128 (3) | H21B···O1viii | 2.8700 |
| C22···O1viii | 3.400 (4) | H21B···H22C | 2.5300 |
| C23···O2vi | 3.329 (4) | H21C···O2iv | 2.7600 |
| C24···O2vi | 3.224 (4) | H21C···C8iv | 2.8200 |
| C24···C18 | 3.157 (4) | H21C···H22B | 2.5400 |
| C1···H22Cii | 2.8800 | H22A···C14 | 2.4500 |
| C1···H18iii | 3.0700 | H22A···C15 | 2.5600 |
| C2···H15ii | 2.8500 | H22A···C24iv | 2.9400 |
| C2···H10A | 2.4500 | H22A···H15 | 2.5400 |
| C3···H10A | 2.6300 | H22A···H24Civ | 2.5000 |
| C4···H16ii | 3.0800 | H22B···C11xii | 3.0900 |
| C5···H16ii | 2.8500 | H22B···H11Bxii | 2.4800 |
| C6···H11B | 2.9800 | H22B···H21C | 2.5400 |
| C6···H16ii | 3.0500 | H22C···O1viii | 2.8300 |
| C6···H11A | 2.6900 | H22C···O3iv | 2.6100 |
| C7···H11A | 2.5400 | H22C···C1viii | 2.8800 |
| C8···H21Ci | 2.8200 | H22C···H21B | 2.5300 |
| C10···H3 | 2.9800 | H23A···O4 | 2.3800 |
| C11···H22Bix | 3.0900 | H23B···O2vi | 2.7100 |
| C11···H6 | 2.7800 | H23C···H5 | 2.4600 |
| C11···H10Ai | 2.9500 | H23C···H24C | 2.2900 |
| C12···H21Ai | 3.0300 | H24A···C18 | 3.0800 |
| C13···H10Bv | 2.9500 | H24A···O2vi | 2.5600 |
| C13···H3v | 3.0100 | H24B···C18 | 2.7900 |
| C14···H22A | 2.4500 | H24B···C19 | 2.5300 |
| C14···H3v | 2.8800 | H24C···H22Ai | 2.5000 |
| C15···H22A | 2.5600 | H24C···H23C | 2.2900 |
| C1—N1—C9 | 119.8 (2) | H12B—C12—H12C | 109.00 |
| C1—N1—C10 | 125.4 (2) | N2—C12—H12A | 110.00 |
| C9—N1—C10 | 114.7 (2) | N2—C12—H12B | 109.00 |
| C8—N2—C11 | 124.9 (2) | N2—C12—H12C | 109.00 |
| C8—N2—C12 | 119.6 (3) | H12A—C12—H12B | 109.00 |
| C11—N2—C12 | 115.6 (2) | H12A—C12—H12C | 110.00 |
| C13—N3—C22 | 124.7 (2) | O3—C13—N3 | 123.2 (3) |
| C21—N3—C22 | 115.2 (2) | O3—C13—C14 | 118.5 (2) |
| C13—N3—C21 | 120.0 (2) | N3—C13—C14 | 118.2 (2) |
| C23—N4—C24 | 116.3 (3) | C13—C14—C15 | 119.7 (2) |
| C20—N4—C23 | 118.4 (3) | C13—C14—C19 | 120.5 (2) |
| C20—N4—C24 | 124.2 (3) | C15—C14—C19 | 119.5 (3) |
| O1—C1—C2 | 118.6 (2) | C14—C15—C16 | 120.7 (3) |
| O1—C1—N1 | 123.9 (3) | C15—C16—C17 | 119.9 (3) |
| N1—C1—C2 | 117.5 (2) | C16—C17—C18 | 120.0 (3) |
| C1—C2—C3 | 119.7 (3) | C17—C18—C19 | 120.5 (2) |
| C1—C2—C7 | 120.2 (2) | C14—C19—C18 | 119.4 (3) |
| C3—C2—C7 | 119.8 (2) | C14—C19—C20 | 119.6 (2) |
| C2—C3—C4 | 120.4 (3) | C18—C19—C20 | 120.6 (2) |
| C3—C4—C5 | 120.0 (3) | O4—C20—N4 | 122.8 (3) |
| C4—C5—C6 | 120.1 (3) | O4—C20—C19 | 118.4 (3) |
| C5—C6—C7 | 120.2 (3) | N4—C20—C19 | 118.9 (3) |
| C6—C7—C8 | 121.0 (3) | C14—C15—H15 | 120.00 |
