Abstract
AIM: To evaluate the efficacy and safety of the FOLFIRI regimen in patients with metastatic pancreatic adenocarcinoma (PAC) after the failure of gemcitabine and platinum salts.
METHODS: All consecutive patients with histologically confirmed, metastatic PAC and World Health Organization performance status (PS) ≤ 2 received FOLFIRI-1 [irinotecan 180 mg/m2 on day 1 and leucovorin 400 mg/m2 followed by 5-fluorouracil (5-FU) 400 mg/m2 bolus, then 5-FU 2400 mg/m2 as a 46-h infusion, biweekly] or FOLFIRI-3 (irinotecan 100 mg/m2 on day 1 and leucovorin 400 mg/m2, then 5-FU 2400 mg/m2 as a 46-h infusion and irinotecan 100 mg/m2 repeated on day 3, biweekly) after failure of gemcitabine and platinum-based chemotherapies as a systematic policy in two institutions between January 2005 and May 2010. Tumor response, time to progression (TTP), overall survival rate (OS) and grade 3-4 toxicities were retrospectively studied. Subgroup analyses were performed to search for prognostic factors.
RESULTS: Sixty-three patients (52.4% male, median age 59 years) were analyzed. Among them, 42.9% were PS 0, 38.1% were PS 1 and 19.0% were PS 2. Fifty one patients (81.0%) had liver metastases. Before the FOLFIRI regimen, patients had received 1 line (n = 19), 2 lines (n = 39) or 3 lines (n = 5) of chemotherapy. Median TTP obtained with the line before FOLFIRI was 3.9 mo (95% CI: 3.4-5.3 mo). A total of 480 cycles was completed (median: 6 cycles, range: 1-51 cycles). The main reason for discontinuing FOLFIRI was tumor progression (90.3%). Tumor control was achieved in 25 patients (39.7%) (partial response: n = 5, stable disease: n = 20) with FOLFIRI. Median TTP was 3.0 mo (95% CI: 2.1-3.9 mo) and median OS was 6.6 mo (95% CI: 5.3-8.1 mo). Dose adaptation was required in 36 patients (57.1%). Fifteen patients (23.8%) had grade 3-4 toxicities, mainly hematological (n = 11) or digestive (n = 4). Febrile neutropenia occurred in 3 patients. There was no toxic death. PS 2 was significantly associated with poor TTP [hazard ratio (HR): 16.036, P < 0.0001] and OS (HR: 4.003, P = 0.004).
CONCLUSION: The FOLFIRI regimen had an acceptable toxicity and an interesting efficacy in our study, limited to patients in good condition (PS 0-1).
Keywords: Pancreatic cancer, Pancreatic adenocarcinoma, Metastases, Chemotherapy, 5-fluorouracil, Irinotecan, Camptothecin, FOLFIRI regimen
INTRODUCTION
Pancreatic adenocarcinoma (PAC) accounts for 2%-3% of all cancers but is the fourth leading cause of cancer death in Western countries[1]. More than 80% of patients present with unresectable disease and most of those with operable tumors who undergo resection have local relapse or metastases[2]. The overall prognosis of metastatic PAC remains poor, with a 5-year survival rate of less than 5%[3].
Gemcitabine became the reference regimen as first-line chemotherapy in patients with metastatic PAC after a randomized trial showed significant improvement in median overall survival (OS) compared with 5-fluorouracil (5-FU) (5.6 mo vs 4.4 mo, P = 0.002)[4]. Over the past decade, multiple phase II and III studies have attempted to improve these results using various combinations of gemcitabine with other agents but no significant benefit on survival has been found compared with gemcitabine alone, except for erlotinib which resulted in a modest but significant improvement in OS (6.2 mo vs 5.9 mo, P = 0.038)[5,6]. A phase III trial comparing the FOLFIRINOX regimen (folinic acid/5-FU, irinotecan and oxaliplatin combination) to gemcitabine as first-line treatment for metastatic PAC showed that this combination was superior to gemcitabine (OS: 11.1 mo vs 6.8 mo, P < 0.001)[7].
In clinical practice, about half of metastatic PAC patients with disease progression under gemcitabine treatment remain in acceptable clinical condition and thus may receive subsequent line(s) of chemotherapy. A retrospective series of 117 patients evaluated the feasibility and benefits of second- and third-line chemotherapies in patients with metastatic PAC after the failure of gemcitabine[8]. Fifty three (45%) received two lines and 24 (21%) received three or more lines. Median time to progression (TTP) and OS from the beginning of the second line were 2.3 mo and 4.7 mo, respectively. The FFCD 0301 phase III trial was the first randomized study to evaluate a chemotherapy strategy with a second line of treatment in the treatment plan. It compared the combination of folinic acid/5-FU and cisplatin followed by gemcitabine or the reverse sequence in metastatic PAC[9]. The second line of therapy was administered at disease progression to 68% of patients who received folinic acid/5-FU and cisplatin as a first line treatment and to 55% in the gemcitabine arm (non-significant). Median progression-free survival (PFS) and OS in the two arms were not significantly different. Although there is no standard regimen in this setting, two randomized studies have indicated that the combination of folinic acid/5-FU and oxaliplatin appeared to be superior to both best supportive care (4.9 mo vs 2.3 mo, P = 0.008) and folinic acid/5-FU alone (6.0 mo vs 3.0 mo, P = 0.014) as a second line of treatment[10]. Other regimens have been tested in non-randomized phase II studies, but the samples were small and information on World Health Organization (WHO) performance status (PS) and disease stage were often lacking[11].
