Abstract
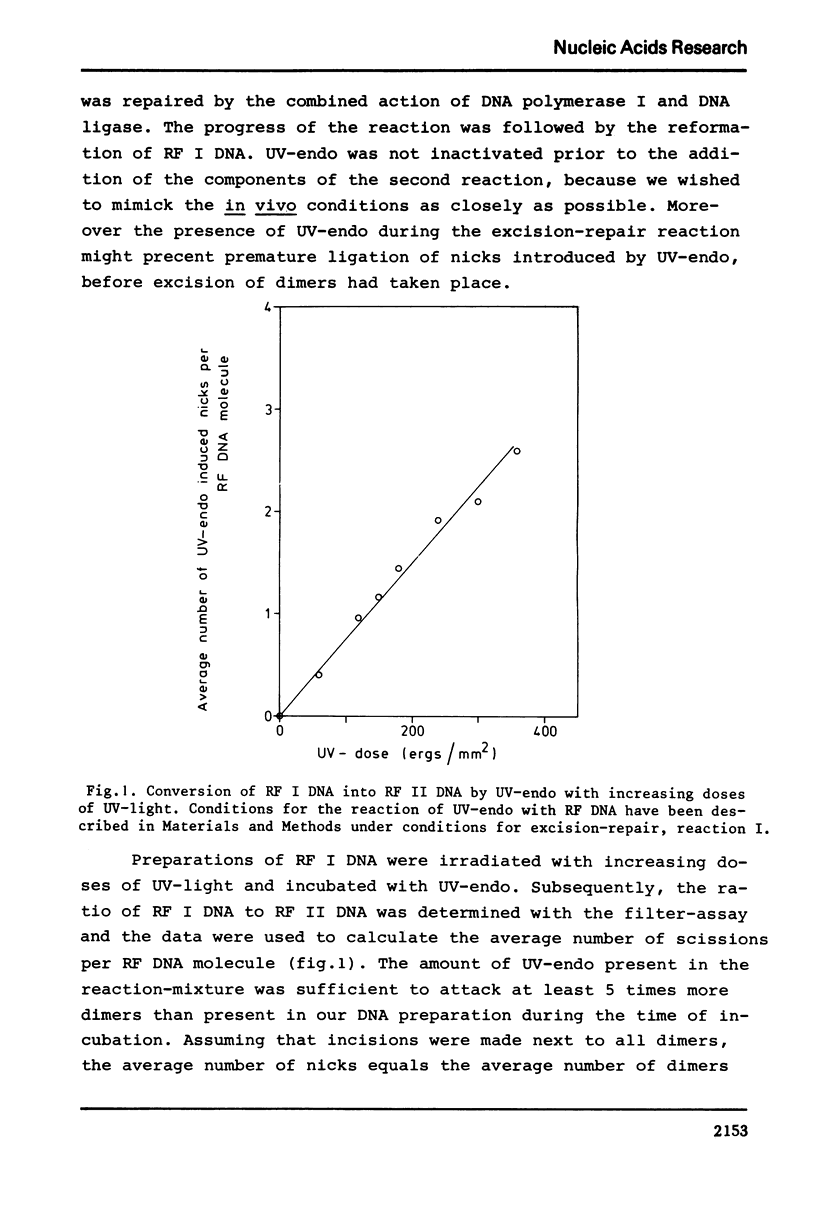
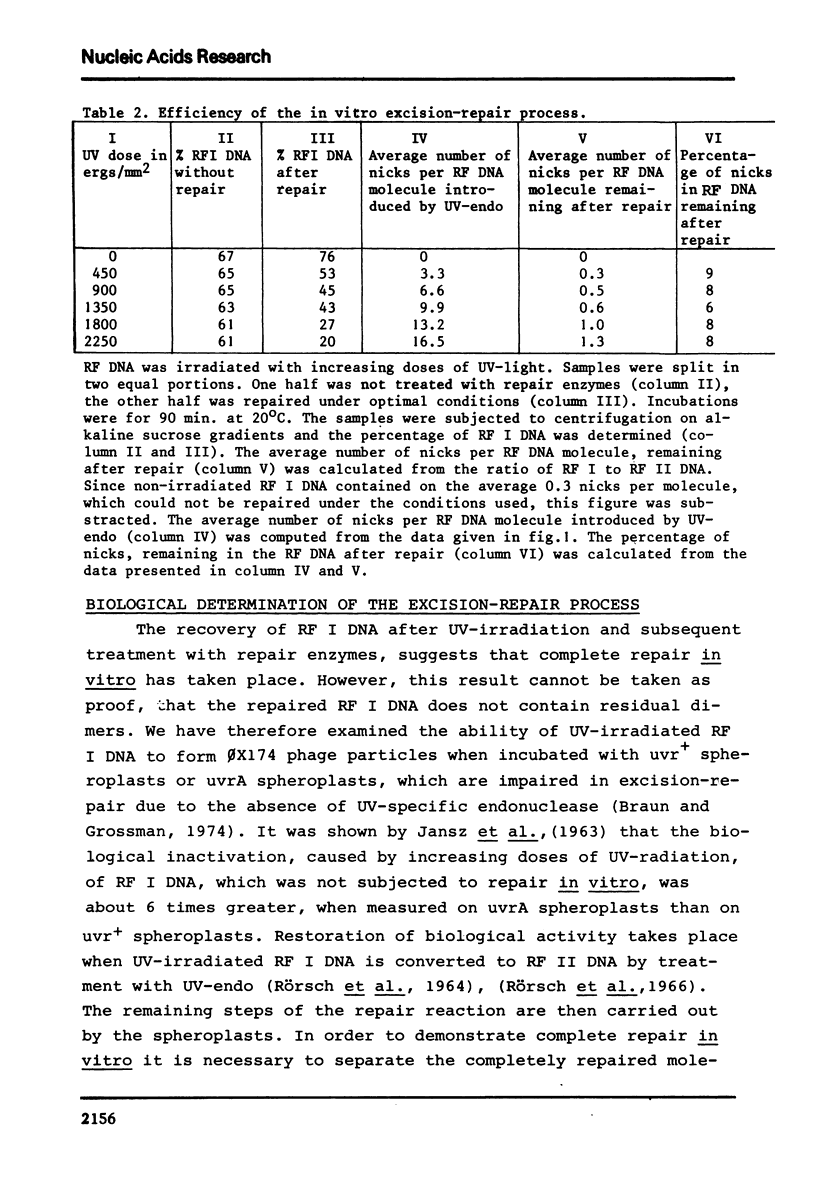
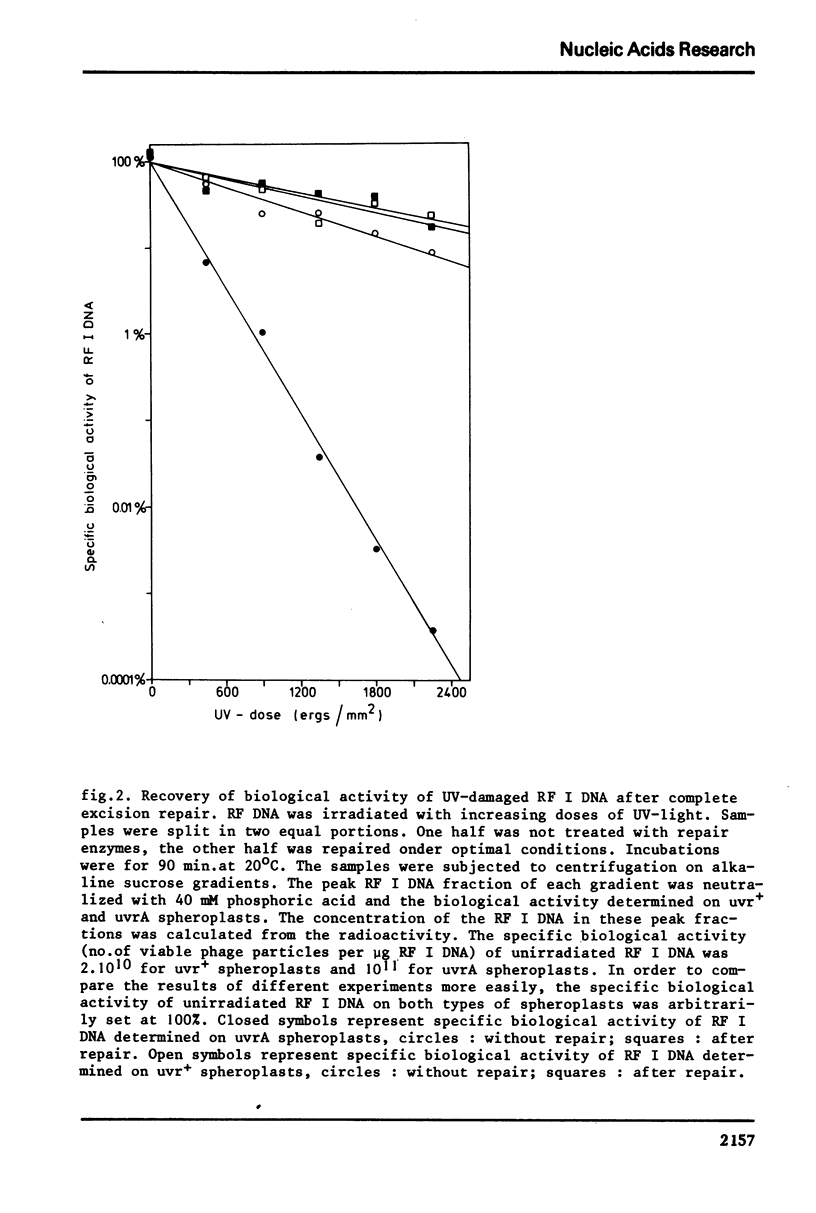
We have studied excision-repair of UV-irradiated phiX174 RFI DNA in vitro with UV-specific endonuclease from Micrococcus luteus (UV-endo), DNA polymerase I from Escherichia coli and DNA ligase from phage T4 infected E. coli. Excision-repair was measured a) by physico-chemical methods, i.e. by determination of the conversion of RF I DNA into RF II DNA by UV-endo and by the subsequent conversion of RF II DNA ligase, b) by biological methods i. e. by measuring the ability of the reaction product to form phages upon incubation with spheroplasts from the appropriate strains of E. coli. Using the first method, we have shown, that more than 90% of the pyrimidine dimers can be repaired in vitro; with the latter method we have shown, that the molecules which are repaired as defined by method a) have regained full biological activity. Exonuclease III was found to be not essential for excision-repair in vitro and also did not stimulate repair. From this result we conclude that UV-endo generates 3'OH endgroups, in agreement with results obtained by Hamilton et al. (1974). The usefulness of the method presented in this paper with regard to the study of excision-repair is discussed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Cook J. S. Photoreactivation in animal cells. Photophysiology. 1970;5:191–233. [PubMed] [Google Scholar]