| C2—C7—C6 | 119.4 (2) | C16—C15—H15 | 120.00 |
| C2—C7—C8 | 119.3 (2) | C15—C16—H16 | 120.00 |
| N2—C8—C7 | 118.2 (2) | C17—C16—H16 | 120.00 |
| O2—C8—N2 | 123.3 (3) | C16—C17—H17 | 120.00 |
| O2—C8—C7 | 118.5 (2) | C18—C17—H17 | 120.00 |
| C2—C3—H3 | 120.00 | C17—C18—H18 | 120.00 |
| C4—C3—H3 | 120.00 | C19—C18—H18 | 120.00 |
| C3—C4—H4 | 120.00 | N3—C21—H21A | 109.00 |
| C5—C4—H4 | 120.00 | N3—C21—H21B | 109.00 |
| C6—C5—H5 | 120.00 | N3—C21—H21C | 109.00 |
| C4—C5—H5 | 120.00 | H21A—C21—H21B | 110.00 |
| C5—C6—H6 | 120.00 | H21A—C21—H21C | 109.00 |
| C7—C6—H6 | 120.00 | H21B—C21—H21C | 110.00 |
| N1—C9—H9B | 109.00 | N3—C22—H22A | 109.00 |
| N1—C9—H9A | 109.00 | N3—C22—H22B | 109.00 |
| H9A—C9—H9C | 109.00 | N3—C22—H22C | 109.00 |
| N1—C9—H9C | 110.00 | H22A—C22—H22B | 109.00 |
| H9A—C9—H9B | 109.00 | H22A—C22—H22C | 109.00 |
| H9B—C9—H9C | 109.00 | H22B—C22—H22C | 109.00 |
| N1—C10—H10C | 109.00 | N4—C23—H23A | 109.00 |
| N1—C10—H10A | 109.00 | N4—C23—H23B | 109.00 |
| N1—C10—H10B | 109.00 | N4—C23—H23C | 109.00 |
| H10A—C10—H10C | 110.00 | H23A—C23—H23B | 110.00 |
| H10B—C10—H10C | 109.00 | H23A—C23—H23C | 109.00 |
| H10A—C10—H10B | 109.00 | H23B—C23—H23C | 110.00 |
| H11A—C11—H11B | 109.00 | N4—C24—H24A | 109.00 |
| H11A—C11—H11C | 109.00 | N4—C24—H24B | 109.00 |
| N2—C11—H11A | 109.00 | N4—C24—H24C | 109.00 |
| N2—C11—H11B | 110.00 | H24A—C24—H24B | 109.00 |
| N2—C11—H11C | 109.00 | H24A—C24—H24C | 109.00 |
| H11B—C11—H11C | 109.00 | H24B—C24—H24C | 109.00 |
| C9—N1—C1—O1 | 3.8 (4) | C3—C4—C5—C6 | −1.4 (4) |
| C10—N1—C1—O1 | −173.5 (3) | C4—C5—C6—C7 | 0.7 (4) |
| C9—N1—C1—C2 | −172.2 (2) | C5—C6—C7—C8 | 175.2 (3) |
| C10—N1—C1—C2 | 10.5 (4) | C5—C6—C7—C2 | 1.3 (4) |
| C12—N2—C8—C7 | −173.4 (2) | C6—C7—C8—N2 | 55.4 (4) |
| C11—N2—C8—O2 | −177.3 (3) | C2—C7—C8—O2 | 51.7 (4) |
| C12—N2—C8—O2 | 4.1 (4) | C2—C7—C8—N2 | −130.7 (3) |
| C11—N2—C8—C7 | 5.2 (4) | C6—C7—C8—O2 | −122.3 (3) |
| C21—N3—C13—O3 | −6.4 (4) | O3—C13—C14—C19 | −58.4 (4) |
| C22—N3—C13—O3 | 168.5 (3) | N3—C13—C14—C15 | −60.6 (4) |
| C21—N3—C13—C14 | 169.5 (2) | N3—C13—C14—C19 | 125.6 (3) |
| C22—N3—C13—C14 | −15.7 (4) | O3—C13—C14—C15 | 115.4 (3) |
| C23—N4—C20—O4 | −8.3 (4) | C15—C14—C19—C18 | 1.0 (4) |
| C23—N4—C20—C19 | 171.2 (3) | C15—C14—C19—C20 | 174.1 (3) |
| C24—N4—C20—C19 | 3.6 (4) | C19—C14—C15—C16 | −0.7 (4) |
| C24—N4—C20—O4 | −175.9 (3) | C13—C14—C19—C18 | 174.9 (3) |