Preclinical studies have shown that the camptothecin analog irinotecan has significant activity in both cultured pancreatic tumor cells and in xenograft models[12,13]. Irinotecan monotherapy in previously untreated PAC patients yielded response rates (RR) of 9%-27%[14-16]. In vitro studies have suggested that there is a synergistic effect between irinotecan and 5-FU[17-19]. A multicenter phase II study with folinic acid/5-FU and irinotecan day 1/day 3 combination (FOLFIRI-3 regimen) showed promising activity in chemotherapy-naive patients with locally advanced or metastatic PAC, with a median PFS and OS of 5.6 mo and 12.1 mo, respectively, and a manageable toxicity profile[20]. A randomized phase II study has compared modified FOLFIRI-3 and a modified FOLFOX schema (folinic acid/5-FU and oxaliplatin combination) as second-line chemotherapy in that setting[21]. The efficacy was similar, with 6-mo OS rate of 27% and 30%, respectively. An Italian group reported a retrospective series of 40 patients with gemcitabine-resistant locally advanced or metastatic PAC treated with a standard FOLFIRI (FOLFIRI-1, folinic acid/5-FU and irinotecan day 1 combination) regimen[22]. Median TTP was 3.7 mo and median OS was 6 mo.
Because no data exist on the efficacy of the FOLFIRI regimen after the failure of both gemcitabine and platinum salts, we performed a retrospective study to evaluate the efficacy and safety of this regimen in patients with advanced PAC in that setting. As locally advanced PAC may have a more favorable natural history than metastatic PAC, we decided to exclude locally advanced PAC patients from the study to have a homogeneous population.
MATERIALS AND METHODS
Patients
All patients with histologically confirmed, metastatic PAC, after failure (progression or major toxicity) of gemcitabine and platinum-based chemotherapies, received an irinotecan-based regimen as a systematic policy after discussion during a weekly multidisciplinary meeting in our institutions (Saint Antoine Hospital, Paris and Beaujon Hospital, Clichy), if they met the following criteria: previous treatment with gemcitabine and platinum salt (combined or given in consecutive lines); WHO PS ≤ 2; at least one bidimensionally measurable lesion according the Response Evaluation Criteria in Solid Tumors (RECIST); absence of severe uncontrolled cardiovascular, metabolic, infectious or renal disease; serum bilirubin level < 1.5 times the upper limit of normal; polynuclear neutrophil count > 1500/mm3; platelet count > 100 000/mm3.
Chemotherapy regimen
The FOLFIRI-1 regimen consisted of irinotecan 180 mg/m2 administered as a 90-min infusion on day 1, together with leucovorin 400 mg/m2 for 2 h followed by an 5-FU 400 mg/m2 bolus, then a 46-h infusion of 5-FU 2400 mg/m2. The FOLFIRI-3 regimen consisted of irinotecan 100 mg/m2 administered as a 60-min infusion on day 1, together with leucovorin 400 mg/m2 for 2 h, then a 46-h infusion (without bolus administration) of 5-FU 2400 mg/m2 and irinotecan 100 mg/m2 repeated on day 3 at the end of 5-FU infusion. Only FOLFIRI-3 (intensified) regimen has been evaluated in phase II studies in PAC[20,21]. However, the FOLFIRI-1 regimen is extensively used in clinical practice for treatment of other gastrointestinal cancers and seems to be less toxic. Thus, the choice between the FOLFIRI-1 or FOLFIRI-3 regimen was left up to the discretion of the investigator. The chemotherapy cycles were repeated every two weeks if the clinical and biochemical assessment was compatible (as mentioned above).
Patients who developed a cholinergic syndrome received preventive treatment with atropine (0.25 mg subcutaneously) during all subsequent cycles. Late-onset diarrhea was treated using high-dose loperamide. When severe neutropenia occurred and/or did not recover to grade ≤ 1 on day 14, a granulocyte-colony stimulating factor was given.
The irinotecan and the 5-FU dosages were reduced by 20% when any grade 3-4 toxicity occurred; other dose adjustments were decided on an individual basis. Treatment was stopped when the tumor progressed or severe toxicity occurred, or at the patient’s request. Further treatments are discussed on an individual basis.
Assessment of therapeutic efficacy
Treatment efficacy was assessed on a clinical evaluation, carbohydrate antigen (CA) 19-9 serum levels and thoraco-abdominal computed tomography (CT). Assessment of treatment efficacy was performed every 2 mo (four cycles) or earlier in patients with clinically suspected progression. Tumor response was assessed using CT according to RECIST[23]. A complete response (CR) was defined as complete disappearance of all assessable disease, partial response (PR) as a decrease of > 30% in the sum of the largest diameters of target lesions, stable disease (SD) as a decrease of < 30% or an increase of < 20% in measurable lesions, and progressive disease (PD) as an increase of > 20% in measurable lesions or the appearance of new malignant lesions. Patients who were not assessable by CT but who presented clinical and/or biochemical (CA 19-9 serum level elevation) evidence of disease progression or who died from a cancer-related cause were also considered as PD. The sum of CR, PR and SD was reported as the tumor control rate (TCR). The sum of CR and PR was reported as overall RR (ORR). OS was defined as the time from the first day of the FOLFIRI regimen to the date of death (all causes) or last follow-up. TTP was defined as the time from the first day of the FOLFIRI regimen to the date of disease progression. Patients without progression were censored at the last follow-up.
Safety
Toxicity was assessed before each cycle with the National Cancer Institute Common Toxicity Criteria (version 3.0). A complete physical examination was performed and a full blood count and serum bilirubin, aminotransferases, alkaline phosphatase and creatinine assays were obtained before each treatment cycle.