- Englund P. T., Kelly R. B., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXI. Binding of deoxyribonucleic acid to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3045–3052. [PubMed] [Google Scholar]
- Friedberg E. C., Lehman I. R. Excision of thymine dimers by proteolytic and amber fragments of E. coli DNA polymerase I. Biochem Biophys Res Commun. 1974 May 7;58(1):132–139. doi: 10.1016/0006-291x(74)90901-2. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., van Sluis C. A., Heijneker H. L., Rörsch A. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. I. General characterization. Mol Gen Genet. 1973 Jul 31;124(1):69–82. doi: 10.1007/BF00267166. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Boyer H. Susceptibility of the phiX-like phages G4 and G14 to R-EcoRi endonuclease. Virology. 1974 Nov;62(1):270–275. doi: 10.1016/0042-6822(74)90321-3. [DOI] [PubMed] [Google Scholar]
- Hamilton L., Mahler I., Grossman L. Enzymatic repair of deoxyribonucleic acid; the biochemical and biological repair properties of a deoxyribonucleic acid polymerase from micrococcus luteus. Biochemistry. 1974 Apr 23;13(9):1886–1896. doi: 10.1021/bi00706a017. [DOI] [PubMed] [Google Scholar]
- Heijneker H. L., Ellens D. J., Tjeerde R. H., Glickman B. W., van Dorp B., Pouwels P. H. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. II. Purification and properties of the mutant enzyme. Mol Gen Genet. 1973 Jul 31;124(1):83–96. doi: 10.1007/BF00267167. [DOI] [PubMed] [Google Scholar]