| O1—C1—C2—C3 | −114.6 (3) | C13—C14—C15—C16 | −174.6 (3) |
| O1—C1—C2—C7 | 59.1 (4) | C13—C14—C19—C20 | −12.1 (4) |
| N1—C1—C2—C3 | 61.6 (4) | C14—C15—C16—C17 | −0.2 (5) |
| N1—C1—C2—C7 | −124.7 (3) | C15—C16—C17—C18 | 0.8 (5) |
| C7—C2—C3—C4 | 2.0 (4) | C16—C17—C18—C19 | −0.4 (4) |
| C1—C2—C7—C6 | −176.4 (3) | C17—C18—C19—C14 | −0.5 (4) |
| C1—C2—C3—C4 | 175.8 (3) | C17—C18—C19—C20 | −173.4 (3) |
| C1—C2—C7—C8 | 9.5 (4) | C14—C19—C20—N4 | 119.4 (3) |
| C3—C2—C7—C6 | −2.7 (4) | C18—C19—C20—O4 | 111.9 (3) |
| C3—C2—C7—C8 | −176.7 (3) | C18—C19—C20—N4 | −67.7 (4) |
| C2—C3—C4—C5 | 0.0 (4) | C14—C19—C20—O4 | −61.1 (4) |
Symmetry codes: (i) x+1, y, z; (ii) x+1, y, z−1; (iii) −x+1, y+1/2, −z+1; (iv) x−1, y, z; (v) x, y, z+1; (vi) −x+1, y−1/2, −z+1; (vii) x, y, z−1; (viii) x−1, y, z+1; (ix) x+2, y, z; (x) −x, y−1/2, −z+1; (xi) −x, y+1/2, −z+1; (xii) x−2, y, z.
Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the C2–C7 ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O3vii | 0.93 | 2.60 | 3.518 (3) | 170 |
| C5—H5···O4i | 0.93 | 2.58 | 3.141 (4) | 119 |
| C6—H6···O4i | 0.93 | 2.51 | 3.105 (4) | 122 |
| C18—H18···O2vi | 0.93 | 2.49 | 3.329 (3) | 150 |
| C24—H24A···O2vi | 0.96 | 2.56 | 3.224 (4) | 127 |
| C16—H16···Cg1 | 0.93 | 2.97 | 3.810 (4) | 124 |
Symmetry codes: (i) x+1, y, z; (vi) −x+1, y−1/2, −z+1; (vii) x, y, z−1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BQ2372).
References
- Altamura, M., Coppini, G., Cuda, F., Dapporto, P., Guerri, A., Guidi, A., Nativi, C., Paoli, P. & Rossi, P. (2005). J. Mol. Struct. 749, 20–30.
- Anderson, R. J., Batsanov, A. S., Belskaia, N., Groundwater, P. W., Meth-Cohn, O. & Zaytsev, A. (2004). Tetrahedron Lett. 45, 943–946.
- Bruker (2006). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Clayden, J., Lai, L. W. & Helliwell, M. (2001). Tetrahedron Asymmetry, 12, 695–698.
- Comins, D. L., Lee, Y. S. & Boyle, P. D. (1998). Tetrahedron Lett., 39, 187–190.
- Dowty, E. (1995). ATOMS Shape Software, Kingsport, Tennessee, USA.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Sakamoto, M., Kobaru, S., Mino, T. & Fujita, T. (2004). Chem. Commun. pp. 1002–1003. [DOI] [PubMed]
- Sheldrick, G. M. (2002). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812035167/bq2372sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812035167/bq2372Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812035167/bq2372Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report