Data collection
The following patient data were collected and analyzed retrospectively: age; gender; primary tumor location; stage at the time of diagnosis; previous surgery and/or radiotherapy; previous lines of chemotherapy; TTP with the previous line; reasons for stopping previous line; presence or absence of liver metastases at the beginning of FOLFIRI regimen; PS at the beginning of FOLFIRI regimen; type of FOLFIRI regimen (FOLFIRI-1 or FOLFIRI-3); number of cycles administered; best tumor response; grade 3-4 toxicities; dose adaptation; TTP and OS from the beginning of FOLFIRI regimen; reasons for stopping FOLFIRI regimen; further treatments.
Statistical analysis
All analyses were performed using Stata software (version 11.0; StataCorp). All statistical tests were two sided with an alpha type one error of 5%. TTP and OS were estimated using the Kaplan-Meier method and described using median or rate of TTP/OS at a specific time point with 95% CI. Log-rank tests were used to compare survival curves.
Univariate and multivariate Cox proportional hazard model analyses were performed with the following variables: stage at diagnosis; previous treatment by radiotherapy; number of previous chemotherapy lines; presence or absence of liver metastases; PS at the beginning of FOLFIRI regimen.
For exploratory purposes, subgroup analyses were performed according to the following variables: primary tumor location; stage at the diagnosis; previous treatment by surgery or radiotherapy; number of previous chemotherapy lines; presence or absence of liver metastases; PS at the beginning of FOLFIRI regimen; type of FOLFIRI regimen (FOLFIRI-1 or FOLFIRI-3).
RESULTS
Patients
Between January 2005 and May 2010, 63 patients with metastatic PAC fulfilled the criteria for this study. Their characteristics are shown in Table 1. Median age was 59 years (range: 24-81 years). Thirty three patients (52.4%) were male. The primary tumor was located in the head of the pancreas in 32/50 patients (64.0%). Twenty three patients (36.5%) had undergone prior surgery and 16 patients (25.4%) had received chemoradiotherapy. Twenty-seven patients (42.9%) were WHO PS 0, 24 patients (38.1%) were PS 1 and 12 patients (19.0%) were PS 2 at the beginning of the FOLFIRI regimen. Fifty one patients (81.0%) had liver metastases.
Table 1.
Characteristics of the 63 patients with metastatic pancreatic cancer treated with FOLFIRI after failure of gemcitabine and platinum-salts
| Characteristics | Data |
| Age (yr) | |
| Median | 59 (range: 24-81) |
| Sex (%) | |
| Male | 33 (52.4) |
| Performans status (%) | |
| PS 0 | 27 (42.9) |
| PS 1 | 24 (38.1) |
| PS 2 | 12 (19.0) |
| Liver metastases (%) | |
| Present | 51 (81.0) |
| Number of previous lines before FOLFIRI (%) | |
| 1 | 19 (30.2) |
| 2 | 39 (61.9) |
| ≥ 3 | 5 (7.9) |
Before receiving the FOLFIRI regimen, patients had received one line (gemcitabine-oxaliplatin: n = 19, 30.2%), two lines (gemcitabine then FOLFOX regimen: n = 39, 61.9%) or three lines (n = 5, 7.9%) of chemotherapy. The previous line had been stopped for tumor progression in 55 patients (87.3%) and due to toxicity (oxaliplatin-related neuropathy) in the remaining 8 patients (12.7%).
Study treatment and drug delivery
Fifty five patients (87.3%) received the FOLFIRI-1 regimen and 8 patients (12.7%) received the FOLFIRI-3 regimen. A total of 480 cycles was completed (median: 6 cycles per patient, range: 1-51 cycles per patient).
The reasons for discontinuing the FOLFIRI regimen was progression in 56/62 patients (90.3%), toxicity in one patient (febrile neutropenia: n = 1, 1.6%), a tumor control ≥ 6 mo or at the patient’s request in 4 patients (6.5%), surgery in one patient (1.6%) who had a major response. Sixteen patients (25.4%) who remained in good condition at the time of FOLFIRI withdrawal received a subsequent line following the multidisciplinary proposal.
Tumor response and survival
Median TTP obtained with the last line of chemotherapy before FOLFIRI was 3.9 mo (95% CI: 3.4-5.3 mo). Tumor control was obtained with the FOLFIRI regimen in 25 patients (39.7%) (CR: n = 0; PR: n = 5, 7.9%; SD: n = 20, 31.8%). ORR was 7.9% (5/63). Median TTP was 3.0 mo (95% CI: 2.1-3.9 mo) and median OS was 6.6 mo (95% CI: 5.3-8.1 mo) (Figure 1A and B).
Figure 1.
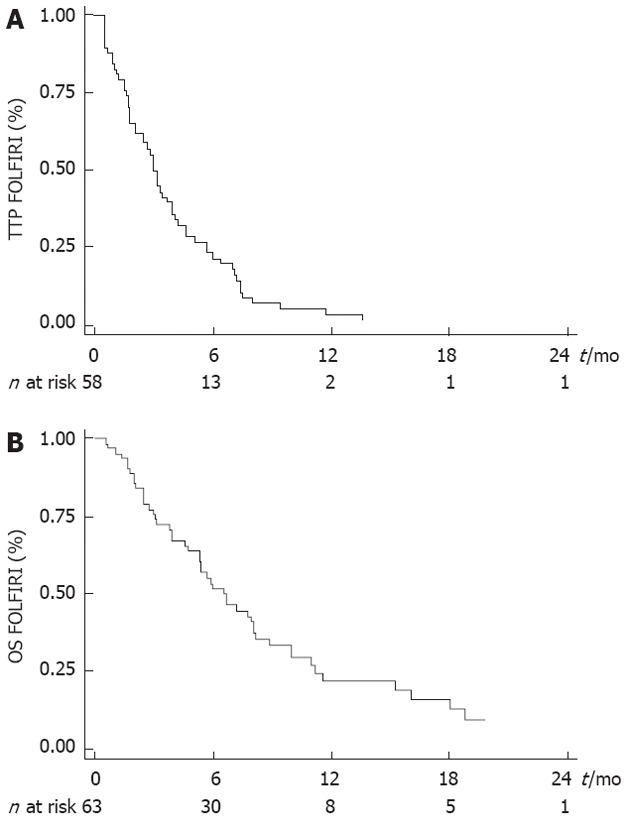
Time to progression (A) and overall survival (B) from the beginning of FOLFIRI for the 63 patients. TTP: Time to progression; OS: Overall survival.