- Heijneker H. L., Pannekoek H., Oosterbaan R. A., Pouwels P. H., Bron S., Arwert F., Venema G. In vitro excision-repair of ultraviolet-irradiated transforming DNA from Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2967–2971. doi: 10.1073/pnas.68.12.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyneker H. L., Klenow H. Involvement of Escherichia coli DNA polymerase-I-associated 5' in equilibrium 3' exonuclease in excision-repair of UV-damaged DNA. Basic Life Sci. 1975;5A:219–223. doi: 10.1007/978-1-4684-2895-7_29. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair and recombination. Br Med Bull. 1973 Sep;29(3):226–235. doi: 10.1093/oxfordjournals.bmb.a071012. [DOI] [PubMed] [Google Scholar]
- JANSZ H. S., POUWELS P. H., VAN ROTTERDAM SENSITIVITY TO ULTRAVIOLET LIGHT OF SINGLE- AND DOUBLE-STRANDED DNA. Biochim Biophys Acta. 1963 Dec 20;76:655–657. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kushner S. R., Kaplan J. C., Ono H., Grossman L. Enzymatic repair of deoxyribonucleic acid. IV. Mechanism of photoproduct excision. Biochemistry. 1971 Aug 31;10(18):3325–3334. doi: 10.1021/bi00794a002. [DOI] [PubMed] [Google Scholar]