Subgroup analysis
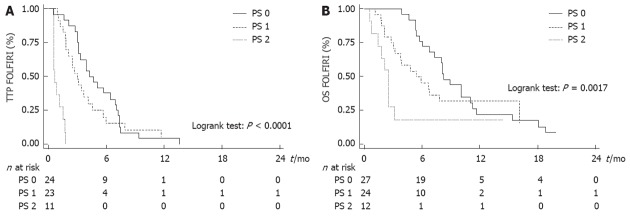
WHO PS was the only variable that was significantly associated with TTP and OS (Tables 2 and 3). Median TTP was 4.2 mo (95% CI: 3.2-7.0 mo) in PS 0 patients, 3.0 mo (95% CI: 1.8-4.1 mo) in PS 1 patients and 0.7 mo (95% CI: 0.5-1.5 mo) in PS 2 patients (Figure 2A). Median OS after the beginning of FOLFIRI was 8.2 mo (95% CI: 6.7-11.0 mo) in PS 0 patients, 5.4 mo (95% CI: 3.0-16.1 mo) in PS 1 patients and 2.5 mo (95% CI: 0.7-3.1 mo) in PS 2 patients (Figure 2B). PS 2 was significantly associated with a poor TTP [hazard ratio (HR) = 16.036, P < 0.0001] and OS (HR = 4.003, P = 0.004) in univariate analysis and in multivariate analysis also. No significant association was found between other variables and survival, except for the number of previous lines with TTP in multivariate analysis (Tables 2 and 3).
Table 2.
Results of univariate and multivariate analysis for time to progression with FOLFIRI
| Progression | Univariate analysis | Multivariate analysis | |||||
| Yes/no (57/1) | HR | 95% CI | P value | HR | 95% CI | P value | |
| Primary tumor location1 | 0.687 | ||||||
| Body or tail | 18/0 | 1 | |||||
| Head | 28/1 | 0.883 | (0.483-1.615) | ||||
| Stage at the diagnosis | 0.666 | 0.652 | |||||
| Resectable | 22/0 | 1 | 1 | ||||
| Locally advanced | 15/1 | 1.235 | (0.631-2.418) | 1.394 | (0.662-2.939) | ||
| Metastatic | 20/0 | 0.905 | (0.490-1.674) | 1.299 | (0.626-2.695) | ||
| Previous surgery | 0.659 | ||||||
| No | 37/1 | 1 | |||||
| Yes | 20/0 | 0.882 | (0.506-1.539) | ||||
| Previous radiotherapy | 0.411 | 0.168 | |||||
| No | 44/0 | 1 | 1 | ||||
| Yes | 13/1 | 1.305 | (0.692-2.459) | 1.770 | (0.786-3.987) | ||
| Number of previous chemotherapy lines | 0.284 | 0.026 | |||||
| 1 | 16/0 | 1 | 1 | ||||
| 2 | 36/1 | 0.649 | (0.350-1.204) | 0.170 | 0.385 | (0.193-0.770) | |
| 3 | 5/0 | 1.128 | (0.411-3.096) | 0.814 | 0.554 | (0.177-1.735) | |
| Liver metastases | 0.531 | 0.564 | |||||
| No | 9/0 | 1 | 1 | ||||
| Yes | 48/1 | 0.793 | (0.383-1.640) | 1.298 | (0.535-3.151) | ||
| WHO performans status | < 0.0001 | < 0.0001 | |||||
| 0 | 24/0 | 1 | 1 | ||||
| 1 | 22/1 | 1.325 | (0.731-2.399) | 1.431 | (0.745-2.748) | ||
| 2 | 11/0 | 16.036 | (5.926-43.394) | 29.255 | (9.278-92.248) | ||
| Type of FOLFIRI regimen | 0.124 | ||||||
| FOLFIRI-1 | 50/0 | 1 | |||||
| FOLFIRI-3 | 7/1 | 0.503 | (0.210-1.207) | ||||
HR: Hazard ratio; WHO: World Health Organization.
Data available in 47 out of 58 cases.
Table 3.
Results of univariate and multivariate analysis for overall survival with FOLFIRI
| Death | Univariate analysis | Multivariate analysis | |||||
| Yes/no (49/14) | HR | 95% CI | P value | HR | 95% CI | P value | |
| Primary tumor location1 | 0.306 | ||||||
| Body or tail | 16/2 | 1 | |||||
| Head | 25/7 | 0.717 | (0.380-1.355) | ||||
| Stage at the diagnosis | 0.754 | 0.644 | |||||
| Resectable | 20/4 | 1 | 1 | ||||
| Locally advanced | 13/6 | 1.012 | (0.497-2.058) | 0.790 | (0.346-1.806) | ||
| Metastatic | 16/4 | 1.271 | (0.643-2.515) | 1.205 | (0.577-2.514) | ||
| Previous surgery | 0.203 | ||||||
| No | 31/9 | 1 | |||||
| Yes | 18/5 | 0.676 | (0.370-1.236) | ||||
| Previous radiotherapy | 0.916 | 0.935 | |||||
| No | 38/9 | 1 | 1 | ||||
| Yes | 11/5 | 0.964 | (0.491-1.894) | 0.965 | (0.412-2.260) | ||
| Number of previous chemotherapy lines | 0.102 | 0.171 | |||||
| 1 | 14/5 | 1 | 1 | ||||
| 2 | 30/9 | 1.234 | (0.649-2.346) | 1.197 | (0.564-2.538) | ||
| 3 | 5/0 | 3.188 | (1.090-9.326) | 2.945 | (0.930-9.324) | ||
| Liver metastases | 0.599 | 0.957 | |||||
| No | 9/3 | 1 | 1 | ||||
| Yes | 40/11 | 1.217 | (0.586-2.528) | 0.978 | (0.444-2.159) | ||
| WHO performance status | 0.004 | 0.004 | |||||
| 0 | 22/5 | 1 | 1 | ||||
| 1 | 18/6 | 1.360 | (0.719-2.573) | 1.284 | (0.650-2.535) | ||
| 2 | 9/3 | 4.003 | (1.770-9.056) | 4.702 | (1.883-11.741) | ||
| Type of FOLFIRI regimen | 0.856 | ||||||
| FOLFIRI-1 | 42/13 | 1 | |||||
| FOLFIRI-3 | 7/1 | 1.083 | (0.458-2.562) | ||||
HR: Hazard ratio; WHO: World Health Organization.