- Mazin A. L., Sulimova G. E., Vanyushin B. F. Granulated hydroxyapatite: preparation and chromatographic properties. Anal Biochem. 1974 Sep;61(1):62–71. doi: 10.1016/0003-2697(74)90333-9. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Okubo S., Takagi Y. Repair of ultraviolet-damaged DNA in Micrococcus lysodeikticus. I. An endonuclease specific for ultraviolet-irradiated DNA. Biochim Biophys Acta. 1971 Jan 1;228(1):67–82. doi: 10.1016/0005-2787(71)90547-8. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Paribok V. P., Tomilin N. V. Recognition of pyrimidine dimers in DNA by the incision enzyme from Micrococcus lysodeikticus. Nat New Biol. 1971 Apr 14;230(15):210–211. doi: 10.1038/newbio230210a0. [DOI] [PubMed] [Google Scholar]
- ROERSCH A., VAN DER KAM P. C., ADEMA J. DARK REACTIVATION OF ULTRAVIOLET IRRADIATED BACTERIOPHAGE DEOXYRIBONUCLEIC ACID IN VITRO. Biochim Biophys Acta. 1964 Feb 17;80:346–348. [PubMed] [Google Scholar]
- Rauth A. M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys J. 1965 May;5(3):257–273. doi: 10.1016/s0006-3495(65)86715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. II. A proteolytic fragment containing the 5' leads to 3' exonuclease function. Restoration of intact enzyme functions from the two proteolytic fragments. J Biol Chem. 1972 Jan 10;247(1):232–240. [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- Strauss B., Searashi T., Robbins M. Repair of DNA studied with a nuclease specific for UV-induced lesions. Proc Natl Acad Sci U S A. 1966 Sep;56(3):932–939. doi: 10.1073/pnas.56.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]