Data available in 50 out of 63 cases.
Figure 2.
Subgroup analysis according to the World Health Organization performance status. A: Time to progression (TTP) according to World Health Organization (WHO) performance status (PS). B: Overall survival (OS) according to WHO PS.
Dose adaptation and safety
Dose adaptation was required in 36 patients (57.1%). The initial dose was reduced in 20 patients (31.7%) (19 patients who received the FOLFIRI-1 regimen and one patient who received the FOLFIRI-3 regimen) for the following reasons: cholestasis (n = 11), PS 2 (n = 8) or age > 75 years (n = 2), pre-existent diarrhea (n = 2) or mucositis (n = 1), and an episode of grades 3-4 hematological toxicity during previous chemotherapy (n = 1). A subsequent reduction was proposed in 19 patients (30.2%) (18 patients who received the FOLFIRI-1 regimen and one patient who received the FOLFIRI-3 regimen). Fifteen (23.8%) of these patients had grade 3-4 toxicities, mainly hematological (n = 11, 17.5%) and/or digestive with diarrhea and/or mucositis (n = 4, 6.3%). Febrile neutropenia occurred in 3 patients (4.8%). There were no related deaths.
DISCUSSION
We have evaluated the efficacy and safety of the FOLFIRI regimen after the failure of both gemcitabine and platinum salts in 63 patients with metastatic PAC treated in two centers. Tumor control was obtained in 39.7% of patients. The median TTP was 3.0 mo and the median OS after the beginning of FOLFIRI was 6.6 mo. Toxicity was frequent with the FOLFIRI regimen (grade 3-4 toxicities in 23.8% of patients, mainly hematological and digestive) but manageable as only one patient had to stop treatment. In the subgroup analysis, the WHO PS was the only variable that was significantly associated with TTP (HR = 16.036, P < 0.0001) and OS (HR = 4.003, P = 0.004). Patients with WHO PS 2 may not benefit from this regimen.
Irinotecan-based chemotherapies have previously been tested in advanced PAC. Irinotecan was tested as a single agent in the first line setting in three phase II trials with interesting results, showing an ORR of 9%-27% and a median OS of 5.2-7.3 mo[14-16]. However, two phase III trials that tested irinotecan combined with gemcitabine as first-line chemotherapy did not show a significant benefit in TTP (2.8-3.5 mo) and OS (6.4-6.6 mo), despite a higher response rate than with standard gemcitabine (ORR: 15%-16%)[24,25]. A randomized phase II study confirmed that the antitumoral activity of the combination of gemcitabine and irinotecan is similar to a regimen of fixed dose gemcitabine, gemcitabine/cisplatin or gemcitabine/docetaxel[26]. Thus, the gemcitabine/irinotecan combination does not seem to be synergistic. In contrast, the efficacy of the combination of irinotecan and 5-FU has been shown to be interesting with acceptable toxicity (Table 4)[20,27-29]. This is supported by in vitro and in vivo data showing synergy between these two drugs[17-19]. These regimens have not yet been tested in a phase III trial compared with gemcitabine. Recently, a phase III trial comparing the FOLFIRINOX regimen (folinic acid/5-FU, irinotecan and oxaliplatin combination) to gemcitabine as first-line treatment for metastatic PAC showed a significant improvement in survival with the FOLFIRINOX regimen with a median PFS and OS of 6.8 mo and 11.1 mo, respectively[7]. Toxicity was significant (grade 3-4 in 54% the patients) but manageable, and no toxic death occurred. Patients included in this study were in good condition (WHO PS 0-1). In addition, an absence of cholestasis was required for inclusion which probably explains the unusually high rate of body/tail tumor localization.
Table 4.
PhaseI-II studies of regimens as first and second lines chemotherapy in advanced pancreatic cancer
| Regimen | Number of patients | TCR/ORR (%/%) | TTP or PFS (mo) | OS (mo) |
| Phase I-II studies1 | ||||
| Irinotecan/gemcitabine/5-FU[27] | 30 | 43/7 | 3.4 | 8.3 |
| G-FLIP (Irinotecan/gemcitabine/5-FU/cisplatin)[28] | 31 | 68/26 | 6.1 | 8.1 |
| FOLFIRI-3 (Irinotecan/5-FU)[20] | 40 | 65/38 | 5.6 | 12.1 |
| Irinotecan/S1[29] | 16 | 75/44 | 4.9 | 11.3 |
| Phase II studies2 | ||||
| Irinotecan/raltitrexed[32] | 19 | 53/16 | 4.0 | 6.5 |
| IROX (Irinotecan/oxaliplatin)[33] | 30 | 33/10 | 4.1 | 5.9 |
| IROX (Irinotecan/oxaliplatin)[34] | 14 | 50/21 | 1.4 | 4.1 |
| G-FLIP (Irinotecan/gemcitabine/5-FU/cisplatin)[35] | 34 | 44/24 | 3.9 | 10.3 |
| Irinotecan/docetaxel[36] | 14 | 21/0 | 1.2 | 4.4 |
| MDI (Irinotecan/mitomycin/docetaxel)[37] | 15 | 20/0 | 1.7 | 6.1 |
Phases I-II studies of irinotecan and 5-fluorouracil (5-FU)-based regimens as first line chemotherapy;
Phase II studies of irinotecan-based regimens as second line chemotherapy. TCR: Tumor control rate; ORR: Overall response rate; TTP: Time to progression; PFS: Progression free survival; OS: Overall survival.
Irinotecan as a single agent has been shown to be a well-tolerated but marginally effective regimen in gemcitabine-pretreated patients. ORR was less than 10% and median OS did not exceed 4-6.6 mo[30,31]. The results with irinotecan-based combination regimens in gemcitabine-resistant advanced PAC were conflicting (Table 4)[32-37]. Data on irinotecan and 5-FU combination regimens in this setting are scarce. A randomized phase II study evaluated modified FOLFIRI-3 vs modified FOLFOX (folinic acid/5-FU and oxaliplatin combination) in patients with gemcitabine-resistant advanced PAC. Efficacy was comparable with both regimens with a 6-mo OS rate of 27% (95% CI: 13%-46%) and 30% (95% CI: 15%-49%), respectively[21]. An Italian group reported a retrospective series of 40 patients with gemcitabine-resistant locally advanced or metastatic PAC treated with the standard FOLFIRI-1 regimen[22]. As in our series, most patients were PS 0-1 (82.5%); 17.5% of patients had locally advanced PAC, while all patients in our series were metastatic. The efficacy was quite similar to our series: TCR: 50%, ORR: 15%, median TTP: 3.7 mo and median OS: 6 mo. In contrast, toxicity was higher, with 27% and 32% of grades 3-4 hematological and digestive toxicities respectively. Toxicity was more frequent in PS 2 patients (71%) than in PS 0-1 patients (45%). The difference in the incidence of severe toxicity between the two series was not due to a difference in the proportion of PS 2 patients (17.5% vs 19%). One explanation might be that the initial dose was frequently adapted in our series (31.7%), particularly in PS 2 patients (8/12, 66.7%). In contrast, there was no tumor control at 6 mo in a study using a combination of irinotecan and fluoropyrimidine (mostly capecitabine) in 34 patients, most of whom where PS 0-1, and only 6% of patients were alive 1 year after the beginning of this chemotherapy regimen[38]. Most patients (97%) had been pretreated with capecitabine. This suggests that the different dose intensity and administration schedule for fluoropyrimidine, as in the XELIRI regimen, and the synergy of capecitabine and irinotecan could not overcome possible acquired resistance to this drug.
Our current study is the largest retrospective series on chemotherapy with a combination of irinotecan and 5-FU in metastatic PAC after the failure of gemcitabine and platinum salts. It was a bi-center study with a homogenous population (metastatic, not locally advanced, PAC) and selection bias was reduced by the systematic treatment policy in both centers. However, patients were heterogeneous regarding the number of previous lines of chemotherapy or the type of FOLFIRI regimen. We could not compare the efficacy of the two FOLFIRI regimens due to the unequal distribution of patients between the two groups, with only 8 patients receiving the FOLFIRI-3 regimen. Moreover, this study included selected patients treated in high volume centers with teams that were experienced in the management of pancreatic tumors and their complications. Endoscopic procedures for the treatment of jaundice, a classic exclusion criteria for irinotecan (40%-60% of PAC), were easily accessible. Another possible bias was the high rate of previous surgery (32.9%). The natural history of operated patients might be more favorable than that of patients with unresectable PAC at diagnosis. The toxicity of the FOLIFIRI regimen was manageable, although dose adaptation was required in more than half of patients (57.1%). Only patients who are PS 0-1 seem to benefit from this regimen.
In conclusion, the FOLFIRI regimen is a valuable option in patients with metastatic PAC after failure of gemcitabine and platinum salts but should be considered for patients in good condition (WHO PS 0-1). Further studies are needed to determine whether FOLFIRI is a valuable option as first line therapy in advanced PAC.
ACKNOWLEDGMENTS
The authors thank Ms. Mélanie Gauthier for her help in the statistical analysis.
COMMENTS
Background
Pancreatic adenocarcinoma (PAC) is the fourth leading cause of cancer death in the Western countries. The overall prognosis of metastatic PAC remains poor with a 5-year survival rate of less than 5%. Gemcitabine is the reference first-line regimen for metastatic PAC treatment. About half of patients with metastatic PAC whose disease progresses under gemcitabine are eligible for subsequent line(s) of chemotherapy and there is no standard regimen in that setting. Preclinical and clinical studies have suggested that the combination of 5-fluorouracil (5-FU) and irinotecan (FOLFIRI regimen) may be beneficial in PAC. The research aimed to evaluate the efficacy and safety of FOLFIRI regimen in patients with metastatic PAC after the failure of gemcitabine and platinum salts.
Research frontiers
Tumor response rate, toxicity of irinotecan-based regimen, time to progression and overall survival were determined for the FOLFIRI regimen.
Innovations and breakthroughs
This is a homogeneous study of consecutive metastatic PAC patients treated by two experienced teams in the management of patients with pancreatic cancer. The present paper suggests that whereas the combination of gemcitabine and irinotecan was not effective enough, that of 5-FU and irinotecan appears to be beneficial regarding both efficacy and tolerability. In addition, the study series provides original data about the appropriate target population for the irinotecan-based regimen, particularly taking into account the PS status.
Applications
Recently, the FOLFIRINOX schema, combining 5-FU, irinotecan and oxaliplatin, was shown to be superior to gemcitabine in a first-line setting, with an overall survival of 11.1 mo (95% CI: 9-13.1 mo) vs 6.8 mo (95% CI: 5.5-7.6 mo, hazard ratio = 0.57) (P < 0.0001), respectively, in selected patients (performance status 0-1, absence of cholestasis). However, due to the hematological toxicity of this combination, many patients are not eligible for first-line therapy. The sequence FOLFOX then FOLFIRI (or the reverse) may be an alternative and should be considered as being better tolerated.
Terminology
For treatment purposes, pancreatic tumors are generally classified as resectable, locally advanced, or metastatic. A locally advanced pancreatic cancer is a tumor involving the arterial axis (celiac trunk, mesenteric artery) and thus is not resectable despite no detectable metastases. This form of cancer should be distinguished from metastatic tumors as the prognosis is different (slightly better, and some patients can have surgical treatment in case of a good tumor response after chemotherapy) and separate analyses are needed. Thus, locally advanced PAC patients were excluded from the study.
Peer review
This is an interesting study in which authors evaluate the efficacy and safety of this regimen in patients with metastatic PAC after the failure of gemcitabine and platinum salts. The results are convincing and suggest that FOLFIRI regimen had an acceptable toxicity and an interesting efficacy in our study, limited to patients in good condition.
Footnotes
Peer reviewer: Dr. Michel M Murr, MD, Director of Bariatric Surgery, Tampa General Hospital, 1 Tampa General Circle, Tampa, FL 33647, United States
S- Editor Wu X L- Editor Cant MR E- Editor Li JY
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. doi: 10.3892/or_00000749. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Bachet JB, Mitry E, Lièvre A, Lepère C, Vaillant JN, Declety G, Parlier H, Emile JF, Julié C, Rougier P. Second- and third-line chemotherapy in patients with metastatic pancreatic adenocarcinoma: feasibility and potential benefits in a retrospective series of 117 patients. Gastroenterol Clin Biol. 2009;33:1036–1044. doi: 10.1016/j.gcb.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Dahan L, Bonnetain F, Ychou M, Mitry E, Gasmi M, Raoul JL, Cattan S, Phelip JM, Hammel P, Chauffert B, et al. Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301) Gut. 2010;59:1527–1534. doi: 10.1136/gut.2010.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oettle H, Pelzer U, Stieler J, Hilbig A, Roll L, Schwaner I, Adler M, Detken S, Drken B, Riess H. Oxaliplatin/folinic acid/5-fluorouracil [24h] (OFF) plus best supportive care versus best supportive care alone (BSC) in second-line therapy of gemcitabine-refractory advanced pancreatic cancer (CONKO 003) J Clin Oncol. 2005:s4031. [Google Scholar]
- 11.Gounaris I, Zaki K, Corrie P. Options for the treatment of gemcitabine-resistant advanced pancreatic cancer. JOP. 2010;11:113–123. [PubMed] [Google Scholar]
- 12.Bissery MC, Vrignaud P, Lavelle F, Chabot GG. Experimental antitumor activity and pharmacokinetics of the camptothecin analog irinotecan (CPT-11) in mice. Anticancer Drugs. 1996;7:437–460. doi: 10.1097/00001813-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Shimazoe T, Sato K, Sugimoto Y, Tsuruo T, Kono A. Differential expression of DNA topoisomerase I gene between CPT-11 acquired- and native-resistant human pancreatic tumor cell lines: detected by RNA/PCR-based quantitation assay. Biochem Biophys Res Commun. 1992;184:618–625. doi: 10.1016/0006-291x(92)90634-w. [DOI] [PubMed] [Google Scholar]
- 14.Sakata Y, Shimada Y, Yoshino M, Kambe M, Futatsuki K, Nakao I, Ogawa N, Wakui A, Taguchi T. [A late phase II study of CPT-11, irinotecan hydrochloride, in patients with advanced pancreatic cancer. CPT-11 Study Group on Gastrointestinal Cancer] Gan To Kagaku Ryoho. 1994;21:1039–1046. [PubMed] [Google Scholar]
- 15.Wagener DJ, Verdonk HE, Dirix LY, Catimel G, Siegenthaler P, Buitenhuis M, Mathieu-Boué A, Verweij J. Phase II trial of CPT-11 in patients with advanced pancreatic cancer, an EORTC early clinical trials group study. Ann Oncol. 1995;6:129–132. doi: 10.1093/oxfordjournals.annonc.a059107. [DOI] [PubMed] [Google Scholar]
- 16.Ueno H, Okusaka T, Funakoshi A, Ishii H, Yamao K, Ishikawa O, Ohkawa S, Saitoh S. A phase II study of weekly irinotecan as first-line therapy for patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2007;59:447–454. doi: 10.1007/s00280-006-0283-9. [DOI] [PubMed] [Google Scholar]
- 17.Guichard S, Cussac D, Hennebelle I, Bugat R, Canal P. Sequence-dependent activity of the irinotecan-5FU combination in human colon-cancer model HT-29 in vitro and in vivo. Int J Cancer. 1997;73:729–734. doi: 10.1002/(sici)1097-0215(19971127)73:5<729::aid-ijc20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Mans DR, Grivicich I, Peters GJ, Schwartsmann G. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999;35:1851–1861. doi: 10.1016/s0959-8049(99)00222-1. [DOI] [PubMed] [Google Scholar]
- 19.Mullany S, Svingen PA, Kaufmann SH, Erlichman C. Effect of adding the topoisomerase I poison 7-ethyl-10-hydroxycamptothecin (SN-38) to 5-fluorouracil and folinic acid in HCT-8 cells: elevated dTTP pools and enhanced cytotoxicity. Cancer Chemother Pharmacol. 1998;42:391–399. doi: 10.1007/s002800050835. [DOI] [PubMed] [Google Scholar]
- 20.Taïeb J, Lecomte T, Aparicio T, Asnacios A, Mansourbakht T, Artru P, Fallik D, Spano JP, Landi B, Lledo G, et al. FOLFIRI.3, a new regimen combining 5-fluorouracil, folinic acid and irinotecan, for advanced pancreatic cancer: results of an Association des Gastro-Enterologues Oncologues (Gastroenterologist Oncologist Association) multicenter phase II study. Ann Oncol. 2007;18:498–503. doi: 10.1093/annonc/mdl427. [DOI] [PubMed] [Google Scholar]
- 21.Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, Lee SS, Seo DW, Lee SK, Kim MH, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658–1663. doi: 10.1038/sj.bjc.6605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebbia V, Maiello E, Giuliani F, Borsellino N, Arcara C, Colucci G. Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol. 2010;33:461–464. doi: 10.1097/COC.0b013e3181b4e3b0. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 25.Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587–592. doi: 10.1038/sj.bjc.6603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulke MH, Tempero MA, Niedzwiecki D, Hollis DR, Kindler HL, Cusnir M, Enzinger PC, Gorsch SM, Goldberg RM, Mayer RJ. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. J Clin Oncol. 2009;27:5506–5512. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endlicher E, Troppmann M, Kullmann A, Golder S, Herold T, Herfarth H, Grossmann J, Schlottmann K, Kullmann F. Irinotecan plus gemcitabine and 5-fluorouracil in advanced pancreatic cancer: a phase II study. Oncology. 2007;72:279–284. doi: 10.1159/000113039. [DOI] [PubMed] [Google Scholar]
- 28.Goel A, Grossbard ML, Malamud S, Homel P, Dietrich M, Rodriguez T, Mirzoyev T, Kozuch P. Pooled efficacy analysis from a phase I-II study of biweekly irinotecan in combination with gemcitabine, 5-fluorouracil, leucovorin and cisplatin in patients with metastatic pancreatic cancer. Anticancer Drugs. 2007;18:263–271. doi: 10.1097/CAD.0b013e3280121334. [DOI] [PubMed] [Google Scholar]
- 29.Shitara K, Komatsu Y, Yuki S, Munakata M, Muto O, Shimaya S, Sakata Y. Pilot study of combination chemotherapy using irinotecan plus S-1 for metastatic pancreatic cancer. Oncology. 2008;75:67–70. doi: 10.1159/000151742. [DOI] [PubMed] [Google Scholar]
- 30.Yi SY, Park YS, Kim HS, Jun HJ, Kim KH, Chang MH, Park MJ, Uhm JE, Lee J, Park SH, et al. Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:1141–1145. doi: 10.1007/s00280-008-0839-y. [DOI] [PubMed] [Google Scholar]
- 31.Klapdor R, Fenner C. Irinotecan(Campto R): efficacy as third/forth line therapy in advanced pancreatic cancer. Anticancer Res. 2000;20:5209–5212. [PubMed] [Google Scholar]
- 32.Ulrich-Pur H, Raderer M, Verena Kornek G, Schüll B, Schmid K, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F, et al. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer. 2003;88:1180–1184. doi: 10.1038/sj.bjc.6600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantore M, Rabbi C, Fiorentini G, Oliani C, Zamagni D, Iacono C, Mambrini A, Del Freo A, Manni A. Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology. 2004;67:93–97. doi: 10.1159/000080993. [DOI] [PubMed] [Google Scholar]
- 34.Oh SY, Kim HJ, Kim TH, Lee GW, Kim HG, Jeong CY, Kwon HC, Kang JH. Pilot study of irinotecan/oxalipltin (IROX) combination chemotherapy for patients with gemcitabine- and 5-fluorouracil- refractory pancreatic cancer. Invest New Drugs. 2010;28:343–349. doi: 10.1007/s10637-009-9265-1. [DOI] [PubMed] [Google Scholar]
- 35.Kozuch P, Grossbard ML, Barzdins A, Araneo M, Robin A, Frager D, Homel P, Marino J, DeGregorio P, Bruckner HW. Irinotecan combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and noncrossresistant treatment for chemotherapy refractory metastatic pancreatic cancer. Oncologist. 2001;6:488–495. doi: 10.1634/theoncologist.6-6-488. [DOI] [PubMed] [Google Scholar]
- 36.Ko AH, Dito E, Schillinger B, Venook AP, Bergsland EK, Tempero MA. Excess toxicity associated with docetaxel and irinotecan in patients with metastatic, gemcitabine-refractory pancreatic cancer: results of a phase II study. Cancer Invest. 2008;26:47–52. doi: 10.1080/07357900701681483. [DOI] [PubMed] [Google Scholar]
- 37.Reni M, Panucci MG, Passoni P, Bonetto E, Nicoletti R, Ronzoni M, Zerbi A, Staudacher C, Di Carlo V, Villa E. Salvage chemotherapy with mitomycin, docetaxel, and irinotecan (MDI regimen) in metastatic pancreatic adenocarcinoma: a phase I and II trial. Cancer Invest. 2004;22:688–696. doi: 10.1081/cnv-200032929. [DOI] [PubMed] [Google Scholar]
- 38.Cereda S, Reni M, Rognone A, Ghidini M, Belli C, Longoni S, Fugazza C, Brioschi M, Nicoletti R, Balzano G, et al. XELIRI or FOLFIRI as salvage therapy in advanced pancreatic cancer. Anticancer Res. 2010;30:4785–4790. [PubMed] [Google Scholar]